

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50057761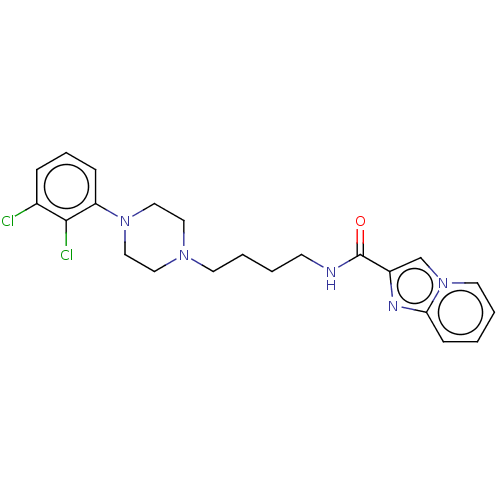 (CHEMBL3322994) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.00380 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Antagonist activity against human D3R expressed in CHO cells assessed as inhibition of dopamine-induced [35S]GTPgammaS binding by dopamine potency sh... | J Med Chem 57: 7042-60 (2014) Article DOI: 10.1021/jm500801r BindingDB Entry DOI: 10.7270/Q27D2WSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Antagonist activity against D3R in human U2OS cells assessed as inhibition of (+)-PD128907-induced beta-arrestin translocation by beta-galactosidase ... | J Med Chem 57: 7042-60 (2014) Article DOI: 10.1021/jm500801r BindingDB Entry DOI: 10.7270/Q27D2WSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50057761 (CHEMBL3322994) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Antagonist activity against D3R in human U2OS cells assessed as inhibition of (+)-PD128907-induced beta-arrestin translocation by beta-galactosidase ... | J Med Chem 57: 7042-60 (2014) Article DOI: 10.1021/jm500801r BindingDB Entry DOI: 10.7270/Q27D2WSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50119386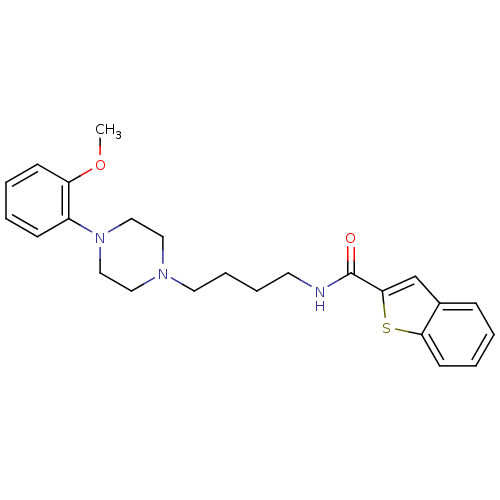 (Benzo[b]thiophene-2-carboxylic acid {4-[4-(2-metho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug AbuseIntramural Research Program Curated by ChEMBL | Assay Description Antagonist activity at human D3 receptor expressed in HEK293 cells assessed as inhibition of quinpirole-stimulated mitogenesis | J Med Chem 52: 2559-70 (2009) Article DOI: 10.1021/jm900095y BindingDB Entry DOI: 10.7270/Q2HX1DKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Antagonist activity at recombinant human D3 receptor expressed in CHOK1 cells assessed as inhibition of dopamine-induced beta arrestin2 recruitment p... | J Med Chem 62: 5132-5147 (2019) Article DOI: 10.1021/acs.jmedchem.9b00412 BindingDB Entry DOI: 10.7270/Q2Z03CM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50057767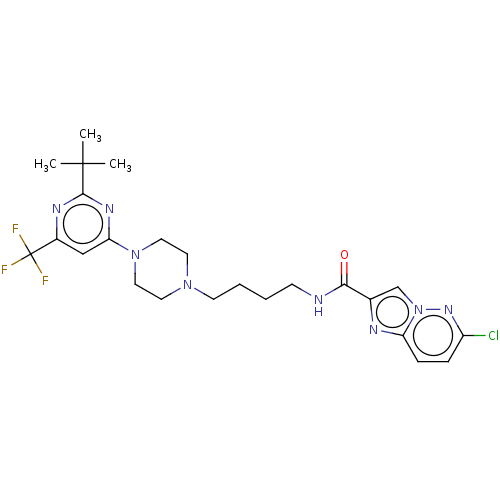 (CHEMBL3323017 | US9598387, Compound 116 | US996974...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Antagonist activity against D3R in human U2OS cells assessed as inhibition of (+)-PD128907-induced beta-arrestin translocation by beta-galactosidase ... | J Med Chem 57: 7042-60 (2014) Article DOI: 10.1021/jm500801r BindingDB Entry DOI: 10.7270/Q27D2WSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50057763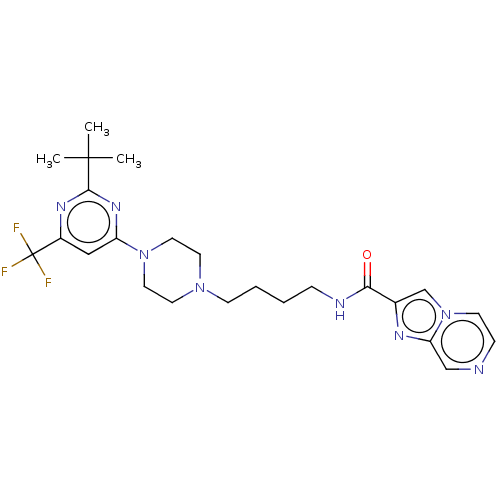 (CHEMBL3323016 | US9598387, Compound 115 | US996974...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Antagonist activity against D3R in human U2OS cells assessed as inhibition of (+)-PD128907-induced beta-arrestin translocation by beta-galactosidase ... | J Med Chem 57: 7042-60 (2014) Article DOI: 10.1021/jm500801r BindingDB Entry DOI: 10.7270/Q27D2WSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50057763 (CHEMBL3323016 | US9598387, Compound 115 | US996974...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Antagonist activity against human D3R expressed in CHO cells assessed as inhibition of dopamine-induced [35S]GTPgammaS binding by dopamine potency sh... | J Med Chem 57: 7042-60 (2014) Article DOI: 10.1021/jm500801r BindingDB Entry DOI: 10.7270/Q27D2WSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50057768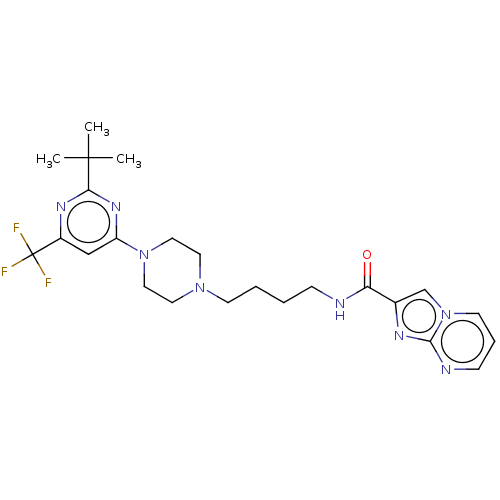 (CHEMBL3323015 | US9598387, Compound 114 | US996974...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Antagonist activity against D3R in human U2OS cells assessed as inhibition of (+)-PD128907-induced beta-arrestin translocation by beta-galactosidase ... | J Med Chem 57: 7042-60 (2014) Article DOI: 10.1021/jm500801r BindingDB Entry DOI: 10.7270/Q27D2WSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50403975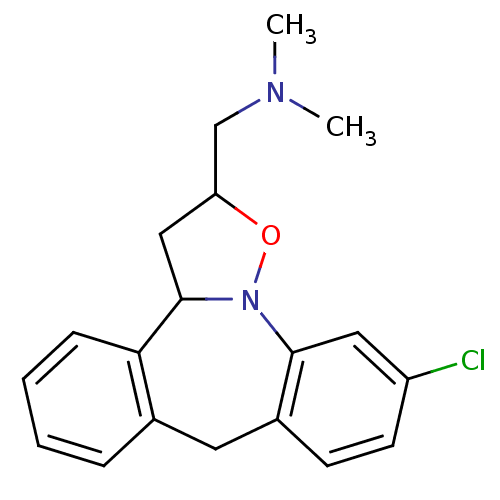 (CHEMBL315772) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity against Dopamine receptor D3 | Bioorg Med Chem Lett 12: 3573-7 (2002) BindingDB Entry DOI: 10.7270/Q26M384X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50403975 (CHEMBL315772) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen-Cilag Curated by ChEMBL | Assay Description Binding affinity for human cloned Dopamine receptor D3 | Bioorg Med Chem Lett 12: 249-53 (2001) Article DOI: 10.1016/S0960-894X(01)00722-3 BindingDB Entry DOI: 10.7270/Q2ZW1N3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50057762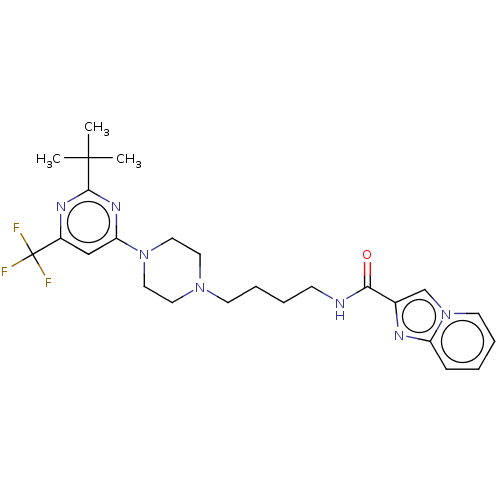 (CHEMBL3323011 | US9598387, Compound 113 | US996974...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Antagonist activity against D3R in human U2OS cells assessed as inhibition of (+)-PD128907-induced beta-arrestin translocation by beta-galactosidase ... | J Med Chem 57: 7042-60 (2014) Article DOI: 10.1021/jm500801r BindingDB Entry DOI: 10.7270/Q27D2WSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50057772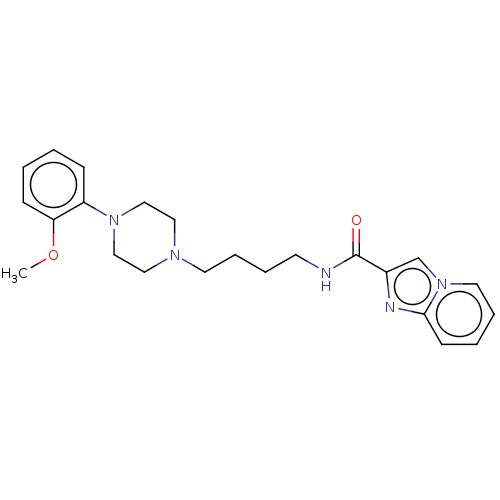 (CHEMBL3322993) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Antagonist activity against D3R in human U2OS cells assessed as inhibition of (+)-PD128907-induced beta-arrestin translocation by beta-galactosidase ... | J Med Chem 57: 7042-60 (2014) Article DOI: 10.1021/jm500801r BindingDB Entry DOI: 10.7270/Q27D2WSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50253619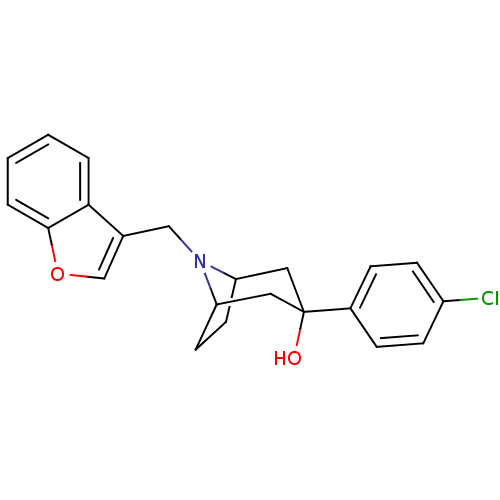 (CHEMBL493278 | endo-8-(Benzofur-3-ylmethyl)-3-(4-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuses Curated by ChEMBL | Assay Description Antagonist activity at dopamine D3 receptor (unknown origin) | J Med Chem 51: 6095-109 (2008) Article DOI: 10.1021/jm800532x BindingDB Entry DOI: 10.7270/Q2HM589Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50219117 (CHEMBL244562 | N-(4-(4-(2,3-dichlorophenyl)piperaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 0.600 | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America as Represented by the Secretary of the Department of Health and Human Services; The University of North Texas Health Science Center at Fort Worth US Patent | Assay Description Methods for performing in vitro dopamine receptor binding studies are described in Huang et al. J. Med. Chem. 44:1815-1826 (2001) and Luedtke et al. ... | US Patent US8748608 (2014) BindingDB Entry DOI: 10.7270/Q2GT5KVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM123842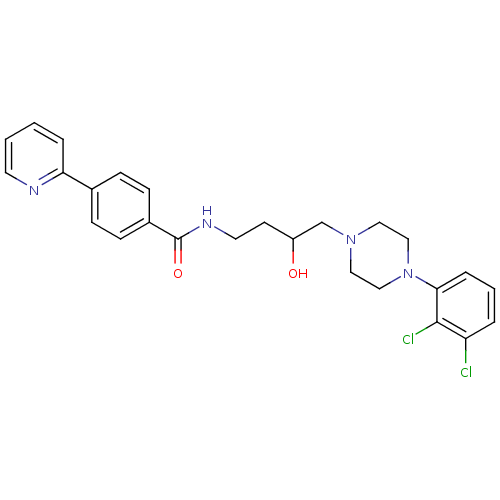 (US8748608, 16) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3 | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America as Represented by the Secretary of the Department of Health and Human Services; The University of North Texas Health Science Center at Fort Worth US Patent | Assay Description Methods for performing in vitro dopamine receptor binding studies are described in Huang et al. J. Med. Chem. 44:1815-1826 (2001) and Luedtke et al. ... | US Patent US8748608 (2014) BindingDB Entry DOI: 10.7270/Q2GT5KVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50219117 (CHEMBL244562 | N-(4-(4-(2,3-dichlorophenyl)piperaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Antagonist activity at human dopamine D3 receptor expressed in HEK293 cells by mitogenesis assay | J Med Chem 50: 4135-46 (2007) Article DOI: 10.1021/jm0704200 BindingDB Entry DOI: 10.7270/Q2WW7HDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50161217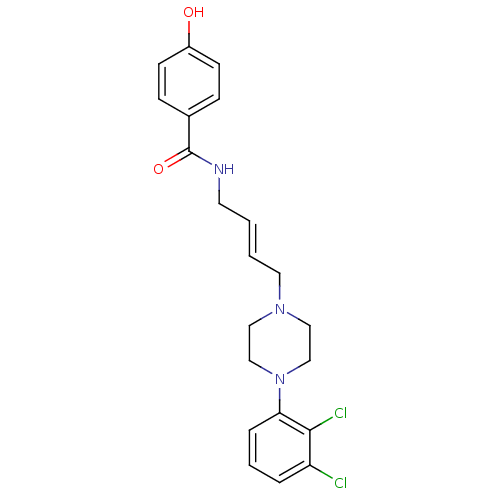 (CHEMBL195057 | N-{4-[4-(2,3-Dichloro-phenyl)-piper...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Mitogenic stimulation or antagonism of 30 nM quinpirole-stimulated mitogenesis in CHO cells expressing human dopamine D3 receptor | J Med Chem 48: 3663-79 (2005) Article DOI: 10.1021/jm040190e BindingDB Entry DOI: 10.7270/Q2474BNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50378016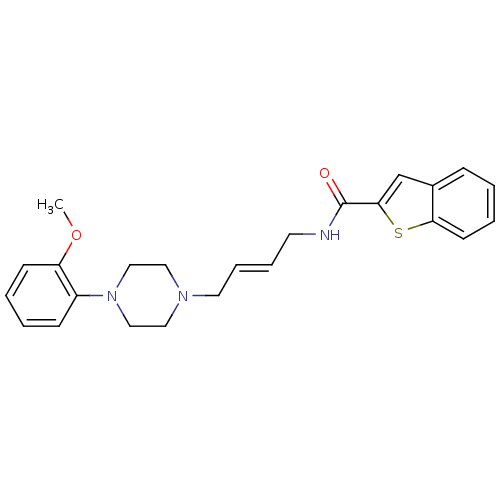 (CHEMBL1627304) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.09 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug AbuseIntramural Research Program Curated by ChEMBL | Assay Description Antagonist activity at human D3 receptor expressed in HEK293 cells assessed as inhibition of quinpirole-stimulated mitogenesis | J Med Chem 52: 2559-70 (2009) Article DOI: 10.1021/jm900095y BindingDB Entry DOI: 10.7270/Q2HX1DKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM123840 (US8748608, 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.10 | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America as Represented by the Secretary of the Department of Health and Human Services; The University of North Texas Health Science Center at Fort Worth US Patent | Assay Description Methods for performing in vitro dopamine receptor binding studies are described in Huang et al. J. Med. Chem. 44:1815-1826 (2001) and Luedtke et al. ... | US Patent US8748608 (2014) BindingDB Entry DOI: 10.7270/Q2GT5KVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50219108 (CHEMBL244774 | N-(4-(4-(2,3-dichlorophenyl)piperaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Antagonist activity at human dopamine D3 receptor expressed in HEK293 cells by mitogenesis assay | J Med Chem 50: 4135-46 (2007) Article DOI: 10.1021/jm0704200 BindingDB Entry DOI: 10.7270/Q2WW7HDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50172414 (2-(2,4-Dichloro-phenoxy)-N-[2-(2-dimethylamino-eth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
7TM Pharma A/S Curated by ChEMBL | Assay Description Inhibitory concentration against dopamine receptor D3 | J Med Chem 48: 5684-97 (2005) Article DOI: 10.1021/jm050103y BindingDB Entry DOI: 10.7270/Q2H41QZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50057762 (CHEMBL3323011 | US9598387, Compound 113 | US996974...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Antagonist activity against human D3R expressed in CHO cells assessed as inhibition of dopamine-induced [35S]GTPgammaS binding by dopamine potency sh... | J Med Chem 57: 7042-60 (2014) Article DOI: 10.1021/jm500801r BindingDB Entry DOI: 10.7270/Q27D2WSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50207145 (CHEMBL3946995 | US9550741, I-1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University Curated by ChEMBL | Assay Description Antagonistic activity at D3 receptor (unknown origin) expressed in cell membranes assessed as inhibition of quinpirole-induced response after 40 mins... | Eur J Med Chem 123: 332-353 (2016) Article DOI: 10.1016/j.ejmech.2016.07.038 BindingDB Entry DOI: 10.7270/Q2GH9KXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50378008 (CHEMBL1627314) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.51 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug AbuseIntramural Research Program Curated by ChEMBL | Assay Description Antagonist activity at human D3 receptor expressed in HEK293 cells assessed as inhibition of quinpirole-stimulated mitogenesis | J Med Chem 52: 2559-70 (2009) Article DOI: 10.1021/jm900095y BindingDB Entry DOI: 10.7270/Q2HX1DKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50378007 (CHEMBL1627315) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug AbuseIntramural Research Program Curated by ChEMBL | Assay Description Antagonist activity at human D3 receptor expressed in HEK293 cells assessed as inhibition of quinpirole-stimulated mitogenesis | J Med Chem 52: 2559-70 (2009) Article DOI: 10.1021/jm900095y BindingDB Entry DOI: 10.7270/Q2HX1DKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50208987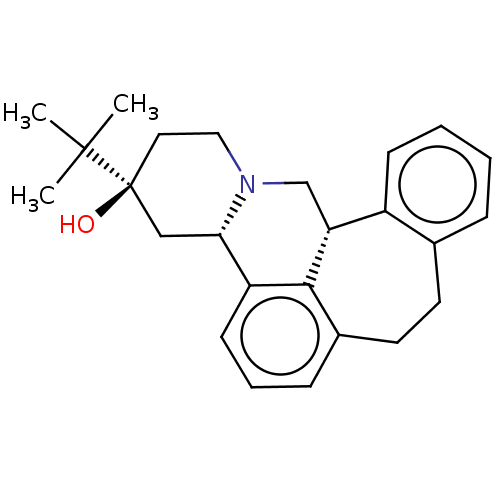 (CHEMBL3885419) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]methyl-spiperone from human recombinant dopamine D3 receptor expressed in CHO cells measured after 60 mins by scintillation count... | Bioorg Med Chem 25: 471-482 (2017) Article DOI: 10.1016/j.bmc.2016.11.014 BindingDB Entry DOI: 10.7270/Q2CF9S3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50008735 ((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]methylspiperone from human recombinant Dopamine D3 receptor expressed in CHO cells | Bioorg Med Chem 24: 1793-810 (2016) Article DOI: 10.1016/j.bmc.2016.03.006 BindingDB Entry DOI: 10.7270/Q2J67JS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50378011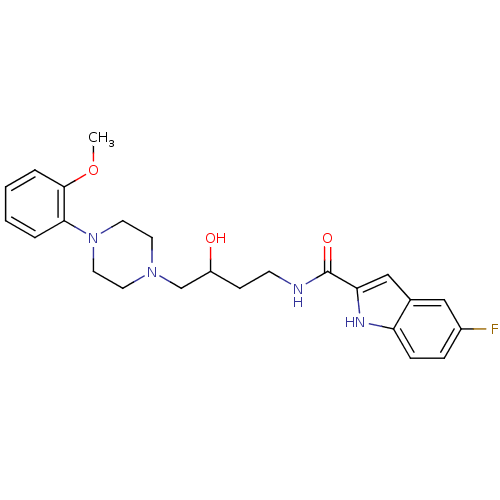 (CHEMBL1627311) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.76 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug AbuseIntramural Research Program Curated by ChEMBL | Assay Description Antagonist activity at human D3 receptor expressed in HEK293 cells assessed as inhibition of quinpirole-stimulated mitogenesis | J Med Chem 52: 2559-70 (2009) Article DOI: 10.1021/jm900095y BindingDB Entry DOI: 10.7270/Q2HX1DKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50119380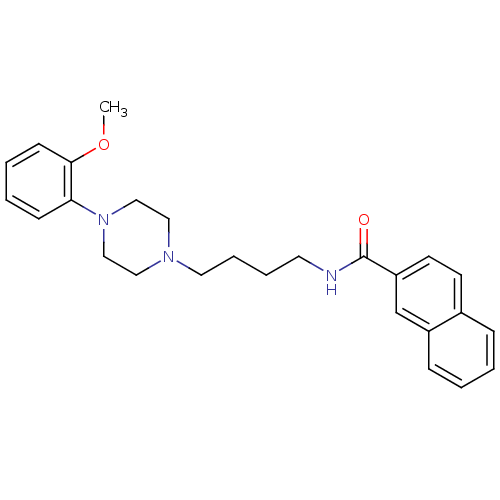 (CHEMBL25236 | CHEMBL540612 | N-(4-(4-(2-methoxyphe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Mitogenic stimulation in CHO cells expressing human Dopamine receptor D3 | J Med Chem 48: 3663-79 (2005) Article DOI: 10.1021/jm040190e BindingDB Entry DOI: 10.7270/Q2474BNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50057773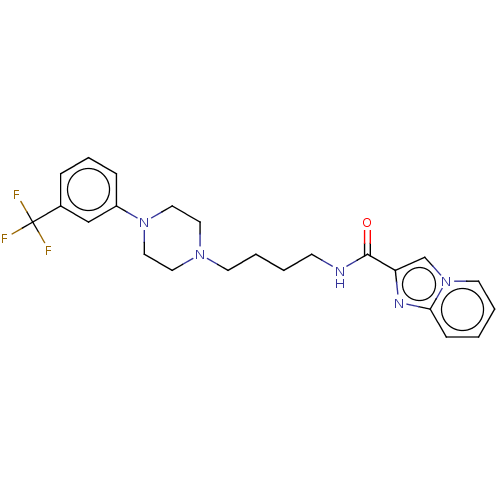 (CHEMBL3322995) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Antagonist activity against D3R in human U2OS cells assessed as inhibition of (+)-PD128907-induced beta-arrestin translocation by beta-galactosidase ... | J Med Chem 57: 7042-60 (2014) Article DOI: 10.1021/jm500801r BindingDB Entry DOI: 10.7270/Q27D2WSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50119380 (CHEMBL25236 | CHEMBL540612 | N-(4-(4-(2-methoxyphe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Mitogenic stimulation in CHO cells expressing human Dopamine receptor D3 | J Med Chem 48: 3663-79 (2005) Article DOI: 10.1021/jm040190e BindingDB Entry DOI: 10.7270/Q2474BNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50178623 (CHEMBL110365 | N-(4-(4-(2-methoxyphenyl)piperazin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Antagonist activity at human dopamine D3 receptor expressed in HEK293 cells by mitogenesis assay | J Med Chem 50: 4135-46 (2007) Article DOI: 10.1021/jm0704200 BindingDB Entry DOI: 10.7270/Q2WW7HDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50161221 (CHEMBL194493 | N-{(E)-4-[4-(2,3-Dichloro-phenyl)-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Mitogenic stimulation or antagonism of 30 nM quinpirole-stimulated mitogenesis in CHO cells expressing human dopamine D3 receptor | J Med Chem 48: 3663-79 (2005) Article DOI: 10.1021/jm040190e BindingDB Entry DOI: 10.7270/Q2474BNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50119377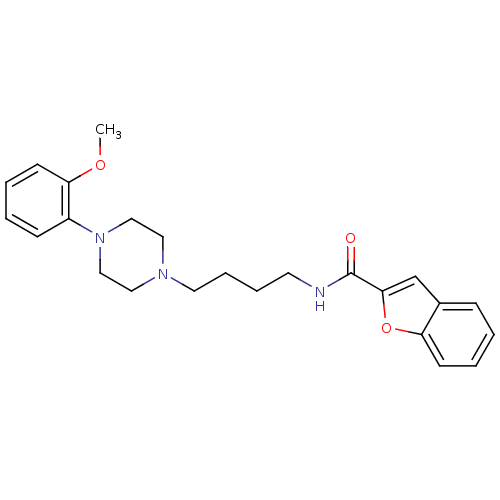 (Benzofuran-2-carboxylic acid {4-[4-(2-methoxy-phen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.39 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug AbuseIntramural Research Program Curated by ChEMBL | Assay Description Antagonist activity at human D3 receptor expressed in HEK293 cells assessed as inhibition of quinpirole-stimulated mitogenesis | J Med Chem 52: 2559-70 (2009) Article DOI: 10.1021/jm900095y BindingDB Entry DOI: 10.7270/Q2HX1DKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50219103 (CHEMBL241973 | N-(4-(4-(2-methoxyphenyl)piperazin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Antagonist activity at human dopamine D3 receptor expressed in HEK293 cells by mitogenesis assay | J Med Chem 50: 4135-46 (2007) Article DOI: 10.1021/jm0704200 BindingDB Entry DOI: 10.7270/Q2WW7HDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50057772 (CHEMBL3322993) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Antagonist activity against human D3R expressed in CHO cells assessed as inhibition of quinpirole-induced mitogenesis after 24 hrs by [3H]thymidine u... | J Med Chem 57: 7042-60 (2014) Article DOI: 10.1021/jm500801r BindingDB Entry DOI: 10.7270/Q27D2WSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50161214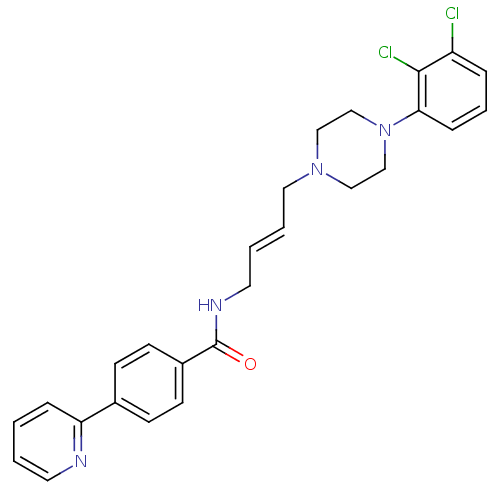 (CHEMBL180010 | N-(4-(4-(2,3-dichlorophenyl)piperaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Antagonist activity at human dopamine D3 receptor expressed in HEK293 cells by mitogenesis assay | J Med Chem 50: 4135-46 (2007) Article DOI: 10.1021/jm0704200 BindingDB Entry DOI: 10.7270/Q2WW7HDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50119380 (CHEMBL25236 | CHEMBL540612 | N-(4-(4-(2-methoxyphe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Partial agonist intrinsic activity at dopamine D3 receptor | J Med Chem 48: 3663-79 (2005) Article DOI: 10.1021/jm040190e BindingDB Entry DOI: 10.7270/Q2474BNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50161214 (CHEMBL180010 | N-(4-(4-(2,3-dichlorophenyl)piperaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.01 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Mitogenic stimulation or antagonism of 30 nM quinpirole-stimulated mitogenesis in CHO cells expressing human dopamine D3 receptor | J Med Chem 48: 3663-79 (2005) Article DOI: 10.1021/jm040190e BindingDB Entry DOI: 10.7270/Q2474BNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human dopamine D3 opioid receptor expressed in HEK293T cells assessed as GalphaoA activation preincubated with compound in D-P... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00611 BindingDB Entry DOI: 10.7270/Q2N301V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM123845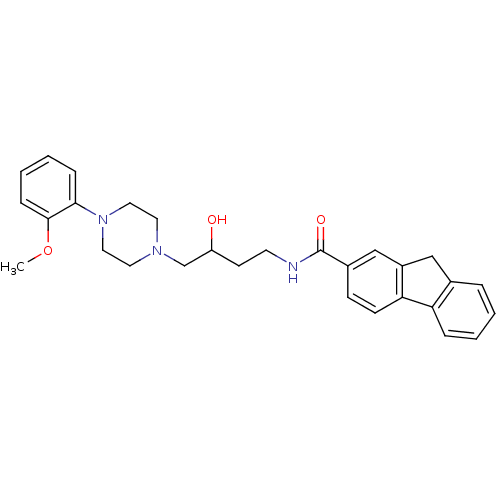 (US8748608, 19) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.80 | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America as Represented by the Secretary of the Department of Health and Human Services; The University of North Texas Health Science Center at Fort Worth US Patent | Assay Description Methods for performing in vitro dopamine receptor binding studies are described in Huang et al. J. Med. Chem. 44:1815-1826 (2001) and Luedtke et al. ... | US Patent US8748608 (2014) BindingDB Entry DOI: 10.7270/Q2GT5KVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50253616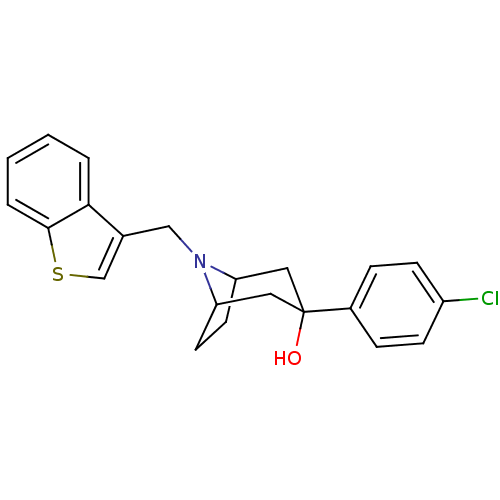 (CHEMBL493276 | endo-8-(Benzothien-3-ylmethyl)-3-(4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuses Curated by ChEMBL | Assay Description Antagonist activity at dopamine D3 receptor (unknown origin) | J Med Chem 51: 6095-109 (2008) Article DOI: 10.1021/jm800532x BindingDB Entry DOI: 10.7270/Q2HM589Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50161227 (CHEMBL370713 | N-{4-[4-(2,3-Dichloro-phenyl)-piper...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.79 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Mitogenic stimulation or antagonism of 30 nM quinpirole-stimulated mitogenesis in CHO cells expressing human dopamine D3 receptor | J Med Chem 48: 3663-79 (2005) Article DOI: 10.1021/jm040190e BindingDB Entry DOI: 10.7270/Q2474BNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50119390 (Benzo[b]thiophene-2-carboxylic acid {4-[4-(2,3-dic...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.94 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug AbuseIntramural Research Program Curated by ChEMBL | Assay Description Antagonist activity at human D3 receptor expressed in HEK293 cells assessed as inhibition of quinpirole-stimulated mitogenesis | J Med Chem 52: 2559-70 (2009) Article DOI: 10.1021/jm900095y BindingDB Entry DOI: 10.7270/Q2HX1DKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50119390 (Benzo[b]thiophene-2-carboxylic acid {4-[4-(2,3-dic...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.94 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Mitogenic stimulation or antagonism of 30 nM quinpirole-stimulated mitogenesis in CHO cells expressing human dopamine D3 receptor | J Med Chem 48: 3663-79 (2005) Article DOI: 10.1021/jm040190e BindingDB Entry DOI: 10.7270/Q2474BNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The IC50 value was reported as apparent, since [3H]NCA was purported to be irreversible. Result indicates the mean of two separate experiments, each ... | J Med Chem 27: 806-10 (1984) BindingDB Entry DOI: 10.7270/Q26111HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50071959 (9H-Fluorene-2-carboxylic acid {4-[4-(2,3-dichloro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Antagonist activity against 100 nM quinpirole-stimulated mitogenesis in CHO cells expressing human dopamine D3 receptor | J Med Chem 48: 3663-79 (2005) Article DOI: 10.1021/jm040190e BindingDB Entry DOI: 10.7270/Q2474BNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM123846 (US8748608, 20) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 11.7 | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America as Represented by the Secretary of the Department of Health and Human Services; The University of North Texas Health Science Center at Fort Worth US Patent | Assay Description Methods for performing in vitro dopamine receptor binding studies are described in Huang et al. J. Med. Chem. 44:1815-1826 (2001) and Luedtke et al. ... | US Patent US8748608 (2014) BindingDB Entry DOI: 10.7270/Q2GT5KVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50219111 (CHEMBL390253 | N-(4-(4-(2,3-dichlorophenyl)piperaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Antagonist activity at human dopamine D3 receptor expressed in HEK293 cells by mitogenesis assay | J Med Chem 50: 4135-46 (2007) Article DOI: 10.1021/jm0704200 BindingDB Entry DOI: 10.7270/Q2WW7HDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 336 total ) | Next | Last >> |