

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50091817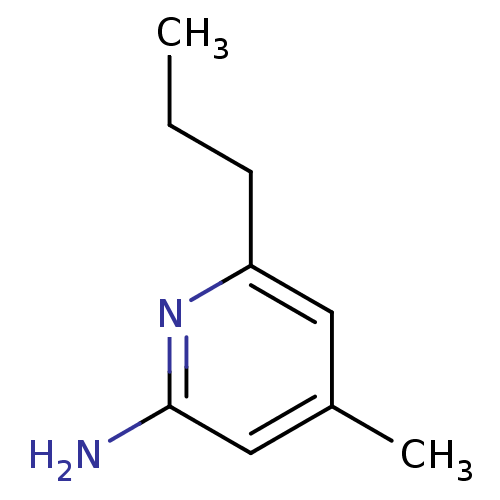 (4-Methyl-6-propyl-pyridin-2-ylamine | 4-Methyl-6-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50091805 (2-amino-4,6-dimethylpyridine | 4,6-Dimethyl-pyridi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 44.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH Curated by ChEMBL | Assay Description Inhibition of human eNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting | Bioorg Med Chem Lett 21: 4228-32 (2011) Article DOI: 10.1016/j.bmcl.2011.05.073 BindingDB Entry DOI: 10.7270/Q2H996GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50091805 (2-amino-4,6-dimethylpyridine | 4,6-Dimethyl-pyridi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rahway Curated by ChEMBL | Assay Description Inhibitory activity against endothelial nitric oxide synthase (eNOS) | Bioorg Med Chem Lett 10: 1975-8 (2001) BindingDB Entry DOI: 10.7270/Q2319V30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50091792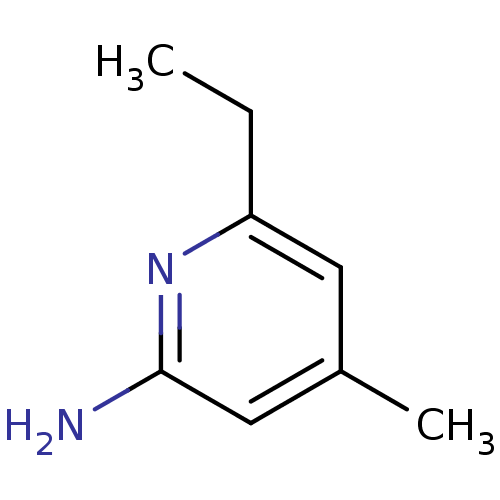 (6-Ethyl-4-methyl-pyridin-2-ylamine | CHEMBL294084) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rahway Curated by ChEMBL | Assay Description Inhibitory activity against endothelial nitric oxide synthase (eNOS) | Bioorg Med Chem Lett 10: 1975-8 (2001) BindingDB Entry DOI: 10.7270/Q2319V30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50098962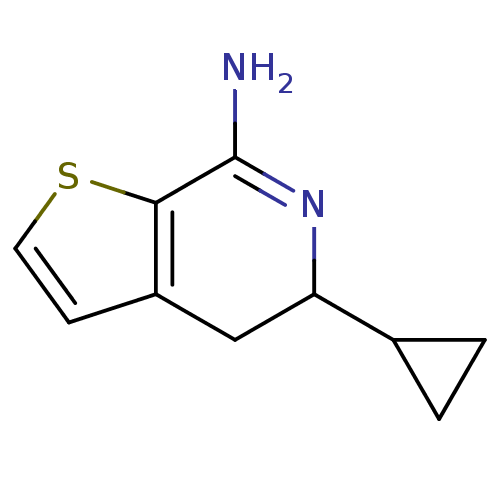 (5-Cyclopropyl-4,5-dihydro-thieno[2,3-c]pyridin-7-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Ability to inhibit the conversion of [3H]-L-Arg to [3H]-L-citrulline catalyzed by e-NOS from HUVECcells | Bioorg Med Chem Lett 11: 1027-30 (2001) BindingDB Entry DOI: 10.7270/Q2F76BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50091817 (4-Methyl-6-propyl-pyridin-2-ylamine | 4-Methyl-6-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description In vitro inhibition of human endothelial nitric oxide synthase. | J Med Chem 47: 3320-3 (2004) Article DOI: 10.1021/jm031035n BindingDB Entry DOI: 10.7270/Q25M656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50091800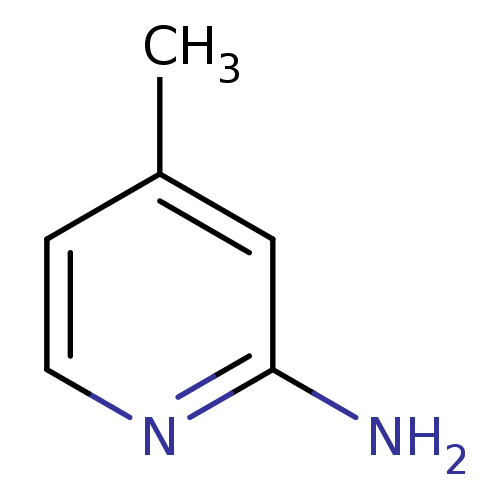 (2-amino-4-methylpyridine | 4-METHYLPYRIDIN-2-AMINE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rahway Curated by ChEMBL | Assay Description Inhibitory activity against endothelial nitric oxide synthase (eNOS) | Bioorg Med Chem Lett 10: 1975-8 (2001) BindingDB Entry DOI: 10.7270/Q2319V30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM36401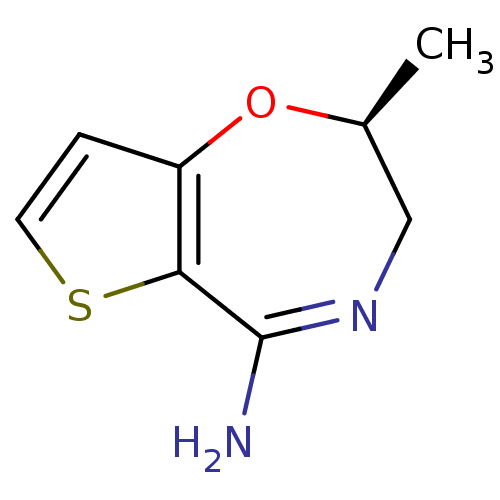 ((2S)-2-Methyl-2,3-dihydrothieno[2,3-f][1,4]oxazepi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50098959 (5-Ethynyl-4,5-dihydro-thieno[2,3-c]pyridin-7-ylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Ability to inhibit the conversion of [3H]-L-Arg to [3H]-L-citrulline catalyzed by e-NOS from HUVECcells | Bioorg Med Chem Lett 11: 1027-30 (2001) BindingDB Entry DOI: 10.7270/Q2F76BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50091791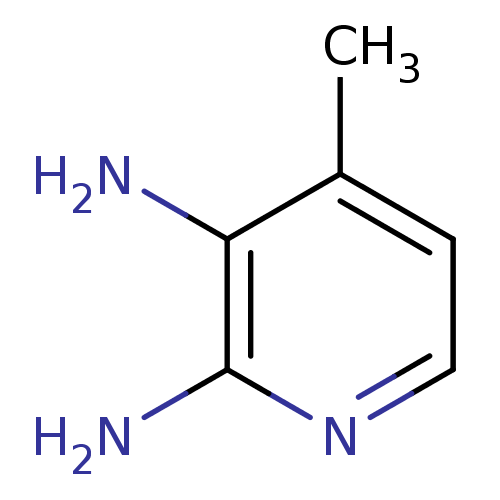 (4-Methyl-pyridine-2,3-diamine | CHEMBL61427) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rahway Curated by ChEMBL | Assay Description Inhibitory activity against endothelial nitric oxide synthase (eNOS) | Bioorg Med Chem Lett 10: 1975-8 (2001) BindingDB Entry DOI: 10.7270/Q2319V30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50091800 (2-amino-4-methylpyridine | 4-METHYLPYRIDIN-2-AMINE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human endothelial nitric oxide synthase | J Med Chem 47: 1575-86 (2004) Article DOI: 10.1021/jm030519g BindingDB Entry DOI: 10.7270/Q2J102MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50098951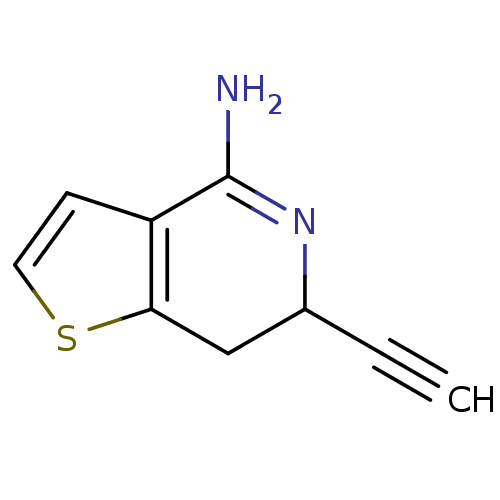 (6-Ethynyl-6,7-dihydro-thieno[3,2-c]pyridin-4-ylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Ability to inhibit the conversion of [3H]-L-Arg to [3H]-L-citrulline catalyzed by e-NOS from HUVECcells | Bioorg Med Chem Lett 11: 1027-30 (2001) BindingDB Entry DOI: 10.7270/Q2F76BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50062133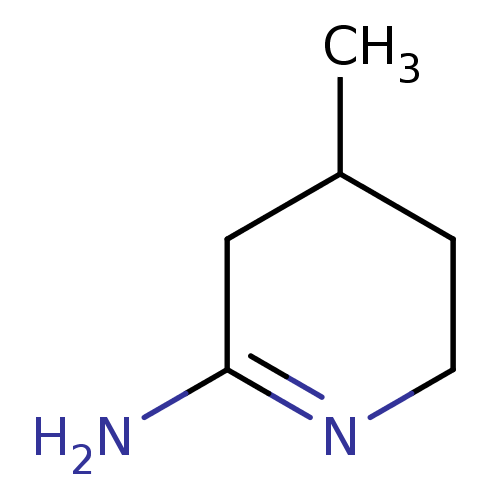 (4-Methyl-piperidin-(2E)-ylideneamine | 4-Methyl-pi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 146 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human Endothelial nitric oxide synthase (eNOS) | Bioorg Med Chem Lett 15: 1997-2001 (2005) Article DOI: 10.1016/j.bmcl.2005.02.067 BindingDB Entry DOI: 10.7270/Q2XK8F3M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50091809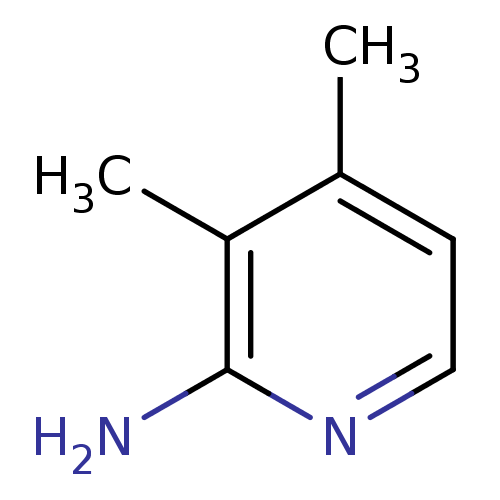 (3,4-Dimethyl-pyridin-2-ylamine | CHEMBL61939) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rahway Curated by ChEMBL | Assay Description Inhibitory activity against endothelial nitric oxide synthase (eNOS) | Bioorg Med Chem Lett 10: 1975-8 (2001) BindingDB Entry DOI: 10.7270/Q2319V30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50091801 (6-Isobutyl-4-methyl-pyridin-2-ylamine | 6-isobutyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human eNOS | J Med Chem 52: 2443-53 (2009) Article DOI: 10.1021/jm801556h BindingDB Entry DOI: 10.7270/Q2QJ7H66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50091801 (6-Isobutyl-4-methyl-pyridin-2-ylamine | 6-isobutyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rahway Curated by ChEMBL | Assay Description Inhibitory activity against endothelial nitric oxide synthase (eNOS) | Bioorg Med Chem Lett 10: 1975-8 (2001) BindingDB Entry DOI: 10.7270/Q2319V30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50098940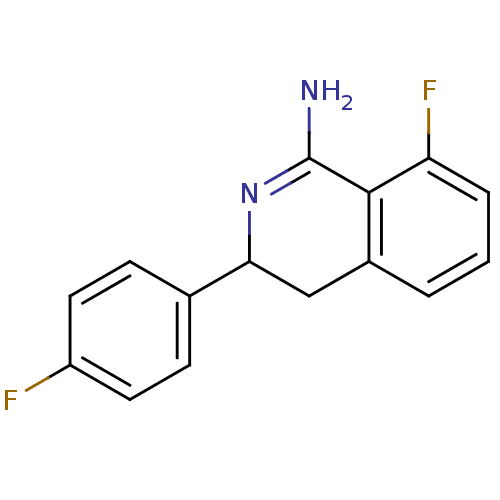 (8-Fluoro-3-(4-fluoro-phenyl)-3,4-dihydro-isoquinol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Ability to inhibit conversion of [3H]-L-Arg to [3H]-L-citrulline catalyzed by endothelial NOS (e NOS) from HUVEC cells | Bioorg Med Chem Lett 11: 1023-6 (2001) BindingDB Entry DOI: 10.7270/Q2K073JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50091805 (2-amino-4,6-dimethylpyridine | 4,6-Dimethyl-pyridi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description In vitro inhibition of human endothelial nitric oxide synthase. | J Med Chem 47: 3320-3 (2004) Article DOI: 10.1021/jm031035n BindingDB Entry DOI: 10.7270/Q25M656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50124519 (5,8-Difluoro-2-furan-2-yl-1,2-dihydro-quinazolin-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R& D Charnwood Curated by ChEMBL | Assay Description Inhibitory activity against human endothelial nitiric oxide synthase | J Med Chem 46: 913-6 (2003) Article DOI: 10.1021/jm0255926 BindingDB Entry DOI: 10.7270/Q2S75H3M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50372207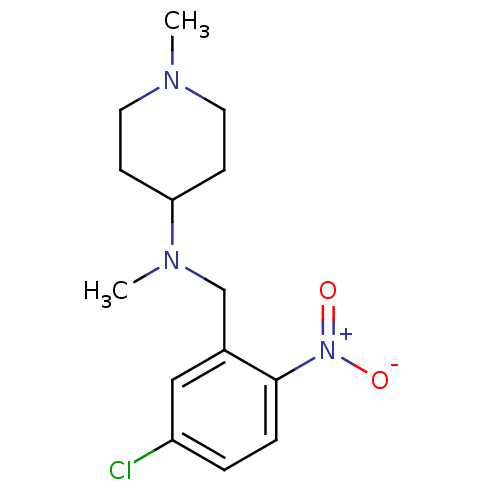 (CHEMBL272708) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Inhibition of human eNOS expressed in insect SF9 cells after 1 hr | Bioorg Med Chem Lett 18: 336-43 (2008) Article DOI: 10.1016/j.bmcl.2007.10.073 BindingDB Entry DOI: 10.7270/Q2H9961Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50392587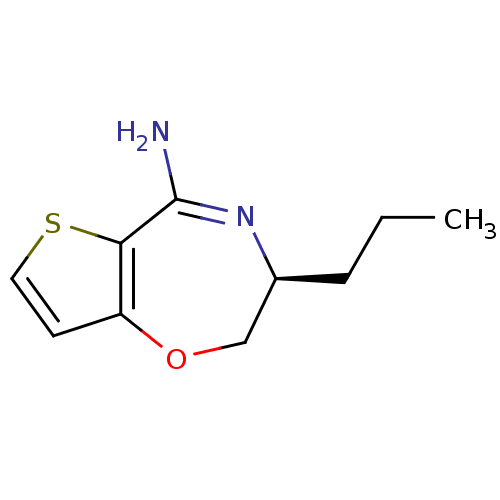 (CHEMBL1230023) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de S£o Paulo Curated by ChEMBL | Assay Description Inhibition of wild type human eNOS using L-Arg as substrate incubated for 1 hr prior to L-Arg addition | Eur J Med Chem 58: 117-27 (2012) Article DOI: 10.1016/j.ejmech.2012.10.010 BindingDB Entry DOI: 10.7270/Q29024XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM36402 ((3R)-3-Propyl-2,3-dihydrothieno[2,3-f][1,4]oxazepi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50091798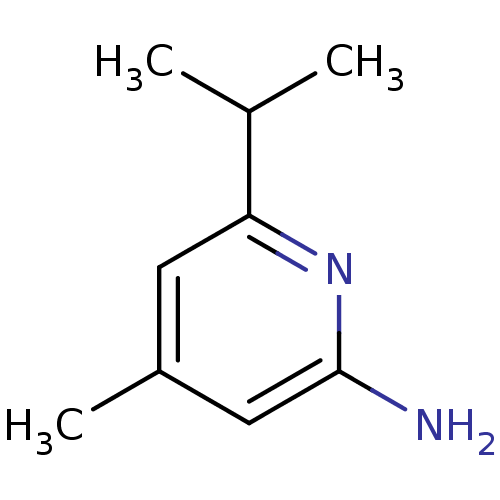 (6-Isopropyl-4-methyl-pyridin-2-ylamine | CHEMBL299...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rahway Curated by ChEMBL | Assay Description Inhibitory activity against endothelial nitric oxide synthase (eNOS) | Bioorg Med Chem Lett 10: 1975-8 (2001) BindingDB Entry DOI: 10.7270/Q2319V30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50062133 (4-Methyl-piperidin-(2E)-ylideneamine | 4-Methyl-pi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rahway Curated by ChEMBL | Assay Description Inhibitory activity against endothelial nitric oxide synthase (eNOS) | Bioorg Med Chem Lett 10: 1975-8 (2001) BindingDB Entry DOI: 10.7270/Q2319V30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50372223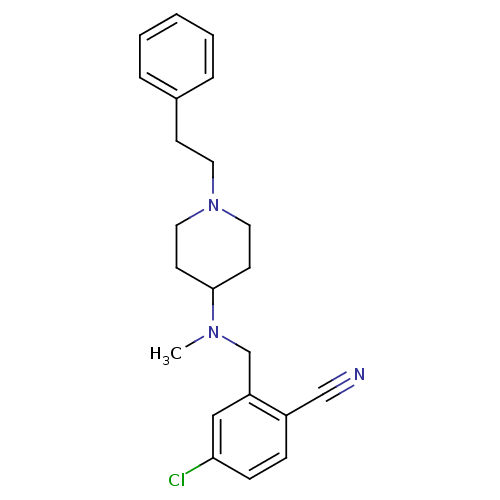 (CHEMBL428069) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Inhibition of human eNOS expressed in insect SF9 cells after 1 hr | Bioorg Med Chem Lett 18: 336-43 (2008) Article DOI: 10.1016/j.bmcl.2007.10.073 BindingDB Entry DOI: 10.7270/Q2H9961Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50116666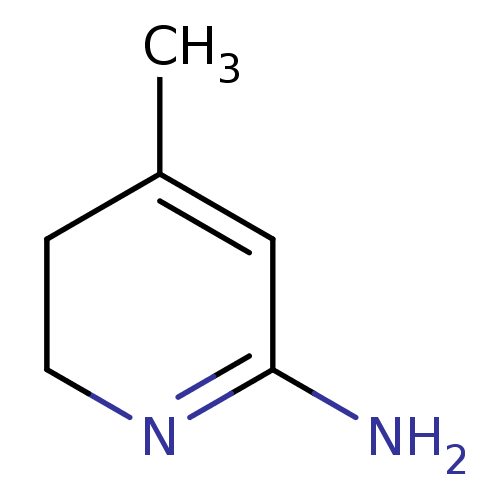 (4-Methyl-3,6-dihydro-1H-pyridin-(2Z)-ylideneamine ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit human Inducible nitric oxide synthase over expressed in A549 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50091813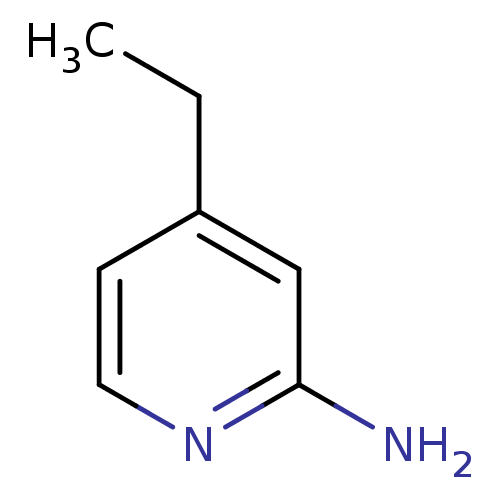 (4-Ethyl-pyridin-2-ylamine | 4-ethylpyridin-2-amine...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rahway Curated by ChEMBL | Assay Description Inhibitory activity against endothelial nitric oxide synthase (eNOS) | Bioorg Med Chem Lett 10: 1975-8 (2001) BindingDB Entry DOI: 10.7270/Q2319V30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50116676 (4,6-Dimethyl-5,6-dihydro-1H-pyridin-(2Z)-ylideneam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Inhibition of human endothelial Nitric Oxide Synthase expressed in Sf-21 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50346529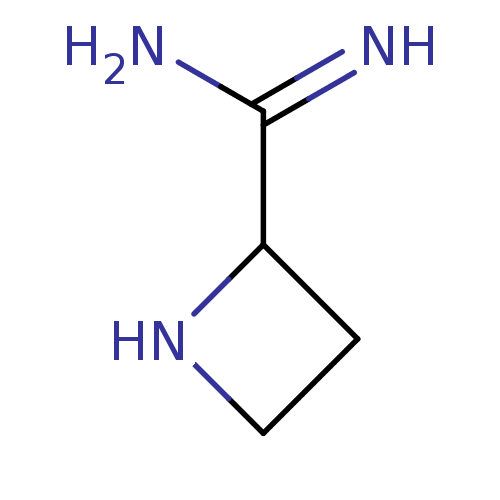 ((R)-azetidine-2-carboximidamide | CHEMBL1783094 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of human recombinant eNOS by microtiter plate assay | Bioorg Med Chem Lett 21: 3037-40 (2011) Article DOI: 10.1016/j.bmcl.2011.03.038 BindingDB Entry DOI: 10.7270/Q2FB538T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50098963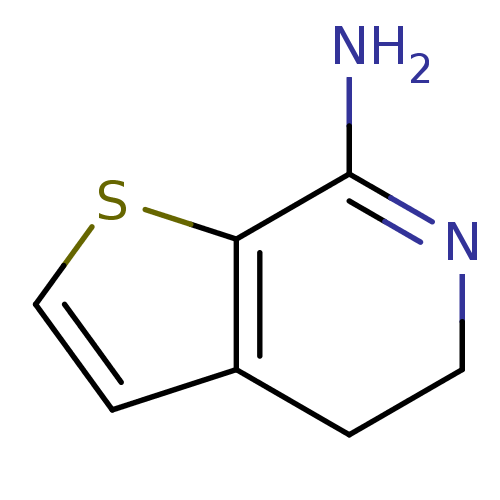 (4,5-Dihydro-thieno[2,3-c]pyridin-7-ylamine | CHEMB...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Ability to inhibit the conversion of [3H]-L-Arg to [3H]-L-citrulline catalyzed by e-NOS from HUVECcells | Bioorg Med Chem Lett 11: 1027-30 (2001) BindingDB Entry DOI: 10.7270/Q2F76BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50091800 (2-amino-4-methylpyridine | 4-METHYLPYRIDIN-2-AMINE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description In vitro inhibition of human endothelial nitric oxide synthase. | J Med Chem 47: 3320-3 (2004) Article DOI: 10.1021/jm031035n BindingDB Entry DOI: 10.7270/Q25M656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50230993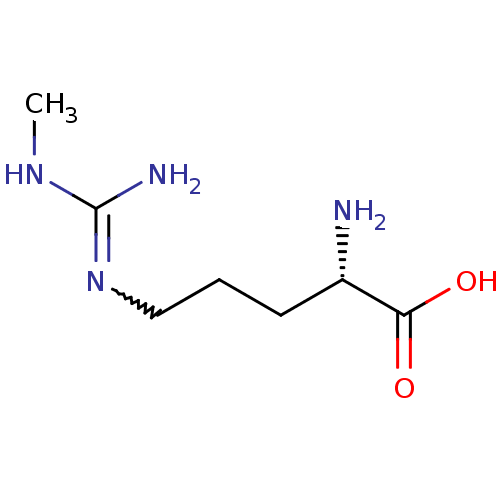 ((2S)-2-amino-5-[(N-methylcarbamimidoyl)amino]penta...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Ability to inhibit the conversion of [3H]-L-Arg to [3H]-L-citrulline catalyzed by e-NOS from HUVECcells | Bioorg Med Chem Lett 11: 1027-30 (2001) BindingDB Entry DOI: 10.7270/Q2F76BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50230993 ((2S)-2-amino-5-[(N-methylcarbamimidoyl)amino]penta...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Ability to inhibit conversion of [3H]-L-Arg to [3H]-L-citrulline catalyzed by endothelial NOS (e NOS) from HUVEC cells | Bioorg Med Chem Lett 11: 1023-6 (2001) BindingDB Entry DOI: 10.7270/Q2K073JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50062142 (4,6-Dimethyl-piperidin-(2Z)-ylideneamine | CHEMBL2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development Curated by ChEMBL | Assay Description inhibition of human endothelial constitutive Endothelial nitric oxide synthase (heNOS) | J Med Chem 41: 96-101 (1998) Article DOI: 10.1021/jm9705059 BindingDB Entry DOI: 10.7270/Q2GM86D2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50116673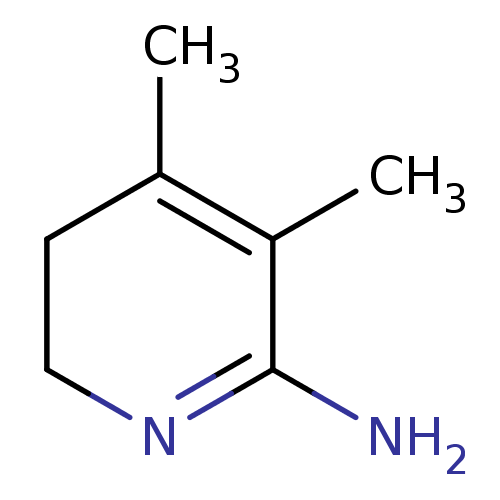 (3,4-Dimethyl-5,6-dihydro-1H-pyridin-(2Z)-ylideneam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Inhibition of human endothelial Nitric Oxide Synthase expressed in Sf-21 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50091800 (2-amino-4-methylpyridine | 4-METHYLPYRIDIN-2-AMINE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50225106 ((2S)-2-amino-5-{[(E)-amino(nitroimino)methyl]amino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering AG Curated by ChEMBL | Assay Description In vitro inhibition of endothelial nitric oxide synthase. | Bioorg Med Chem Lett 12: 2561-4 (2002) BindingDB Entry DOI: 10.7270/Q2VD6XS8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50225106 ((2S)-2-amino-5-{[(E)-amino(nitroimino)methyl]amino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering AG Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant human Endothelial nitric oxide synthase | Bioorg Med Chem Lett 13: 1981-4 (2003) BindingDB Entry DOI: 10.7270/Q2FN16QG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50030279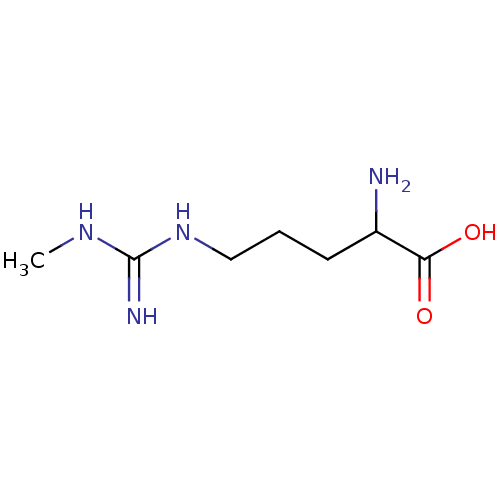 (2-Amino-5-(N'-methyl-guanidino)-pentanoic acid | C...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R& D Charnwood Curated by ChEMBL | Assay Description Inhibitory activity against human endothelial nitiric oxide synthase | J Med Chem 46: 913-6 (2003) Article DOI: 10.1021/jm0255926 BindingDB Entry DOI: 10.7270/Q2S75H3M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50116674 (6-Allyl-4-methyl-5,6-dihydro-1H-pyridin-(2Z)-ylide...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Inhibition of human endothelial Nitric Oxide Synthase expressed in Sf-21 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50081083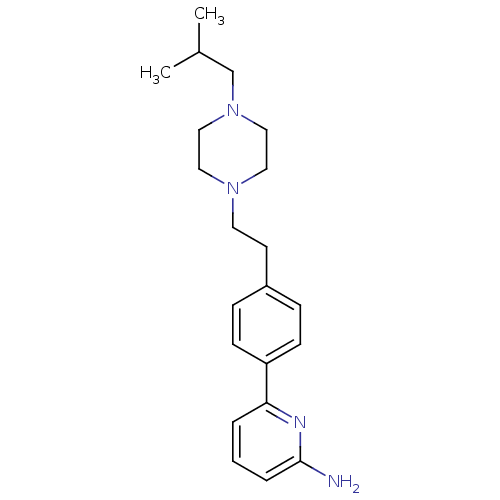 (6-{4-[2-(4-Isobutyl-piperazin-1-yl)-ethyl]-phenyl}...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 372 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Groton Curated by ChEMBL | Assay Description Inhibitory activity against human endothelial nitric oxide synthase (eNOS) | Bioorg Med Chem Lett 9: 2569-72 (1999) BindingDB Entry DOI: 10.7270/Q2BC3XRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50138983 ((S)-2-Amino-5-(2-methyl-isothioureido)-pentanoic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya City University Curated by ChEMBL | Assay Description Inhibitory concentration against human endothelial nitric oxide synthase expressed in Sf-9 cells | Bioorg Med Chem Lett 15: 2881-5 (2005) Article DOI: 10.1016/j.bmcl.2005.03.078 BindingDB Entry DOI: 10.7270/Q2PK0FP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50401279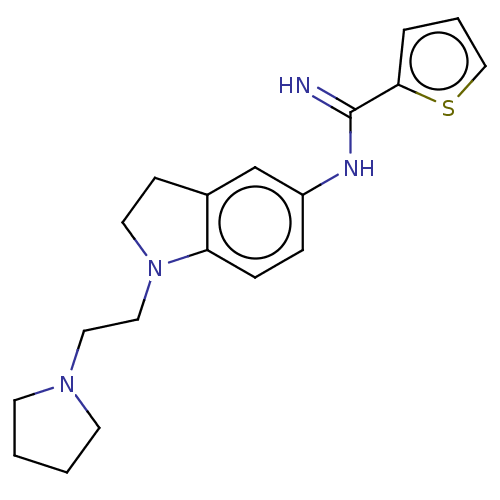 (CHEMBL3215893) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human iNOS expressed in baculovirus-infected Sf9 cells assessed as conversion of [3H]-larginine to [3H]-L-citrulline preinc... | Eur J Med Chem 55: 94-107 (2012) Article DOI: 10.1016/j.ejmech.2012.07.006 BindingDB Entry DOI: 10.7270/Q2GQ6ZWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50116666 (4-Methyl-3,6-dihydro-1H-pyridin-(2Z)-ylideneamine ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Inhibition of human endothelial Nitric Oxide Synthase expressed in Sf-21 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50081080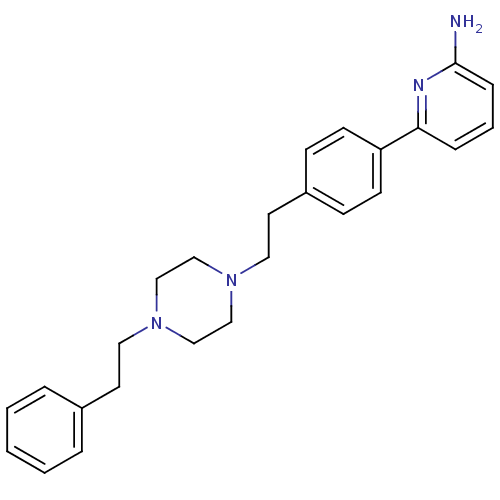 (6-{4-[2-(4-Phenethyl-piperazin-1-yl)-ethyl]-phenyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 448 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Groton Curated by ChEMBL | Assay Description Inhibitory activity against human endothelial nitric oxide synthase (eNOS) | Bioorg Med Chem Lett 9: 2569-72 (1999) BindingDB Entry DOI: 10.7270/Q2BC3XRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50372230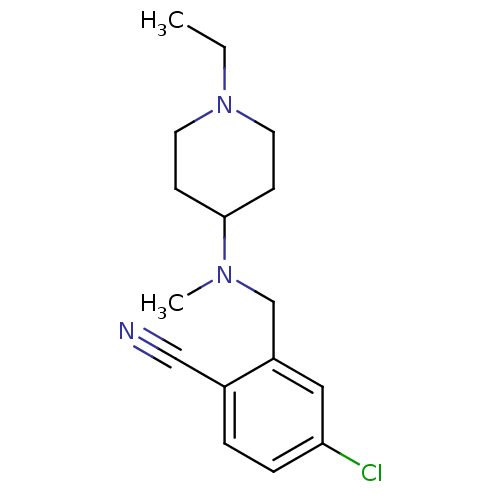 (CHEMBL258310) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Inhibition of human eNOS expressed in insect SF9 cells after 1 hr | Bioorg Med Chem Lett 18: 336-43 (2008) Article DOI: 10.1016/j.bmcl.2007.10.073 BindingDB Entry DOI: 10.7270/Q2H9961Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50155790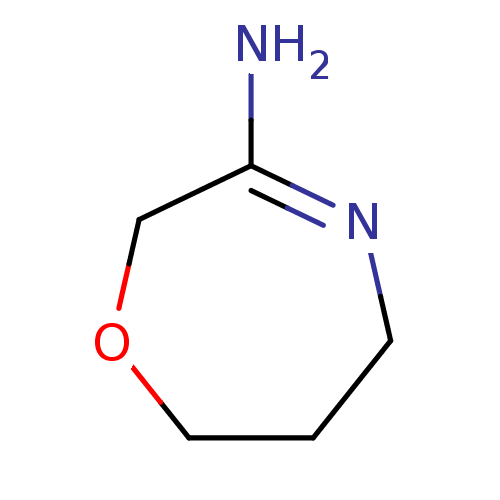 (CHEMBL362772 | [1,4]Oxazepan-(3E)-ylideneamine) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against Endothelial nitric oxide synthase | Bioorg Med Chem Lett 14: 5907-11 (2004) Article DOI: 10.1016/j.bmcl.2004.09.019 BindingDB Entry DOI: 10.7270/Q2JQ10HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50164777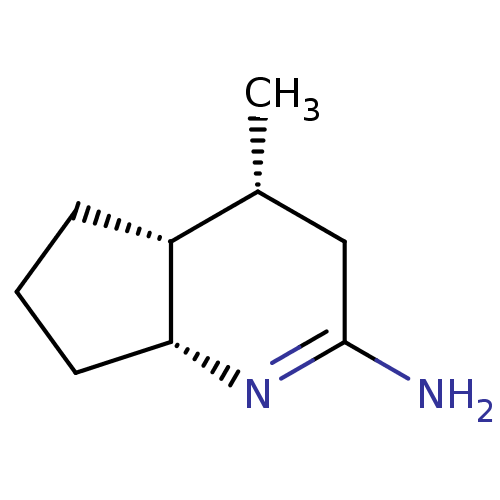 ((4R,4aR,7aR)-4-Methyl-octahydro-[1]pyrindin-(2E)-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human Endothelial nitric oxide synthase (eNOS) | Bioorg Med Chem Lett 15: 1997-2001 (2005) Article DOI: 10.1016/j.bmcl.2005.02.067 BindingDB Entry DOI: 10.7270/Q2XK8F3M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50164777 ((4R,4aR,7aR)-4-Methyl-octahydro-[1]pyrindin-(2E)-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human Endothelial nitric oxide synthase (eNOS) | Bioorg Med Chem Lett 15: 1997-2001 (2005) Article DOI: 10.1016/j.bmcl.2005.02.067 BindingDB Entry DOI: 10.7270/Q2XK8F3M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50049252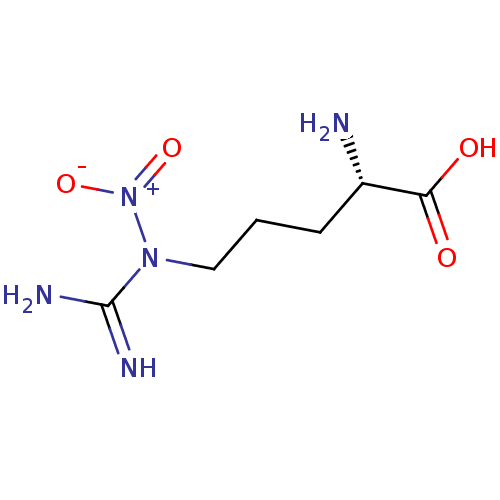 (2-Amino-5-(N-nitro-guanidino)-pentanoic acid | CHE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human Endothelial nitric oxide synthase | J Med Chem 39: 669-72 (1996) Article DOI: 10.1021/jm950766n BindingDB Entry DOI: 10.7270/Q261110M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 768 total ) | Next | Last >> |