

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM249169 (US9453048, 11 | US9453048, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Assays were performed in black, flat-bottom, 384-well plates. The final assay volume was 50 μL prepared from additions of N-His-Tb-BIR3(241-356,... | US Patent US9453048 (2016) BindingDB Entry DOI: 10.7270/Q2057DV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM249163 (US9453048, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Assays were performed in black, flat-bottom, 384-well plates. The final assay volume was 50 μL prepared from additions of N-His-Tb-BIR3(241-356,... | US Patent US9453048 (2016) BindingDB Entry DOI: 10.7270/Q2057DV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM249161 (US9453048, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Assays were performed in black, flat-bottom, 384-well plates. The final assay volume was 50 μL prepared from additions of N-His-Tb-BIR3(241-356,... | US Patent US9453048 (2016) BindingDB Entry DOI: 10.7270/Q2057DV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM249167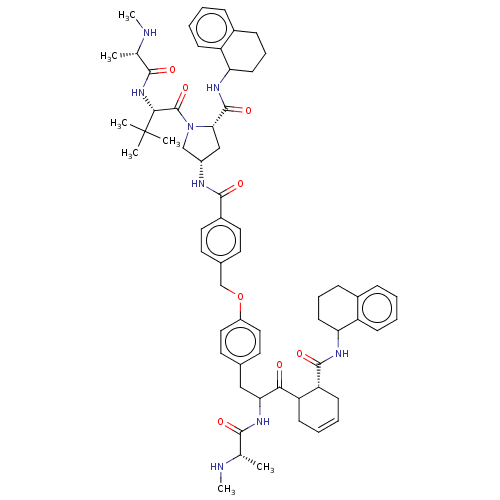 (US9453048, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Assays were performed in black, flat-bottom, 384-well plates. The final assay volume was 50 μL prepared from additions of N-His-Tb-BIR3(241-356,... | US Patent US9453048 (2016) BindingDB Entry DOI: 10.7270/Q2057DV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM249174 (US9453048, 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Assays were performed in black, flat-bottom, 384-well plates. The final assay volume was 50 μL prepared from additions of N-His-Tb-BIR3(241-356,... | US Patent US9453048 (2016) BindingDB Entry DOI: 10.7270/Q2057DV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM249162 (US9453048, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Assays were performed in black, flat-bottom, 384-well plates. The final assay volume was 50 μL prepared from additions of N-His-Tb-BIR3(241-356,... | US Patent US9453048 (2016) BindingDB Entry DOI: 10.7270/Q2057DV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM249172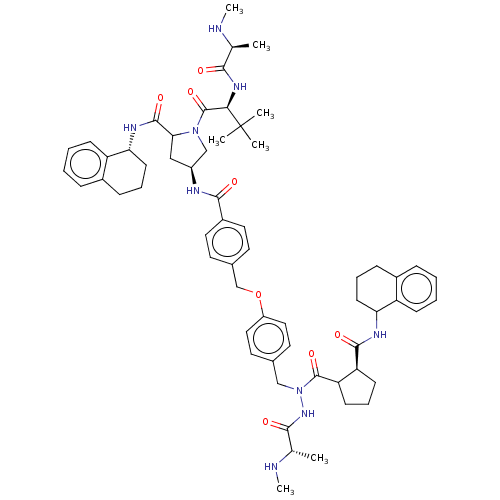 (US9453048, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Assays were performed in black, flat-bottom, 384-well plates. The final assay volume was 50 μL prepared from additions of N-His-Tb-BIR3(241-356,... | US Patent US9453048 (2016) BindingDB Entry DOI: 10.7270/Q2057DV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM249168 (US9453048, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Assays were performed in black, flat-bottom, 384-well plates. The final assay volume was 50 μL prepared from additions of N-His-Tb-BIR3(241-356,... | US Patent US9453048 (2016) BindingDB Entry DOI: 10.7270/Q2057DV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM249165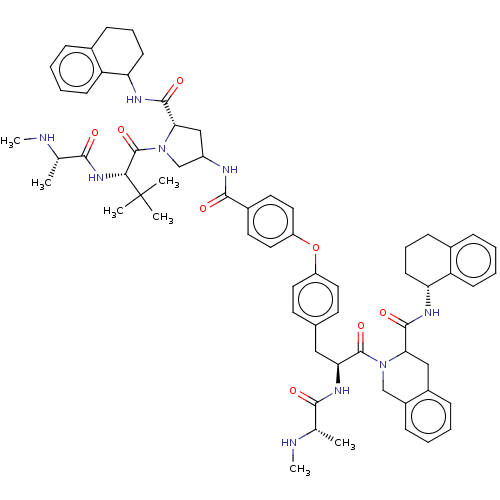 (US9453048, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Assays were performed in black, flat-bottom, 384-well plates. The final assay volume was 50 μL prepared from additions of N-His-Tb-BIR3(241-356,... | US Patent US9453048 (2016) BindingDB Entry DOI: 10.7270/Q2057DV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM249160 (US9453048, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Assays were performed in black, flat-bottom, 384-well plates. The final assay volume was 50 μL prepared from additions of N-His-Tb-BIR3(241-356,... | US Patent US9453048 (2016) BindingDB Entry DOI: 10.7270/Q2057DV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM249166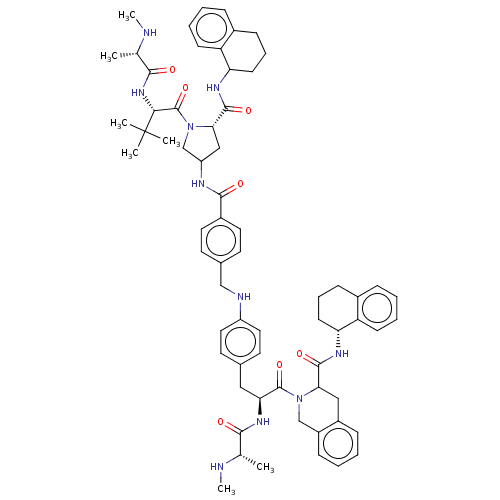 (US9453048, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Assays were performed in black, flat-bottom, 384-well plates. The final assay volume was 50 μL prepared from additions of N-His-Tb-BIR3(241-356,... | US Patent US9453048 (2016) BindingDB Entry DOI: 10.7270/Q2057DV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM249171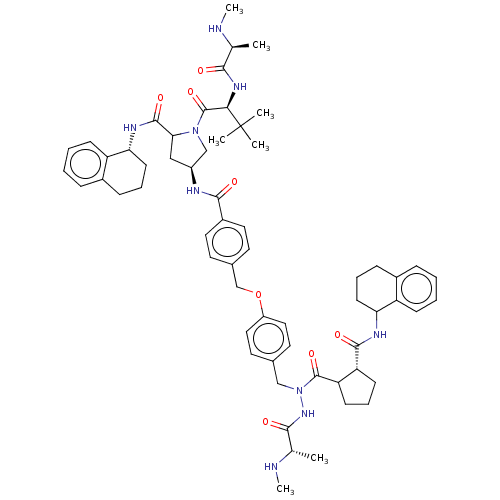 (US9453048, 13 | US9453048, 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Assays were performed in black, flat-bottom, 384-well plates. The final assay volume was 50 μL prepared from additions of N-His-Tb-BIR3(241-356,... | US Patent US9453048 (2016) BindingDB Entry DOI: 10.7270/Q2057DV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM249164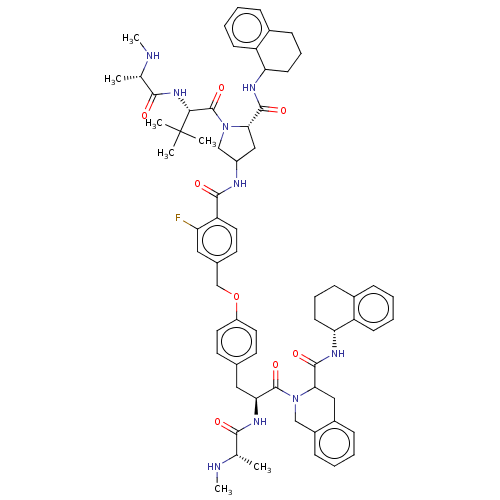 (US9453048, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Assays were performed in black, flat-bottom, 384-well plates. The final assay volume was 50 μL prepared from additions of N-His-Tb-BIR3(241-356,... | US Patent US9453048 (2016) BindingDB Entry DOI: 10.7270/Q2057DV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM249178 (US9453048, 20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Assays were performed in black, flat-bottom, 384-well plates. The final assay volume was 50 μL prepared from additions of N-His-Tb-BIR3(241-356,... | US Patent US9453048 (2016) BindingDB Entry DOI: 10.7270/Q2057DV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM249177 (US9453048, 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Assays were performed in black, flat-bottom, 384-well plates. The final assay volume was 50 μL prepared from additions of N-His-Tb-BIR3(241-356,... | US Patent US9453048 (2016) BindingDB Entry DOI: 10.7270/Q2057DV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM249176 (US9453048, 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Assays were performed in black, flat-bottom, 384-well plates. The final assay volume was 50 μL prepared from additions of N-His-Tb-BIR3(241-356,... | US Patent US9453048 (2016) BindingDB Entry DOI: 10.7270/Q2057DV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM249171 (US9453048, 13 | US9453048, 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Assays were performed in black, flat-bottom, 384-well plates. The final assay volume was 50 μL prepared from additions of N-His-Tb-BIR3(241-356,... | US Patent US9453048 (2016) BindingDB Entry DOI: 10.7270/Q2057DV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM249169 (US9453048, 11 | US9453048, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Assays were performed in black, flat-bottom, 384-well plates. The final assay volume was 50 μL prepared from additions of N-His-Tb-BIR3(241-356,... | US Patent US9453048 (2016) BindingDB Entry DOI: 10.7270/Q2057DV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM249175 (US9453048, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Assays were performed in black, flat-bottom, 384-well plates. The final assay volume was 50 μL prepared from additions of N-His-Tb-BIR3(241-356,... | US Patent US9453048 (2016) BindingDB Entry DOI: 10.7270/Q2057DV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM205417 (US9249151, 63) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description R3 domains of human XIAP (covering amino acids 241 to 356; XIAP BIR3) and cIAPl (covering amino acids 256 to 363; cIAPl BIR3) were expressed and puri... | US Patent US9249151 (2016) BindingDB Entry DOI: 10.7270/Q24748P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM205375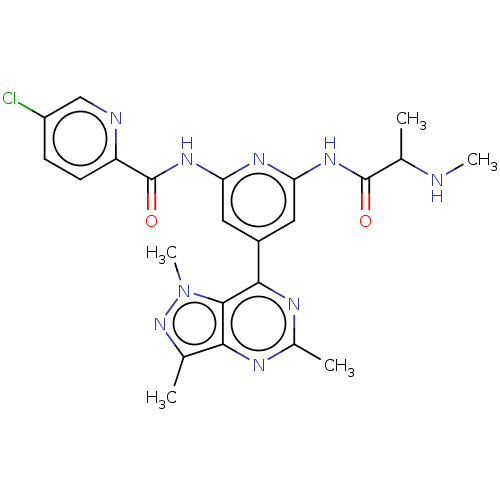 (US9249151, 21 | US9249151, 60) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description R3 domains of human XIAP (covering amino acids 241 to 356; XIAP BIR3) and cIAPl (covering amino acids 256 to 363; cIAPl BIR3) were expressed and puri... | US Patent US9249151 (2016) BindingDB Entry DOI: 10.7270/Q24748P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50425719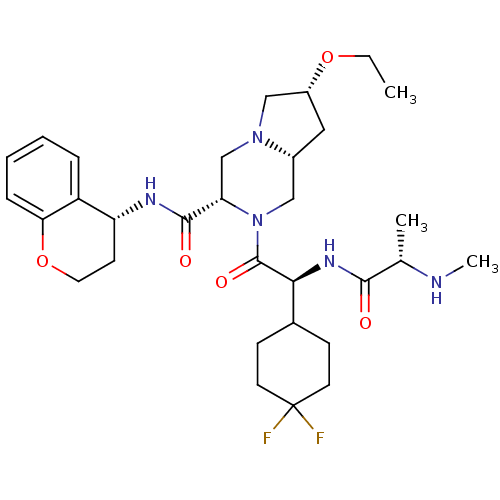 (CHEMBL2316217) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... | J Med Chem 56: 1228-46 (2013) Article DOI: 10.1021/jm301674z BindingDB Entry DOI: 10.7270/Q23N24QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50425721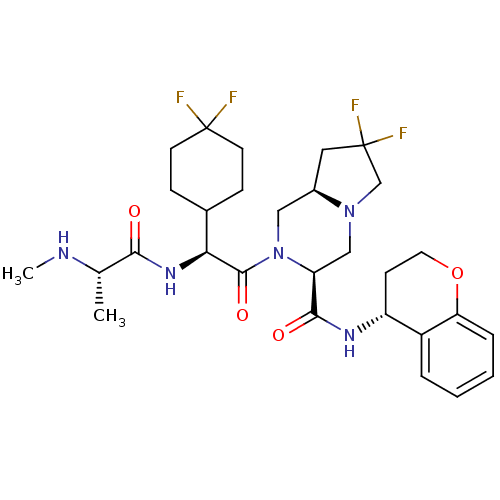 (CHEMBL2311586) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... | J Med Chem 56: 1228-46 (2013) Article DOI: 10.1021/jm301674z BindingDB Entry DOI: 10.7270/Q23N24QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50425728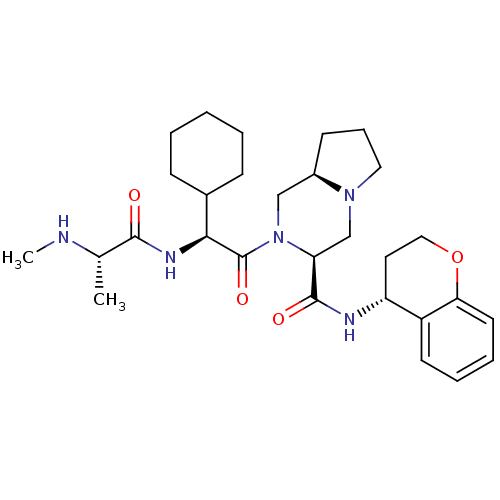 (CHEMBL2365533) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... | J Med Chem 56: 1228-46 (2013) Article DOI: 10.1021/jm301674z BindingDB Entry DOI: 10.7270/Q23N24QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50425722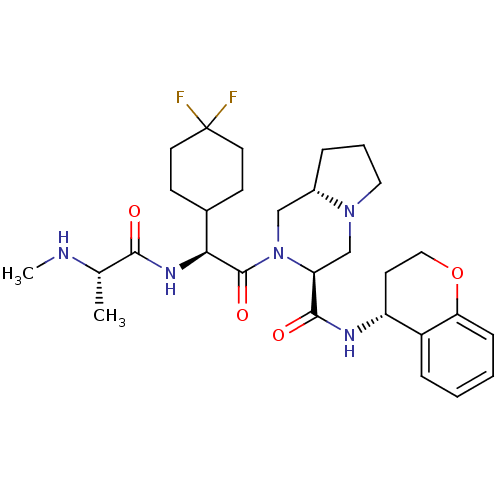 (CHEMBL2316215) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... | J Med Chem 56: 1228-46 (2013) Article DOI: 10.1021/jm301674z BindingDB Entry DOI: 10.7270/Q23N24QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50425725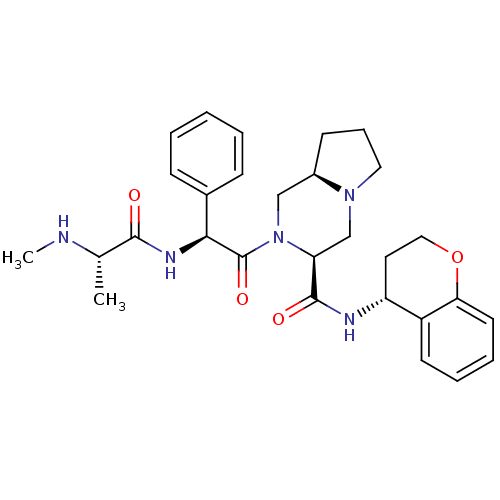 (CHEMBL2316224) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... | J Med Chem 56: 1228-46 (2013) Article DOI: 10.1021/jm301674z BindingDB Entry DOI: 10.7270/Q23N24QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50425730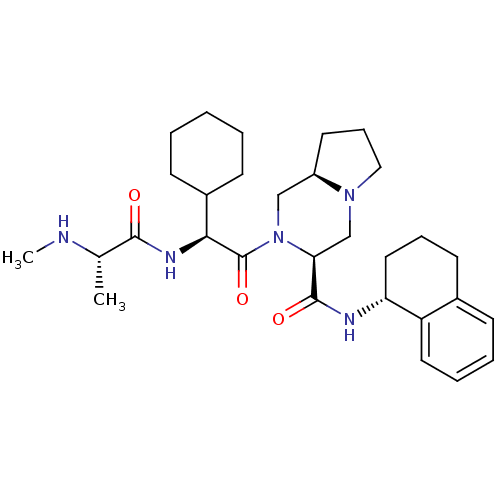 (CHEMBL2316219) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... | J Med Chem 56: 1228-46 (2013) Article DOI: 10.1021/jm301674z BindingDB Entry DOI: 10.7270/Q23N24QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50112346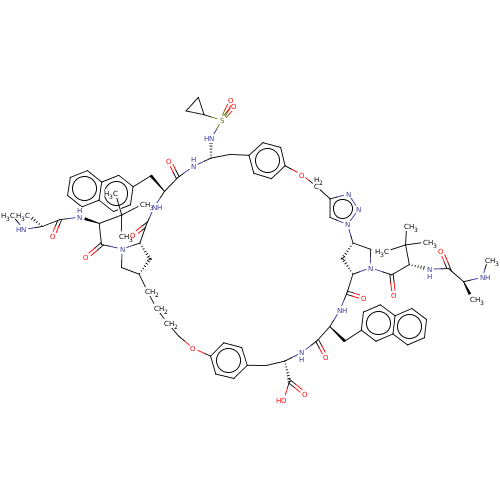 (CHEMBL3609325) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research Curated by ChEMBL | Assay Description Inhibition of cIAP BIR2-3 domain (unknown origin) | ACS Med Chem Lett 6: 770-5 (2015) Article DOI: 10.1021/acsmedchemlett.5b00091 BindingDB Entry DOI: 10.7270/Q29P33D2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50425724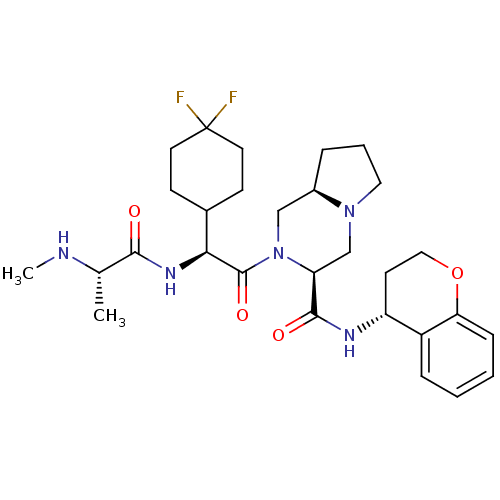 (CHEMBL2316213) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... | J Med Chem 56: 1228-46 (2013) Article DOI: 10.1021/jm301674z BindingDB Entry DOI: 10.7270/Q23N24QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50425723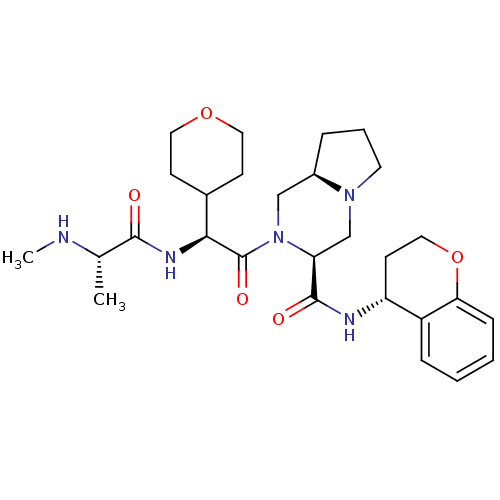 (CHEMBL2316214) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... | J Med Chem 56: 1228-46 (2013) Article DOI: 10.1021/jm301674z BindingDB Entry DOI: 10.7270/Q23N24QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50425727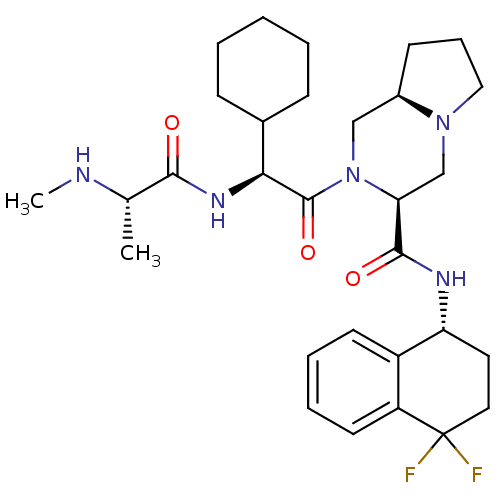 (CHEMBL2316222) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... | J Med Chem 56: 1228-46 (2013) Article DOI: 10.1021/jm301674z BindingDB Entry DOI: 10.7270/Q23N24QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50425720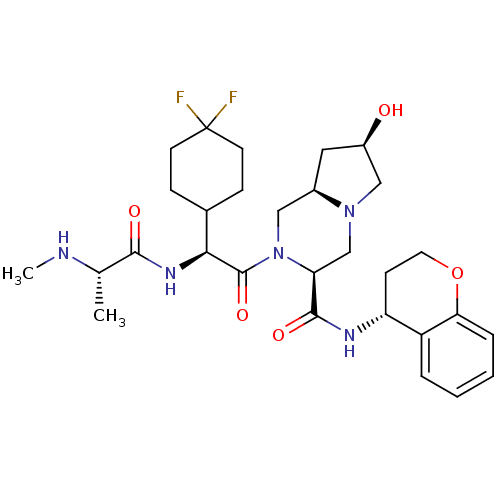 (CHEMBL2316216) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... | J Med Chem 56: 1228-46 (2013) Article DOI: 10.1021/jm301674z BindingDB Entry DOI: 10.7270/Q23N24QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50343521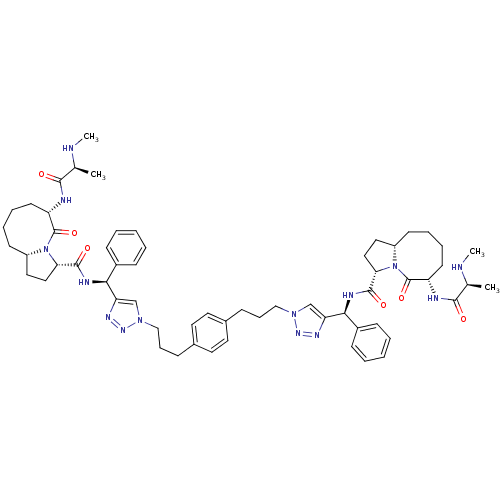 ((S,3S,3'S,6S,6'S,1'aS,1'a'S)-N,N'-((1S,1'S)-(1,1'-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Competitive inhibition of human cIAP2 BIR3 domain after 2 to 3 hrs by fluorescence polarization assay | J Med Chem 54: 3306-18 (2011) Article DOI: 10.1021/jm101651b BindingDB Entry DOI: 10.7270/Q2RJ4JSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50574939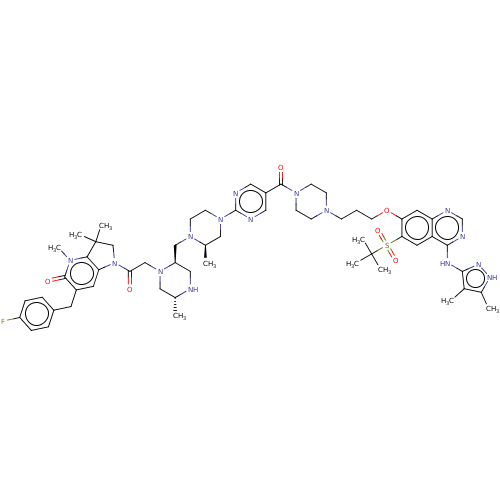 (CHEMBL4870089) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human 6His-thr cIAP1 BIR3 (253 to 363) domain expressed in Escherichia coli measured after 105 mins in dark by time-resolved fluo... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01118 BindingDB Entry DOI: 10.7270/Q2VD739F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50326216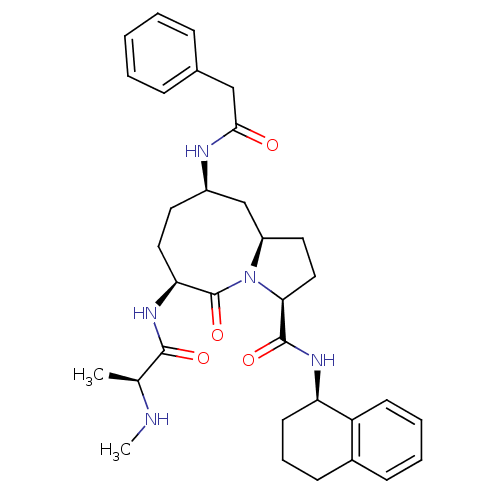 ((3S,6S,9R,10aR)-6-((S)-2-(Methylamino)propanamido)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of fluorescent SM5F peptide from His-tagged human cIAP2 BIR3 domain expressed in Escherichia coli BL21(DE3) cells by fluorescence polari... | J Med Chem 53: 6361-7 (2010) Article DOI: 10.1021/jm100487z BindingDB Entry DOI: 10.7270/Q27H1JTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50279272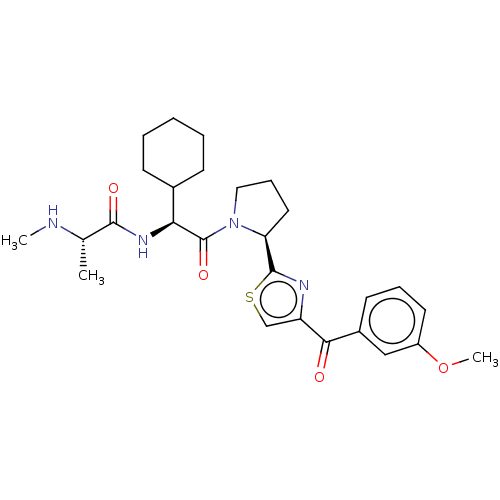 (CHEMBL4164385) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibitory concentration against SO561945 (HIV 1 mutant RT) viral viral infection of MT-4 cells | ACS Med Chem Lett 8: 1042-1047 (2017) Article DOI: 10.1021/acsmedchemlett.7b00247 BindingDB Entry DOI: 10.7270/Q2XG9TNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM240880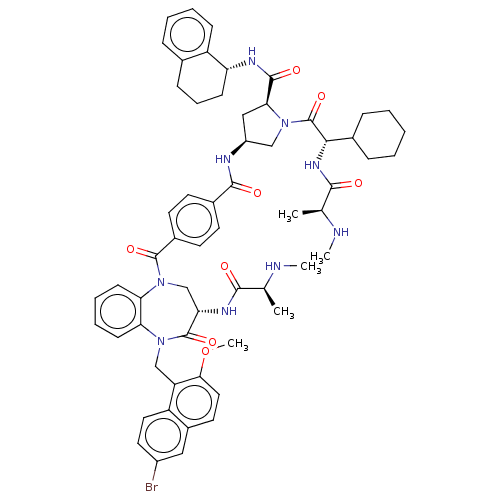 (US9409888, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.33 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Hoffmann-La Roche Inc. US Patent | Assay Description Ten nanomolar of 6× Histidine-tagged BIR2 domain, corresponding to amino acids 124-240 of XIAP, or BIR3 domain, corresponding to amino acids 241-356 ... | US Patent US9409888 (2016) BindingDB Entry DOI: 10.7270/Q2R78D4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50425726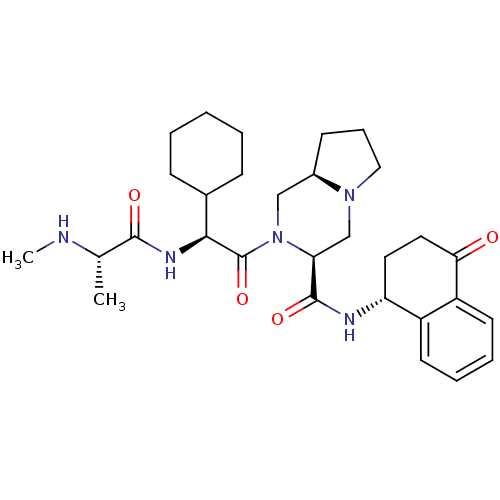 (CHEMBL2316223) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... | J Med Chem 56: 1228-46 (2013) Article DOI: 10.1021/jm301674z BindingDB Entry DOI: 10.7270/Q23N24QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50574934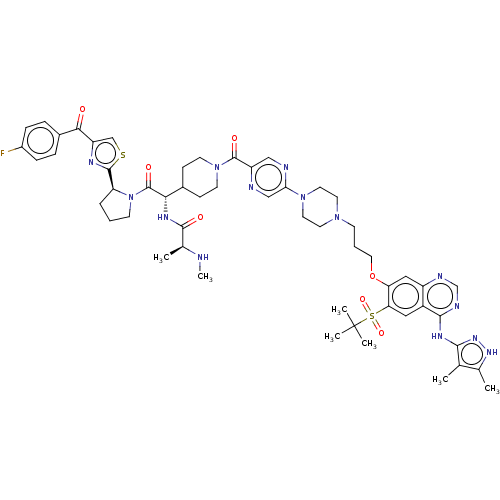 (CHEMBL4876228) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human 6His-thr cIAP1 BIR3 (253 to 363) domain expressed in Escherichia coli measured after 105 mins in dark by time-resolved fluo... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01118 BindingDB Entry DOI: 10.7270/Q2VD739F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50078358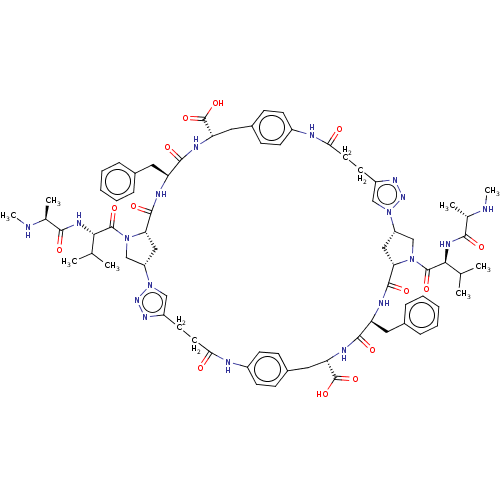 (CHEMBL3414723) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Ensemble Therapeutics Corp Curated by ChEMBL | Assay Description Inhibition of cIAP1 BIR3 (154 to 352 residues) (unknown origin) by fluoresceinated dimeric SMAC peptide based fluorescence polarization assay | J Med Chem 58: 2855-61 (2015) Article DOI: 10.1021/jm501892g BindingDB Entry DOI: 10.7270/Q2RF5WQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50343523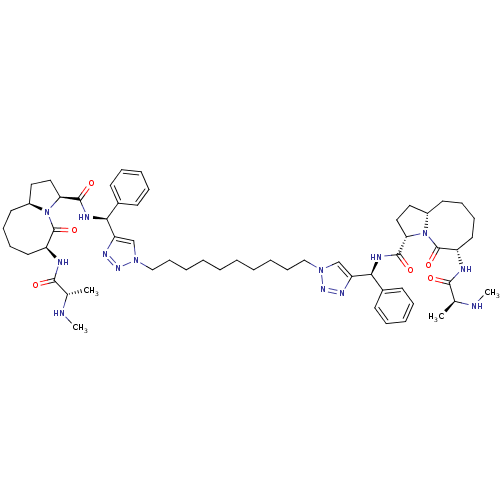 ((S,3S,3'S,6S,6'S,1'aS,1'a'S)-N,N'-((1S,1'S)-(1,1'-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Competitive inhibition of human cIAP2 BIR3 domain after 2 to 3 hrs by fluorescence polarization assay | J Med Chem 54: 3306-18 (2011) Article DOI: 10.1021/jm101651b BindingDB Entry DOI: 10.7270/Q2RJ4JSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50393630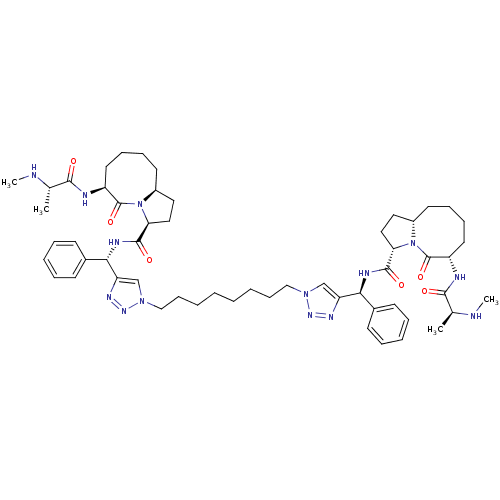 (CHEMBL2158599) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to BIR3 domain of cIAP2 by fluorescence polarization assay | J Med Chem 55: 106-14 (2012) Article DOI: 10.1021/jm201072x BindingDB Entry DOI: 10.7270/Q2BG2Q3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50393632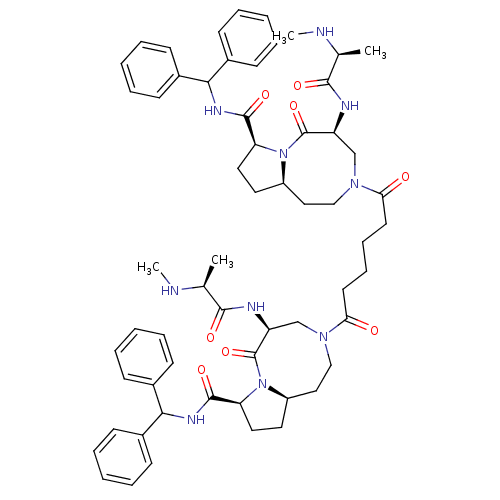 (CHEMBL2158601) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to BIR3 domain of cIAP2 by fluorescence polarization assay | J Med Chem 55: 106-14 (2012) Article DOI: 10.1021/jm201072x BindingDB Entry DOI: 10.7270/Q2BG2Q3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50343522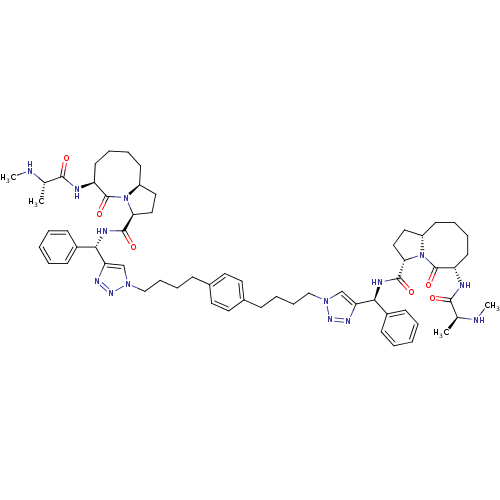 ((S,3S,3'S,6S,6'S,10aS,10a'S)-N,N'-((1S,1'S)-(1,1'-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to BIR3 domain of cIAP2 by fluorescence polarization assay | J Med Chem 55: 106-14 (2012) Article DOI: 10.1021/jm201072x BindingDB Entry DOI: 10.7270/Q2BG2Q3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50343522 ((S,3S,3'S,6S,6'S,10aS,10a'S)-N,N'-((1S,1'S)-(1,1'-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Competitive inhibition of human cIAP2 BIR3 domain after 2 to 3 hrs by fluorescence polarization assay | J Med Chem 54: 3306-18 (2011) Article DOI: 10.1021/jm101651b BindingDB Entry DOI: 10.7270/Q2RJ4JSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50343519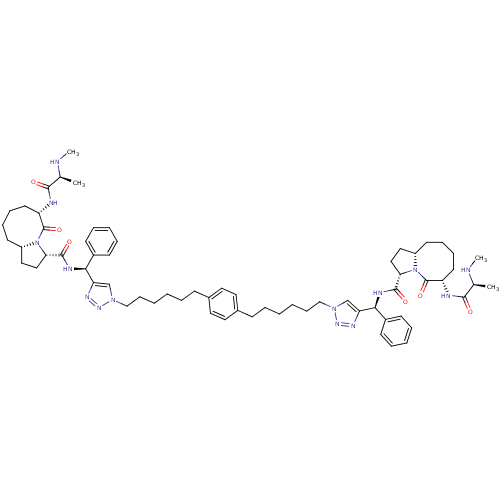 ((S,3S,3'S,6S,6'S,1'aS,1'a'S)-N,N'-((1S,1'S)-(1,1'-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Competitive inhibition of human cIAP2 BIR3 domain after 2 to 3 hrs by fluorescence polarization assay | J Med Chem 54: 3306-18 (2011) Article DOI: 10.1021/jm101651b BindingDB Entry DOI: 10.7270/Q2RJ4JSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50343518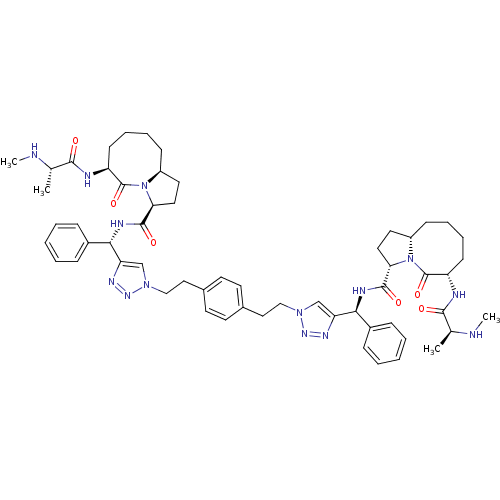 ((S,3S,3'S,6S,6'S,1'aS,1'a'S)-N,N'-((1S,1'S)-(1,1'-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Competitive inhibition of human cIAP2 BIR3 domain after 2 to 3 hrs by fluorescence polarization assay | J Med Chem 54: 3306-18 (2011) Article DOI: 10.1021/jm101651b BindingDB Entry DOI: 10.7270/Q2RJ4JSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50343524 ((S,3S,3'S,6S,6'S,1'aS,1'a'S)-N,N'-((1S,1'S)-(1,1'-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Competitive inhibition of human cIAP2 BIR3 domain after 2 to 3 hrs by fluorescence polarization assay | J Med Chem 54: 3306-18 (2011) Article DOI: 10.1021/jm101651b BindingDB Entry DOI: 10.7270/Q2RJ4JSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50530582 (CHEMBL4581366) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of N-terminal 6x-His-tagged recombinant human cIAP2-BIR3 (244 to 349 residues) expressed in Escherichia coli incubated for 2 hrs after 6 h... | J Med Chem 62: 9188-9200 (2019) Article DOI: 10.1021/acs.jmedchem.9b01108 BindingDB Entry DOI: 10.7270/Q2F47SKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50530582 (CHEMBL4581366) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of N-terminal 6x-His-tagged recombinant human cIAP2-BIR3 (244 to 349 residues) expressed in Escherichia coli incubated for 2 hrs after 6 h... | J Med Chem 62: 9188-9200 (2019) Article DOI: 10.1021/acs.jmedchem.9b01108 BindingDB Entry DOI: 10.7270/Q2F47SKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 865 total ) | Next | Last >> |