 Found 953 hits of ki for UniProtKB: P07858
Found 953 hits of ki for UniProtKB: P07858 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin B
(Homo sapiens (Human)) | BDBM50167289
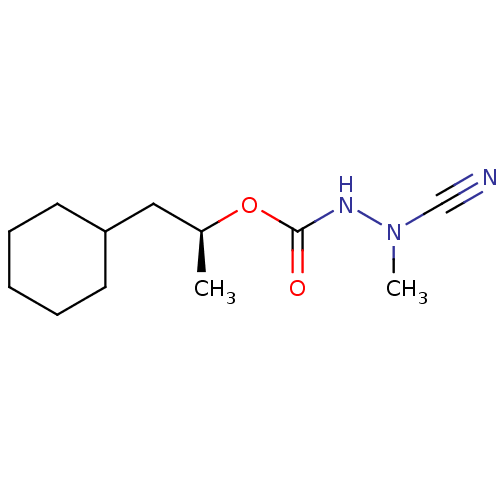
((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-methylhy...)Show InChI InChI=1S/C12H21N3O2/c1-10(8-11-6-4-3-5-7-11)17-12(16)14-15(2)9-13/h10-11H,3-8H2,1-2H3,(H,14,16)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin B in fluorescence assay using Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50284944
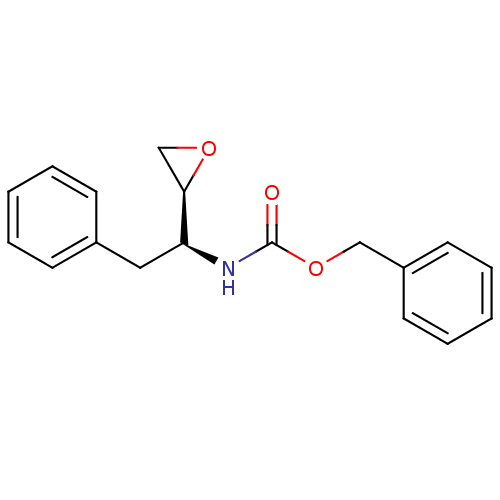
(((S)-1-(S)-Oxiranyl-2-phenyl-ethyl)-carbamic acid ...)Show InChI InChI=1S/C18H19NO3/c20-18(22-12-15-9-5-2-6-10-15)19-16(17-13-21-17)11-14-7-3-1-4-8-14/h1-10,16-17H,11-13H2,(H,19,20)/t16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
| 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Kinetic parameter (Ki 1/min) was evaluated for the inactivation of cathepsin B |
Bioorg Med Chem Lett 5: 1767-1772 (1995)
Article DOI: 10.1016/0960-894X(95)00312-H
BindingDB Entry DOI: 10.7270/Q2MW2H4X |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50167288

((1S)-1-benzylpropyl 2-cyano-2-methylhydrazinecarbo...)Show InChI InChI=1S/C13H17N3O2/c1-3-12(9-11-7-5-4-6-8-11)18-13(17)15-16(2)10-14/h4-8,12H,3,9H2,1-2H3,(H,15,17)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin B in fluorescence assay using Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50335289
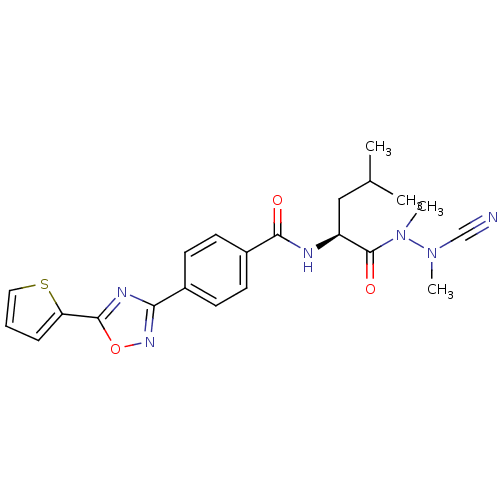
(CHEMBL1651361 | N-{4-[5-(2-Thienyl)-1,2,4-oxadiazo...)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(cc1)-c1noc(n1)-c1cccs1)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C22H24N6O3S/c1-14(2)12-17(22(30)28(4)27(3)13-23)24-20(29)16-9-7-15(8-10-16)19-25-21(31-26-19)18-6-5-11-32-18/h5-11,14,17H,12H2,1-4H3,(H,24,29)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50335279
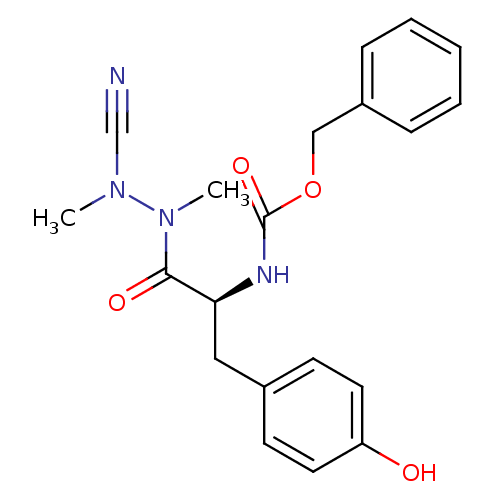
(CHEMBL1651353 | N-(Benzyloxycarbonyl)-tyrosyl-meth...)Show SMILES CN(C#N)N(C)C(=O)[C@H](Cc1ccc(O)cc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C20H22N4O4/c1-23(14-21)24(2)19(26)18(12-15-8-10-17(25)11-9-15)22-20(27)28-13-16-6-4-3-5-7-16/h3-11,18,25H,12-13H2,1-2H3,(H,22,27)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50030745
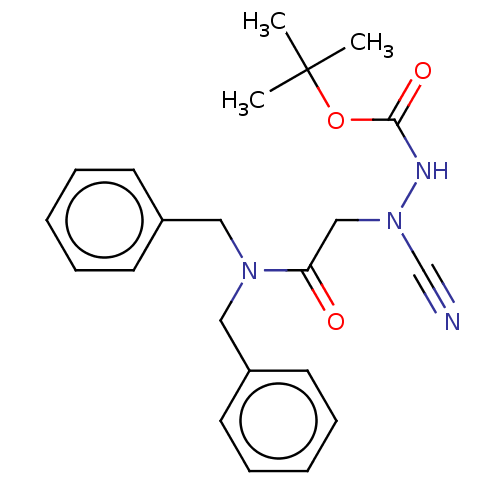
(CHEMBL3342185 | acs.jmedchem.1c00409_ST.412)Show SMILES CC(C)(C)OC(=O)NN(CC(=O)N(Cc1ccccc1)Cc1ccccc1)C#N Show InChI InChI=1S/C22H26N4O3/c1-22(2,3)29-21(28)24-26(17-23)16-20(27)25(14-18-10-6-4-7-11-18)15-19-12-8-5-9-13-19/h4-13H,14-16H2,1-3H3,(H,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.406 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin B using Z-Arg-Arg-pNA chromogenic substrate fluorogenic substrate incubated for 30 mins |
ACS Med Chem Lett 5: 1076-81 (2014)
Article DOI: 10.1021/ml500238q
BindingDB Entry DOI: 10.7270/Q20P11NT |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50304794

((S)-benzyl 1-(2-cyano-1,2-dimethylhydrazinyl)-4-me...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C17H24N4O3/c1-13(2)10-15(16(22)21(4)20(3)12-18)19-17(23)24-11-14-8-6-5-7-9-14/h5-9,13,15H,10-11H2,1-4H3,(H,19,23)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50030746
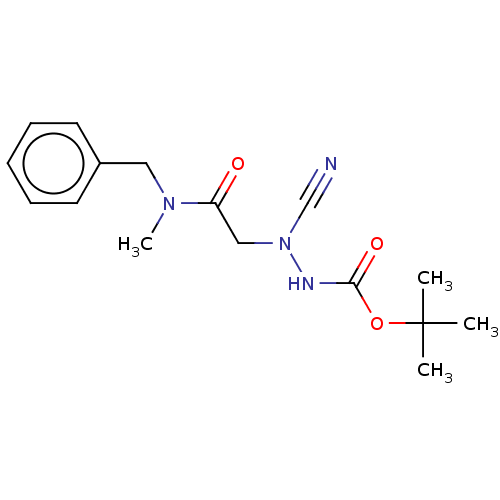
(CHEMBL3342184 | acs.jmedchem.1c00409_ST.413)Show InChI InChI=1S/C16H22N4O3/c1-16(2,3)23-15(22)18-20(12-17)11-14(21)19(4)10-13-8-6-5-7-9-13/h5-9H,10-11H2,1-4H3,(H,18,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.459 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin B using Z-Arg-Arg-pNA chromogenic substrate fluorogenic substrate incubated for 30 mins |
ACS Med Chem Lett 5: 1076-81 (2014)
Article DOI: 10.1021/ml500238q
BindingDB Entry DOI: 10.7270/Q20P11NT |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50335278

(CHEMBL1651352 | N-(Benzyloxycarbonyl)-cyclohexylal...)Show SMILES CN(C#N)N(C)C(=O)[C@H](CC1CCCCC1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C20H28N4O3/c1-23(15-21)24(2)19(25)18(13-16-9-5-3-6-10-16)22-20(26)27-14-17-11-7-4-8-12-17/h4,7-8,11-12,16,18H,3,5-6,9-10,13-14H2,1-2H3,(H,22,26)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50335280

(CHEMBL1651354 | N-(Benzylcarbamoyl)-leucyl-methyla...)Show SMILES CC(C)C[C@H](NC(=O)NCc1ccccc1)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C17H25N5O2/c1-13(2)10-15(16(23)22(4)21(3)12-18)20-17(24)19-11-14-8-6-5-7-9-14/h5-9,13,15H,10-11H2,1-4H3,(H2,19,20,24)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50304793
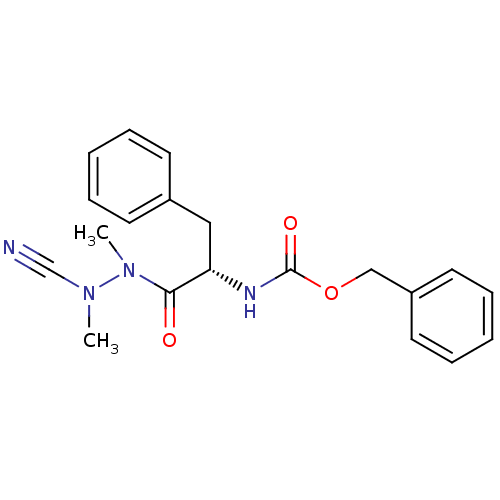
((S)-benzyl 1-(2-cyano-1,2-dimethylhydrazinyl)-1-ox...)Show SMILES CN(C#N)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C20H22N4O3/c1-23(15-21)24(2)19(25)18(13-16-9-5-3-6-10-16)22-20(26)27-14-17-11-7-4-8-12-17/h3-12,18H,13-14H2,1-2H3,(H,22,26)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50510762
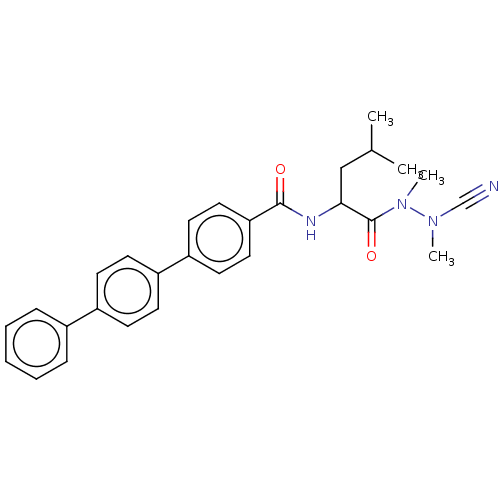
(CHEMBL4442025)Show SMILES CC(C)CC(NC(=O)c1ccc(cc1)-c1ccc(cc1)-c1ccccc1)C(=O)N(C)N(C)C#N Show InChI InChI=1S/C28H30N4O2/c1-20(2)18-26(28(34)32(4)31(3)19-29)30-27(33)25-16-14-24(15-17-25)23-12-10-22(11-13-23)21-8-6-5-7-9-21/h5-17,20,26H,18H2,1-4H3,(H,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin B using Z-Phe-Arg-AMC as substrate incubated for 30 mins by fluorescence based assay |
Bioorg Med Chem 27: 1-15 (2019)
Article DOI: 10.1016/j.bmc.2018.10.017
BindingDB Entry DOI: 10.7270/Q21G0QK2 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50335277

(CHEMBL1651351 | N-(Benzyloxycarbonyl)-isoleucyl-me...)Show SMILES CC[C@H](C)[C@H](NC(=O)OCc1ccccc1)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C17H24N4O3/c1-5-13(2)15(16(22)21(4)20(3)12-18)19-17(23)24-11-14-9-7-6-8-10-14/h6-10,13,15H,5,11H2,1-4H3,(H,19,23)/t13-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50290289
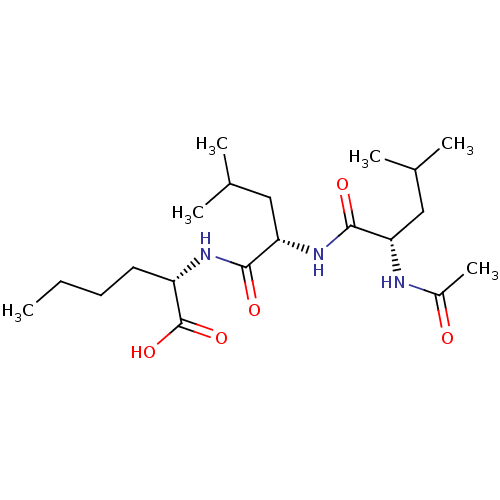
((S)-2-[(S)-2-((S)-2-Acetylamino-4-methyl-pentanoyl...)Show SMILES CCCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(C)=O)C(O)=O Show InChI InChI=1S/C20H37N3O5/c1-7-8-9-15(20(27)28)22-19(26)17(11-13(4)5)23-18(25)16(10-12(2)3)21-14(6)24/h12-13,15-17H,7-11H2,1-6H3,(H,21,24)(H,22,26)(H,23,25)(H,27,28)/t15-,16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Equilibrium dissociation constant of the compound for the inhibition of human cathepsin B was determined |
Bioorg Med Chem Lett 7: 2507-2512 (1997)
Article DOI: 10.1016/S0960-894X(97)10004-X
BindingDB Entry DOI: 10.7270/Q28K793P |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50335281

(CHEMBL1651355 | N-(Phenylcarbamoyl)-leucyl-methyla...)Show SMILES CC(C)C[C@H](NC(=O)Nc1ccccc1)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C16H23N5O2/c1-12(2)10-14(15(22)21(4)20(3)11-17)19-16(23)18-13-8-6-5-7-9-13/h5-9,12,14H,10H2,1-4H3,(H2,18,19,23)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50335283

(CHEMBL1651357 | N-{4-[5-(2-Thienyl)-1,2,4-oxadiazo...)Show SMILES CC(C)C[C@H](NC(=O)NCc1ccc(cc1)-c1noc(n1)-c1cccs1)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C23H27N7O3S/c1-15(2)12-18(22(31)30(4)29(3)14-24)26-23(32)25-13-16-7-9-17(10-8-16)20-27-21(33-28-20)19-6-5-11-34-19/h5-11,15,18H,12-13H2,1-4H3,(H2,25,26,32)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50167290
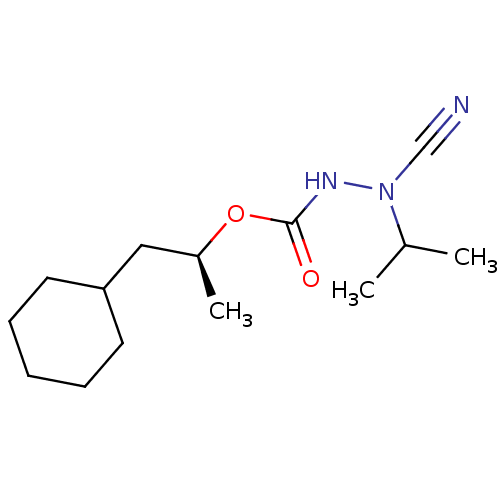
((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-isopropy...)Show InChI InChI=1S/C14H25N3O2/c1-11(2)17(10-15)16-14(18)19-12(3)9-13-7-5-4-6-8-13/h11-13H,4-9H2,1-3H3,(H,16,18)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin B in fluorescence assay using Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50137733
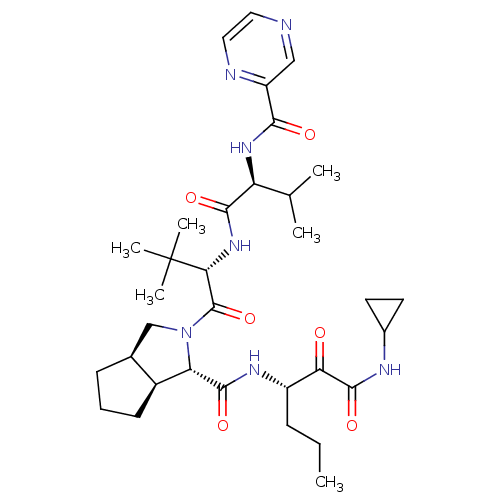
((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...)Show SMILES CCC[C@H](NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)(C)C)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C33H49N7O6/c1-7-9-22(26(41)31(45)36-20-12-13-20)37-30(44)25-21-11-8-10-19(21)17-40(25)32(46)27(33(4,5)6)39-29(43)24(18(2)3)38-28(42)23-16-34-14-15-35-23/h14-16,18-22,24-25,27H,7-13,17H2,1-6H3,(H,36,45)(H,37,44)(H,38,42)(H,39,43)/t19-,21-,22-,24-,25-,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against cathepsin B |
Bioorg Med Chem Lett 14: 257-61 (2003)
BindingDB Entry DOI: 10.7270/Q20P0ZDG |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50230555

(CHEMBL5267078)Show SMILES [H][C@]12CCCC[C@@]1([H])c1cc(Cl)cc(C(=O)N[C@@]3([H])CN4CCC3CC4)c1O2 |wU:6.7,1.0,wD:17.18,(7.76,-5.43,;7.44,-3.92,;8.95,-4.25,;9.98,-3.08,;9.49,-1.63,;7.97,-1.31,;6.95,-2.47,;6.65,-.96,;5.43,-2.47,;4.41,-1.31,;2.9,-1.64,;1.87,-.49,;2.41,-3.08,;3.43,-4.24,;2.94,-5.69,;3.97,-6.85,;1.43,-5.99,;-.11,-5.92,;-.11,-7.46,;-1.02,-7.16,;-2.55,-7,;-3.16,-5.57,;-2.25,-4.34,;-.71,-4.52,;-.88,-6.04,;-2.4,-5.85,;4.95,-3.94,;6.2,-4.84,)| Show InChI InChI=1S/C20H25ClN2O2/c21-13-9-15-14-3-1-2-4-18(14)25-19(15)16(10-13)20(24)22-17-11-23-7-5-12(17)6-8-23/h9-10,12,14,17-18H,1-8,11H2,(H,22,24)/t14-,17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against beta-1 adrenergic receptor determined as pA2 in guinea pig atria |
J Med Chem 20: 430-9 (1977)
Article DOI: 10.1021/jm00213a022
BindingDB Entry DOI: 10.7270/Q2ZC85WV |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM93204
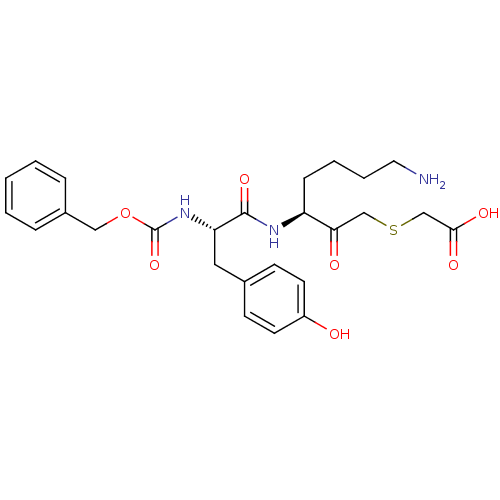
(Mercaptomethyl ketone Inhibitor, 53)Show SMILES NCCCC[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)OCc1ccccc1)C(=O)CSCC(O)=O |r| Show InChI InChI=1S/C26H33N3O7S/c27-13-5-4-8-21(23(31)16-37-17-24(32)33)28-25(34)22(14-18-9-11-20(30)12-10-18)29-26(35)36-15-19-6-2-1-3-7-19/h1-3,6-7,9-12,21-22,30H,4-5,8,13-17,27H2,(H,28,34)(H,29,35)(H,32,33)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
University of California-Berkeley
| Assay Description
A fluorometric high-throughput assay for activity against cathepsin B was performed in 96-well microtiter plates. The assay were performed in Dynate... |
J Comb Chem 5: 869-80 (2003)
Article DOI: 10.1021/cc034008r
BindingDB Entry DOI: 10.7270/Q28914GH |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50410979
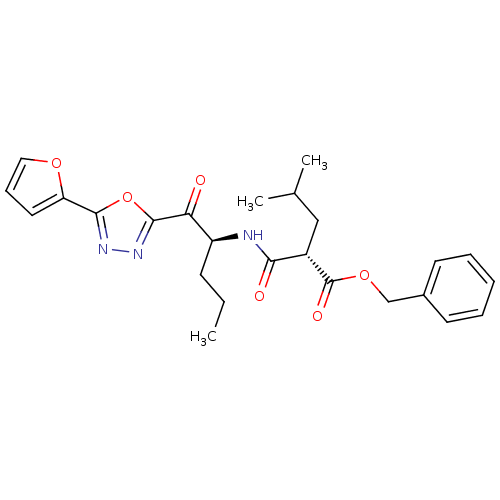
(CHEMBL207347)Show SMILES CCC[C@H](NC(=O)[C@H](CC(C)C)C(=O)OCc1ccccc1)C(=O)c1nnc(o1)-c1ccco1 Show InChI InChI=1S/C25H29N3O6/c1-4-9-19(21(29)24-28-27-23(34-24)20-12-8-13-32-20)26-22(30)18(14-16(2)3)25(31)33-15-17-10-6-5-7-11-17/h5-8,10-13,16,18-19H,4,9,14-15H2,1-3H3,(H,26,30)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin B |
Bioorg Med Chem Lett 16: 2909-14 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.001
BindingDB Entry DOI: 10.7270/Q2QN6808 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50374367
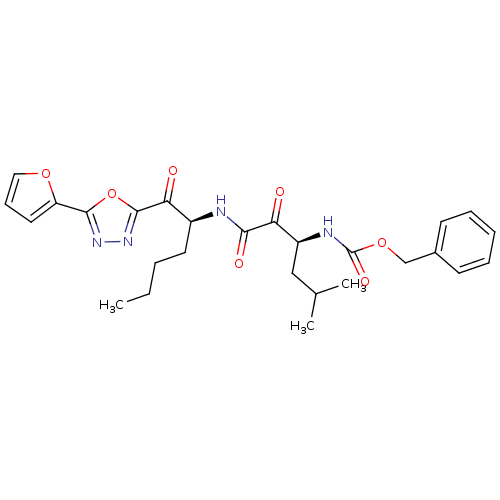
(CHEMBL271004)Show SMILES CCCC[C@H](NC(=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1)C(=O)c1nnc(o1)-c1ccco1 Show InChI InChI=1S/C27H32N4O7/c1-4-5-12-19(23(33)26-31-30-25(38-26)21-13-9-14-36-21)28-24(34)22(32)20(15-17(2)3)29-27(35)37-16-18-10-7-6-8-11-18/h6-11,13-14,17,19-20H,4-5,12,15-16H2,1-3H3,(H,28,34)(H,29,35)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin B |
Bioorg Med Chem 16: 1562-95 (2008)
Article DOI: 10.1016/j.bmc.2007.11.015
BindingDB Entry DOI: 10.7270/Q21J9BNH |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50510761
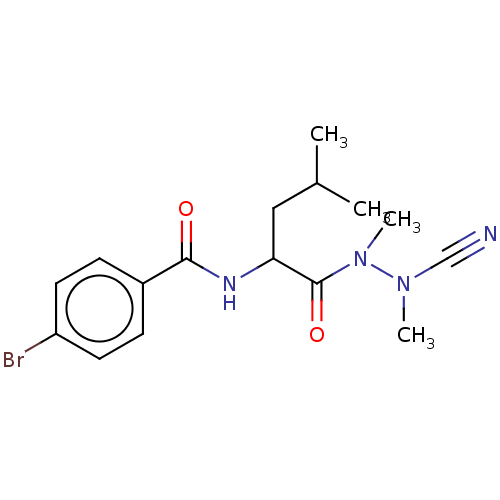
(CHEMBL4440655)Show SMILES CC(C)CC(NC(=O)c1ccc(Br)cc1)C(=O)N(C)N(C)C#N Show InChI InChI=1S/C16H21BrN4O2/c1-11(2)9-14(16(23)21(4)20(3)10-18)19-15(22)12-5-7-13(17)8-6-12/h5-8,11,14H,9H2,1-4H3,(H,19,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin B using Z-Phe-Arg-AMC as substrate incubated for 30 mins by fluorescence based assay |
Bioorg Med Chem 27: 1-15 (2019)
Article DOI: 10.1016/j.bmc.2018.10.017
BindingDB Entry DOI: 10.7270/Q21G0QK2 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50335284
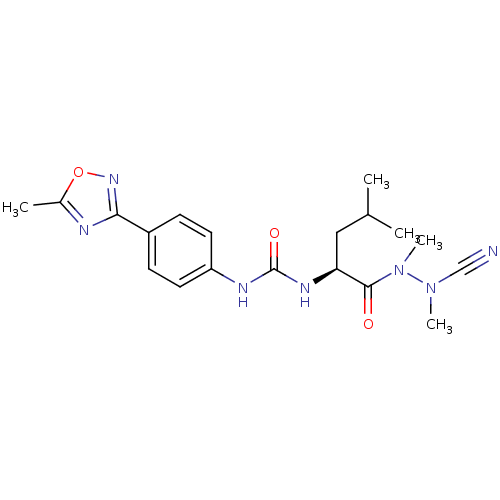
(CHEMBL1651349 | N-[4-(5-Methyl-1,2,4-oxadiazol-3-y...)Show SMILES CC(C)C[C@H](NC(=O)Nc1ccc(cc1)-c1noc(C)n1)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C19H25N7O3/c1-12(2)10-16(18(27)26(5)25(4)11-20)23-19(28)22-15-8-6-14(7-9-15)17-21-13(3)29-24-17/h6-9,12,16H,10H2,1-5H3,(H2,22,23,28)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50335285
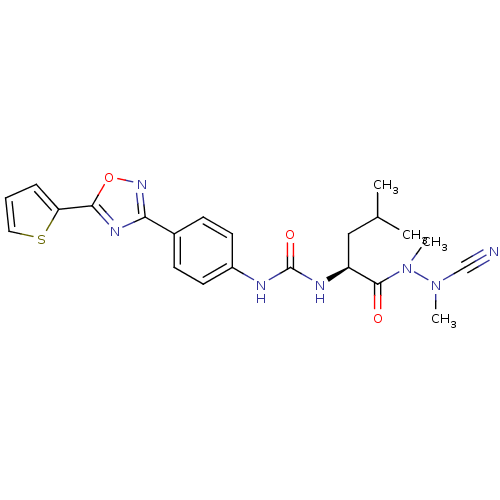
(CHEMBL1651350 | N-{4-[5-(2-Thienyl)-1,2,4-oxadiazo...)Show SMILES CC(C)C[C@H](NC(=O)Nc1ccc(cc1)-c1noc(n1)-c1cccs1)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C22H25N7O3S/c1-14(2)12-17(21(30)29(4)28(3)13-23)25-22(31)24-16-9-7-15(8-10-16)19-26-20(32-27-19)18-6-5-11-33-18/h5-11,14,17H,12H2,1-4H3,(H2,24,25,31)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50335282
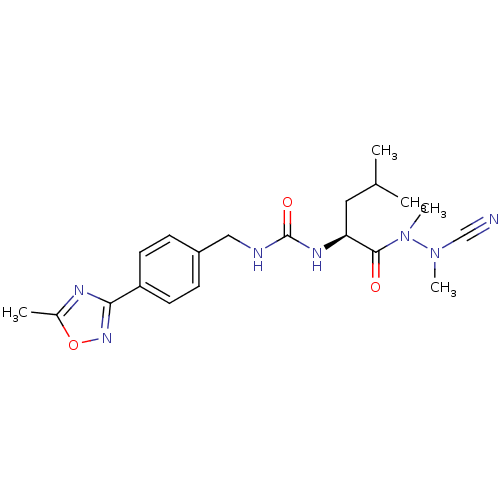
(CHEMBL1651356 | N-[4-(5-Methyl-1,2,4-oxadiazol-3-y...)Show SMILES CC(C)C[C@H](NC(=O)NCc1ccc(cc1)-c1noc(C)n1)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C20H27N7O3/c1-13(2)10-17(19(28)27(5)26(4)12-21)24-20(29)22-11-15-6-8-16(9-7-15)18-23-14(3)30-25-18/h6-9,13,17H,10-11H2,1-5H3,(H2,22,24,29)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM16510
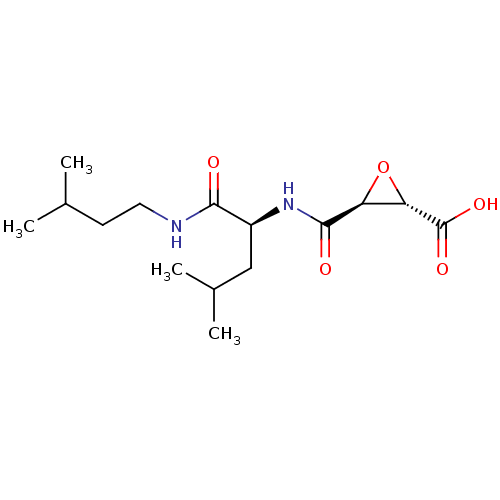
((2S,3S)-3-[[(1S)-1-(isoamylcarbamoyl)-3-methyl-but...)Show SMILES CC(C)CCNC(=O)[C@H](CC(C)C)NC(=O)[C@H]1O[C@@H]1C(O)=O |r| Show InChI InChI=1S/C15H26N2O5/c1-8(2)5-6-16-13(18)10(7-9(3)4)17-14(19)11-12(22-11)15(20)21/h8-12H,5-7H2,1-4H3,(H,16,18)(H,17,19)(H,20,21)/t10-,11-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 3.36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Chemical Laboratory (CSIR-NCL)
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin B |
Bioorg Med Chem 19: 7129-35 (2011)
Article DOI: 10.1016/j.bmc.2011.09.058
BindingDB Entry DOI: 10.7270/Q2KW5GGT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin B
(Homo sapiens (Human)) | BDBM50030747
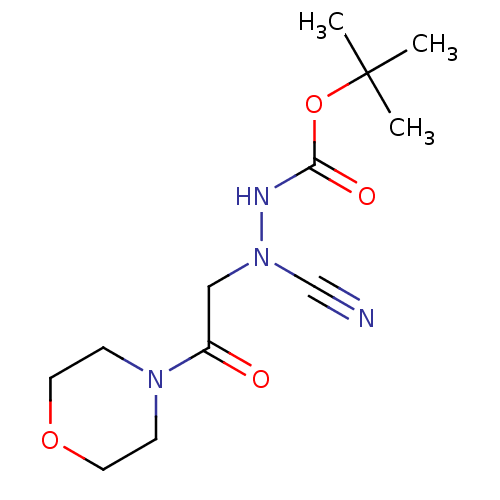
(CHEMBL3342183)Show InChI InChI=1S/C12H20N4O4/c1-12(2,3)20-11(18)14-16(9-13)8-10(17)15-4-6-19-7-5-15/h4-8H2,1-3H3,(H,14,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin B using Z-Arg-Arg-pNA chromogenic substrate fluorogenic substrate incubated for 30 mins |
ACS Med Chem Lett 5: 1076-81 (2014)
Article DOI: 10.1021/ml500238q
BindingDB Entry DOI: 10.7270/Q20P11NT |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50137736

((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...)Show SMILES CCC[C@H](NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)(C)C)C(=O)C(=O)NC(C)CC Show InChI InChI=1S/C34H53N7O6/c1-9-12-23(27(42)32(46)37-20(5)10-2)38-31(45)26-22-14-11-13-21(22)18-41(26)33(47)28(34(6,7)8)40-30(44)25(19(3)4)39-29(43)24-17-35-15-16-36-24/h15-17,19-23,25-26,28H,9-14,18H2,1-8H3,(H,37,46)(H,38,45)(H,39,43)(H,40,44)/t20?,21-,22-,23-,25-,26-,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against cathepsin B |
Bioorg Med Chem Lett 14: 257-61 (2003)
BindingDB Entry DOI: 10.7270/Q20P0ZDG |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50137720

((1S,3aR,6aS)-2-((S)-3-Methyl-2-{(S)-3-methyl-2-[(p...)Show SMILES CCCC(NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)C)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C32H47N7O6/c1-6-8-22(27(40)31(44)35-20-11-12-20)36-30(43)26-21-10-7-9-19(21)16-39(26)32(45)25(18(4)5)38-29(42)24(17(2)3)37-28(41)23-15-33-13-14-34-23/h13-15,17-22,24-26H,6-12,16H2,1-5H3,(H,35,44)(H,36,43)(H,37,41)(H,38,42)/t19-,21-,22?,24-,25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against cathepsin B |
Bioorg Med Chem Lett 14: 257-61 (2003)
BindingDB Entry DOI: 10.7270/Q20P0ZDG |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50065404
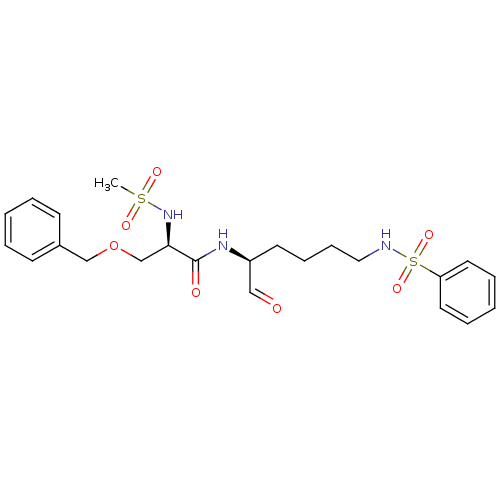
((R)-N-((S)-5-Benzenesulfonylamino-1-formyl-pentyl)...)Show SMILES CS(=O)(=O)N[C@H](COCc1ccccc1)C(=O)N[C@@H](CCCCNS(=O)(=O)c1ccccc1)C=O Show InChI InChI=1S/C23H31N3O7S2/c1-34(29,30)26-22(18-33-17-19-10-4-2-5-11-19)23(28)25-20(16-27)12-8-9-15-24-35(31,32)21-13-6-3-7-14-21/h2-7,10-11,13-14,16,20,22,24,26H,8-9,12,15,17-18H2,1H3,(H,25,28)/t20-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin B receptor in human liver |
J Med Chem 41: 2663-6 (1998)
Article DOI: 10.1021/jm980035y
BindingDB Entry DOI: 10.7270/Q2S46SNF |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50335276
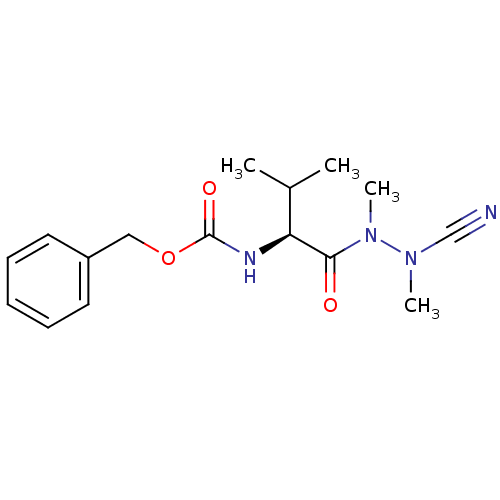
(CHEMBL1651242 | N-(Benzyloxycarbonyl)-valyl-methyl...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C16H22N4O3/c1-12(2)14(15(21)20(4)19(3)11-17)18-16(22)23-10-13-8-6-5-7-9-13/h5-9,12,14H,10H2,1-4H3,(H,18,22)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50053813
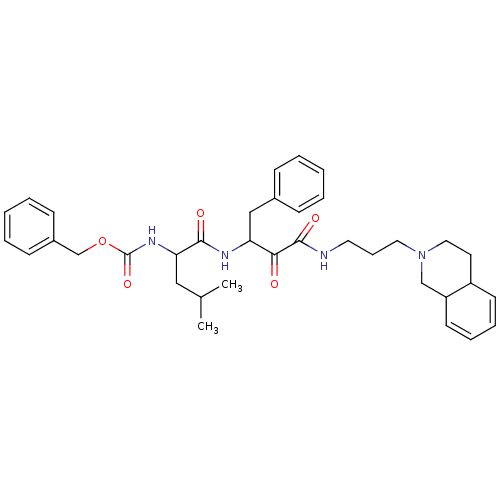
((1-{1-Benzyl-2-oxo-2-[3-(3,4,4a,8a-tetrahydro-1H-i...)Show SMILES CC(C)CC(NC(=O)OCc1ccccc1)C(=O)NC(Cc1ccccc1)C(=O)C(=O)NCCCN1CCC2C=CC=CC2C1 |c:41,43| Show InChI InChI=1S/C36H46N4O5/c1-26(2)22-32(39-36(44)45-25-28-14-7-4-8-15-28)34(42)38-31(23-27-12-5-3-6-13-27)33(41)35(43)37-19-11-20-40-21-18-29-16-9-10-17-30(29)24-40/h3-10,12-17,26,29-32H,11,18-25H2,1-2H3,(H,37,43)(H,38,42)(H,39,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin B |
J Med Chem 39: 4089-98 (1996)
Article DOI: 10.1021/jm950541c
BindingDB Entry DOI: 10.7270/Q2TX3DGR |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM93203
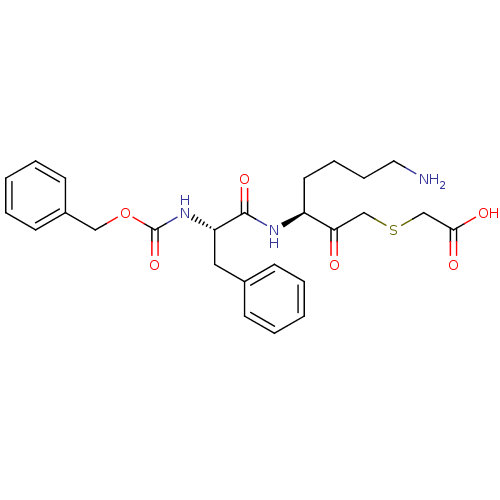
(Mercaptomethyl ketone Inhibitor, 52)Show SMILES NCCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1)C(=O)CSCC(O)=O |r| Show InChI InChI=1S/C26H33N3O6S/c27-14-8-7-13-21(23(30)17-36-18-24(31)32)28-25(33)22(15-19-9-3-1-4-10-19)29-26(34)35-16-20-11-5-2-6-12-20/h1-6,9-12,21-22H,7-8,13-18,27H2,(H,28,33)(H,29,34)(H,31,32)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
University of California-Berkeley
| Assay Description
A fluorometric high-throughput assay for activity against cathepsin B was performed in 96-well microtiter plates. The assay were performed in Dynate... |
J Comb Chem 5: 869-80 (2003)
Article DOI: 10.1021/cc034008r
BindingDB Entry DOI: 10.7270/Q28914GH |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50510759

(CHEMBL4459206)Show SMILES CC(C)CC(NC(=O)c1cccc(c1)-c1ccncc1)C(=O)N(C)N(C)C#N Show InChI InChI=1S/C21H25N5O2/c1-15(2)12-19(21(28)26(4)25(3)14-22)24-20(27)18-7-5-6-17(13-18)16-8-10-23-11-9-16/h5-11,13,15,19H,12H2,1-4H3,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin B using Z-Phe-Arg-AMC as substrate incubated for 30 mins by fluorescence based assay |
Bioorg Med Chem 27: 1-15 (2019)
Article DOI: 10.1016/j.bmc.2018.10.017
BindingDB Entry DOI: 10.7270/Q21G0QK2 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50213272
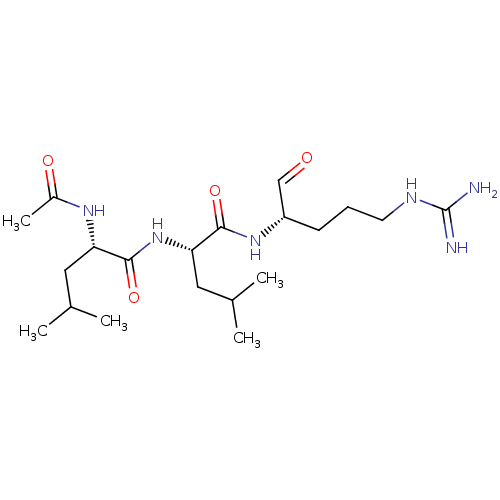
(CHEBI:6426 | Leupeptin)Show SMILES CC(C)C[C@H](NC(C)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C=O |r| Show InChI InChI=1S/C20H38N6O4/c1-12(2)9-16(24-14(5)28)19(30)26-17(10-13(3)4)18(29)25-15(11-27)7-6-8-23-20(21)22/h11-13,15-17H,6-10H2,1-5H3,(H,24,28)(H,25,29)(H,26,30)(H4,21,22,23)/t15-,16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The City University of New York
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin B |
Bioorg Med Chem 21: 2975-87 (2013)
Article DOI: 10.1016/j.bmc.2013.03.062
BindingDB Entry DOI: 10.7270/Q2PG1VNW |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50137730
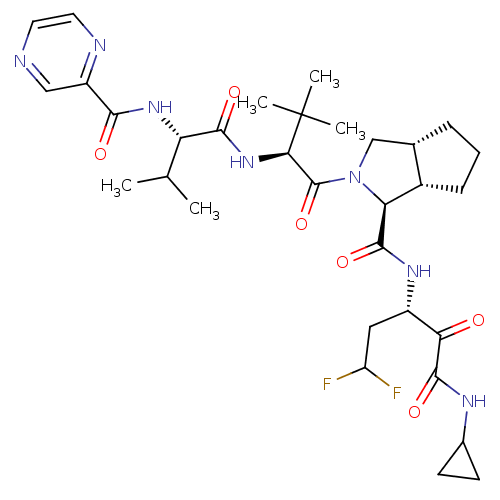
((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...)Show SMILES CC(C)[C@H](NC(=O)c1cnccn1)C(=O)N[C@H](C(=O)N1C[C@@H]2CCC[C@@H]2[C@H]1C(=O)N[C@@H](CC(F)F)C(=O)C(=O)NC1CC1)C(C)(C)C Show InChI InChI=1S/C32H45F2N7O6/c1-16(2)23(39-27(43)21-14-35-11-12-36-21)28(44)40-26(32(3,4)5)31(47)41-15-17-7-6-8-19(17)24(41)29(45)38-20(13-22(33)34)25(42)30(46)37-18-9-10-18/h11-12,14,16-20,22-24,26H,6-10,13,15H2,1-5H3,(H,37,46)(H,38,45)(H,39,43)(H,40,44)/t17-,19-,20-,23-,24-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against cathepsin B |
Bioorg Med Chem Lett 14: 257-61 (2003)
BindingDB Entry DOI: 10.7270/Q20P0ZDG |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50549374

(CHEMBL4746374)Show SMILES C[C@@H](OCc1ccccc1)[C@H](NC(=O)[C@H](Cc1cccc(Cl)c1)NC(=O)c1cccc(c1)-c1cccnc1)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human cathepsin B using Z-Phe-Arg-AMC fluorogenic peptide as substrate preincubated for 2 mins followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115827
BindingDB Entry DOI: 10.7270/Q2CR5XZ7 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50392215
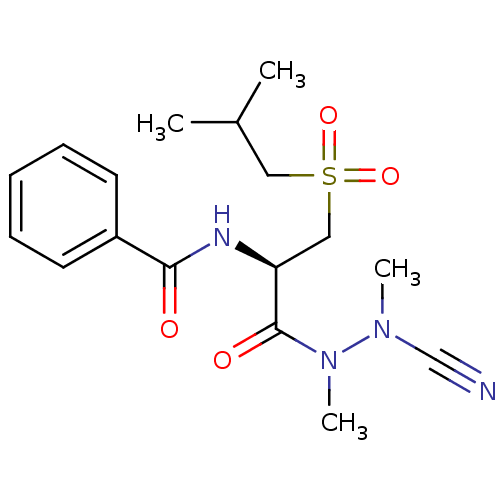
(CHEMBL2153161)Show SMILES CC(C)CS(=O)(=O)C[C@H](NC(=O)c1ccccc1)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C17H24N4O4S/c1-13(2)10-26(24,25)11-15(17(23)21(4)20(3)12-18)19-16(22)14-8-6-5-7-9-14/h5-9,13,15H,10-11H2,1-4H3,(H,19,22)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin B using Z-Arg-Arg-pNA as substrate after 80 mins by spectrophotometric analysis |
J Med Chem 55: 5982-6 (2012)
Article DOI: 10.1021/jm300734k
BindingDB Entry DOI: 10.7270/Q2833T40 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50069984

((R)-1-((S)-2-((S)-2-(benzyloxycarbonyl)-4-methylpe...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1)B(O)O Show InChI InChI=1S/C25H42BN3O6/c1-16(2)12-20(24(31)29-22(26(33)34)14-18(5)6)27-23(30)21(13-17(3)4)28-25(32)35-15-19-10-8-7-9-11-19/h7-11,16-18,20-22,33-34H,12-15H2,1-6H3,(H,27,30)(H,28,32)(H,29,31)/t20-,21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ProScript, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cathepsin B |
Bioorg Med Chem Lett 8: 333-8 (1999)
BindingDB Entry DOI: 10.7270/Q2RV0MVQ |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50286441

((S)-2-((S)-2-Acetylamino-4-(S)-methyl-pentanoylami...)Show SMILES CC(C)C[C@H](NC(C)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O Show InChI InChI=1S/C20H38N6O5/c1-11(2)9-15(24-13(5)27)17(28)26-16(10-12(3)4)18(29)25-14(19(30)31)7-6-8-23-20(21)22/h11-12,14-16H,6-10H2,1-5H3,(H,24,27)(H,25,29)(H,26,28)(H,30,31)(H4,21,22,23)/t14-,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kurukshetra University
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin B |
Eur J Med Chem 77: 231-42 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.007
BindingDB Entry DOI: 10.7270/Q2319XDV |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50286441

((S)-2-((S)-2-Acetylamino-4-(S)-methyl-pentanoylami...)Show SMILES CC(C)C[C@H](NC(C)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O Show InChI InChI=1S/C20H38N6O5/c1-11(2)9-15(24-13(5)27)17(28)26-16(10-12(3)4)18(29)25-14(19(30)31)7-6-8-23-20(21)22/h11-12,14-16H,6-10H2,1-5H3,(H,24,27)(H,25,29)(H,26,28)(H,30,31)(H4,21,22,23)/t14-,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kurukshetra University
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin B |
Bioorg Med Chem 22: 4233-45 (2014)
Article DOI: 10.1016/j.bmc.2014.05.037
BindingDB Entry DOI: 10.7270/Q25Q4XQH |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50137388
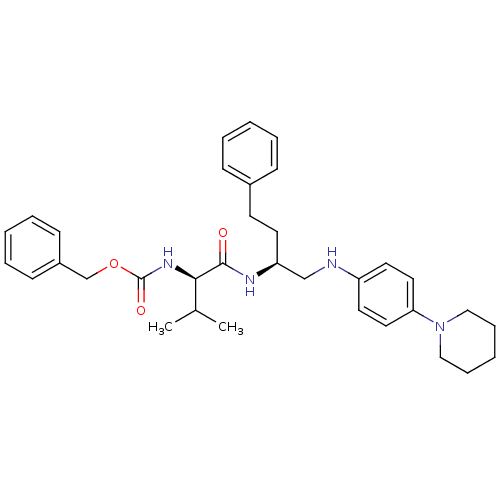
(((R)-2-Methyl-1-{3-phenyl-1-[(4-piperidin-1-yl-phe...)Show SMILES CC(C)[C@@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](CCc1ccccc1)CNc1ccc(cc1)N1CCCCC1 Show InChI InChI=1S/C34H44N4O3/c1-26(2)32(37-34(40)41-25-28-14-8-4-9-15-28)33(39)36-30(17-16-27-12-6-3-7-13-27)24-35-29-18-20-31(21-19-29)38-22-10-5-11-23-38/h3-4,6-9,12-15,18-21,26,30,32,35H,5,10-11,16-17,22-25H2,1-2H3,(H,36,39)(H,37,40)/t30-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cathepsin B |
Bioorg Med Chem Lett 14: 87-90 (2003)
BindingDB Entry DOI: 10.7270/Q2930SKK |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50053865
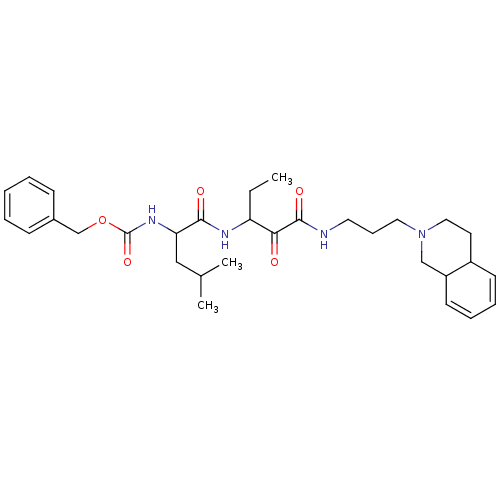
((3-Methyl-1-{1-[3-(3,4,4a,8a-tetrahydro-1H-isoquin...)Show SMILES CCC(NC(=O)C(CC(C)C)NC(=O)OCc1ccccc1)C(=O)C(=O)NCCCN1CCC2C=CC=CC2C1 |c:35,37| Show InChI InChI=1S/C31H44N4O5/c1-4-26(28(36)30(38)32-16-10-17-35-18-15-24-13-8-9-14-25(24)20-35)33-29(37)27(19-22(2)3)34-31(39)40-21-23-11-6-5-7-12-23/h5-9,11-14,22,24-27H,4,10,15-21H2,1-3H3,(H,32,38)(H,33,37)(H,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin B |
J Med Chem 39: 4089-98 (1996)
Article DOI: 10.1021/jm950541c
BindingDB Entry DOI: 10.7270/Q2TX3DGR |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50201259

(CHEMBL3907022)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1)\C=C\[N+]([O-])=O |r| Show InChI InChI=1S/C21H23N3O5/c1-16(12-13-24(27)28)22-20(25)19(14-17-8-4-2-5-9-17)23-21(26)29-15-18-10-6-3-7-11-18/h2-13,16,19H,14-15H2,1H3,(H,22,25)(H,23,26)/b13-12+/t16-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jaume I
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin B |
ACS Med Chem Lett 7: 1073-1076 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00276
BindingDB Entry DOI: 10.7270/Q2M32XR1 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50230554
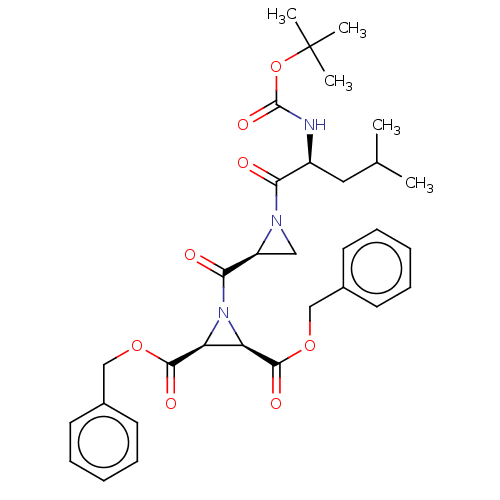
(CHEMBL5283523)Show InChI InChI=1S/C20H23ClN2O2/c1-23-10-4-5-14(23)8-9-22-20(24)17-12-13(21)11-16-15-6-2-3-7-18(15)25-19(16)17/h4-5,10-12,15,18H,2-3,6-9H2,1H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against beta-1 adrenergic receptor determined as pA2 in guinea pig atria |
J Med Chem 20: 430-9 (1977)
Article DOI: 10.1021/jm00213a022
BindingDB Entry DOI: 10.7270/Q2ZC85WV |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50186088
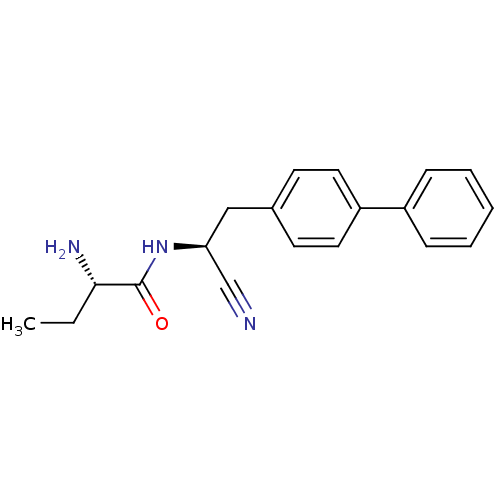
((S)-2-amino-N-((S)-2-(biphenyl-4-yl)-1-cyanoethyl)...)Show SMILES CC[C@H](N)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C#N |r| Show InChI InChI=1S/C19H21N3O/c1-2-18(21)19(23)22-17(13-20)12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,17-18H,2,12,21H2,1H3,(H,22,23)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B by fluorescence assay |
Bioorg Med Chem 17: 1064-70 (2009)
Article DOI: 10.1016/j.bmc.2008.02.002
BindingDB Entry DOI: 10.7270/Q20R9P6K |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50137390
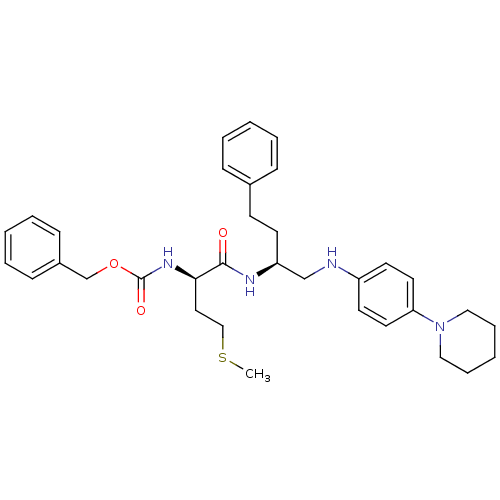
(((R)-3-Methylsulfanyl-1-{3-phenyl-1-[(4-piperidin-...)Show SMILES CSCC[C@@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](CCc1ccccc1)CNc1ccc(cc1)N1CCCCC1 Show InChI InChI=1S/C34H44N4O3S/c1-42-24-21-32(37-34(40)41-26-28-13-7-3-8-14-28)33(39)36-30(16-15-27-11-5-2-6-12-27)25-35-29-17-19-31(20-18-29)38-22-9-4-10-23-38/h2-3,5-8,11-14,17-20,30,32,35H,4,9-10,15-16,21-26H2,1H3,(H,36,39)(H,37,40)/t30-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cathepsin B |
Bioorg Med Chem Lett 14: 87-90 (2003)
BindingDB Entry DOI: 10.7270/Q2930SKK |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50549371

(CHEMBL4757831)Show SMILES C[C@@H](OCc1ccccc1)[C@H](NC(=O)[C@H](Cc1cccc(Cl)c1)NC(=O)c1ccc(Br)cc1)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human cathepsin B using Z-Phe-Arg-AMC fluorogenic peptide as substrate preincubated for 2 mins followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115827
BindingDB Entry DOI: 10.7270/Q2CR5XZ7 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50549379

(CHEMBL4749112)Show SMILES C[C@@H](OCc1ccccc1)[C@H](NC(=O)[C@H](Cc1cccc(Br)c1)NC(=O)c1ccc(cc1)-c1cccnc1)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human cathepsin B using Z-Phe-Arg-AMC fluorogenic peptide as substrate preincubated for 2 mins followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115827
BindingDB Entry DOI: 10.7270/Q2CR5XZ7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data