 Found 28 hits of ec50 for UniProtKB: P30553
Found 28 hits of ec50 for UniProtKB: P30553 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM82235
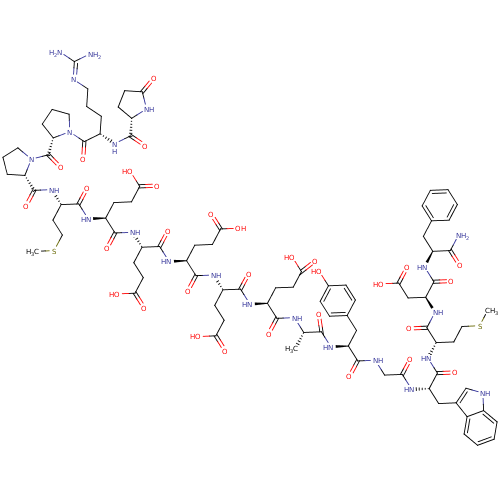
(Gastrin I | Gastrin-17 | Gastrin-I-(1-17))Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H]1CCC(=O)N1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,wU:4.4,138.143,38.41,52.60,70.78,88.95,96.98,wD:8.20,130.135,26.37,43.51,61.69,79.87,103.106,110.122,121.125,(26,6.14,;24.67,5.35,;24.67,3.8,;23.32,3.01,;23.32,1.49,;22,.73,;20.68,1.49,;20.68,3.04,;19.35,.73,;19.35,-.76,;20.68,-1.52,;21.94,-.7,;23.15,-1.69,;22.59,-3.1,;23.29,-4.48,;22.45,-5.77,;20.9,-5.71,;20.2,-4.31,;21.04,-2.98,;18,1.49,;16.68,.7,;16.68,-.76,;15.36,1.49,;14.03,.7,;12.68,1.46,;12.68,3.01,;11.36,.68,;11.36,-.79,;12.71,-1.55,;12.71,-3.1,;14.03,-3.86,;15.36,-3.07,;16.71,-3.86,;15.36,-1.55,;14.03,-.79,;10.01,1.44,;8.66,.68,;8.66,-.82,;7.33,1.44,;7.3,2.98,;6.01,.65,;4.66,1.44,;4.66,2.98,;3.34,.65,;3.34,-.84,;4.66,-1.6,;4.66,-3.15,;6.01,-3.91,;3.34,-3.91,;2.01,1.44,;.66,.65,;.66,-.84,;-.61,1.44,;-.61,2.96,;.66,3.74,;.66,5.32,;2.01,6.11,;-.61,6.11,;-1.93,.65,;-3.28,1.41,;-3.28,2.96,;-4.6,.65,;-4.6,-.84,;-3.28,-1.6,;-3.28,-3.15,;-1.93,-3.94,;-4.6,-3.94,;-5.93,1.41,;-7.28,.65,;-7.28,-.84,;-8.6,1.44,;-8.6,2.96,;-7.28,3.74,;-7.28,5.32,;-5.93,6.11,;-8.6,6.11,;-9.95,.65,;-11.33,1.44,;-11.33,2.98,;-12.65,.65,;-12.65,-.82,;-11.33,-1.6,;-11.33,-3.15,;-9.95,-3.94,;-12.65,-3.91,;-13.98,1.44,;-15.33,.68,;-15.33,-.79,;-16.65,1.44,;-16.65,2.96,;-15.3,3.77,;-15.3,5.29,;-13.98,6.08,;-17.97,.68,;-19.32,1.44,;-19.32,2.98,;-20.62,.68,;-19.69,1.83,;-20.39,3.26,;-21.91,2.96,;-21.91,1.44,;-23.24,.68,;-23.24,-.96,;-24.7,1.52,;-23.97,.34,;-24.56,-.98,;-26.16,-.7,;-26.16,.68,;-27.52,1.44,;-27.52,2.98,;-28.84,.68,;-28.84,-.82,;-27.52,-1.55,;-27.52,-3.1,;-26.14,-3.86,;-26.11,-5.4,;-27.52,-6.16,;-24.78,-6.16,;-30.19,1.44,;-31.51,.68,;-31.51,-.82,;-32.84,1.41,;-31.85,.28,;-32.55,-1.1,;-34.07,-.79,;-35.37,-1.58,;-34.07,.68,;24.67,.73,;24.67,-.73,;26,1.49,;27.35,.7,;27.35,-.76,;28.7,-1.55,;30.02,-.76,;28.7,-3.07,;28.7,1.49,;28.7,3.04,;30.02,.7,;31.34,1.49,;31.34,3.01,;32.7,3.83,;32.7,5.38,;34.02,6.16,;35.37,5.38,;35.37,3.83,;34.02,3.01,;32.7,.7,;34.02,1.49,;32.7,-.76,)| Show InChI InChI=1S/C94H128N22O31S2/c1-48(79(133)113-65(43-50-19-21-52(117)22-20-50)80(134)100-47-71(119)103-66(44-51-46-99-54-15-8-7-14-53(51)54)89(143)109-61(35-40-148-2)88(142)114-67(45-77(130)131)90(144)112-64(78(95)132)42-49-12-5-4-6-13-49)101-81(135)56(24-30-72(120)121)104-83(137)57(25-31-73(122)123)105-84(138)58(26-32-74(124)125)106-85(139)59(27-33-75(126)127)107-86(140)60(28-34-76(128)129)108-87(141)62(36-41-149-3)110-91(145)68-17-10-38-115(68)93(147)69-18-11-39-116(69)92(146)63(16-9-37-98-94(96)97)111-82(136)55-23-29-70(118)102-55/h4-8,12-15,19-22,46,48,55-69,99,117H,9-11,16-18,23-45,47H2,1-3H3,(H2,95,132)(H,100,134)(H,101,135)(H,102,118)(H,103,119)(H,104,137)(H,105,138)(H,106,139)(H,107,140)(H,108,141)(H,109,143)(H,110,145)(H,111,136)(H,112,144)(H,113,133)(H,114,142)(H,120,121)(H,122,123)(H,124,125)(H,126,127)(H,128,129)(H,130,131)(H4,96,97,98)/t48-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a |
University Medical Centre Ljubljana
Curated by ChEMBL
| Assay Description
Agonist activity at gastrin receptor in rat AR4-2J cells assessed as intracellular calcium mobilization by fluorescence assay |
J Med Chem 54: 2602-9 (2011)
Article DOI: 10.1021/jm101279a
BindingDB Entry DOI: 10.7270/Q2668DK5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50348753
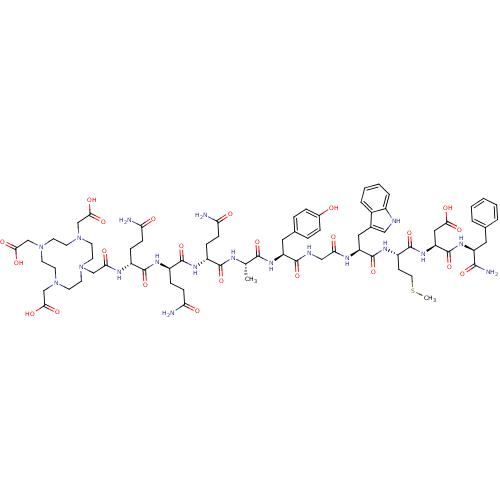
(CHEMBL1806528)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](C)NC(=O)[C@@H](CCC(N)=O)NC(=O)[C@@H](CCC(N)=O)NC(=O)[C@@H](CCC(N)=O)NC(=O)CN1CCN(CC(O)=O)CCN(CC(O)=O)CCN(CC(O)=O)CC1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C74H103N19O23S/c1-42(81-69(111)50(17-20-58(76)96)84-71(113)51(18-21-59(77)97)85-70(112)49(16-19-57(75)95)82-61(99)38-90-23-25-91(39-63(102)103)27-29-93(41-65(106)107)30-28-92(26-24-90)40-64(104)105)67(109)88-54(33-44-12-14-46(94)15-13-44)68(110)80-37-60(98)83-55(34-45-36-79-48-11-7-6-10-47(45)48)73(115)86-52(22-31-117-2)72(114)89-56(35-62(100)101)74(116)87-53(66(78)108)32-43-8-4-3-5-9-43/h3-15,36,42,49-56,79,94H,16-35,37-41H2,1-2H3,(H2,75,95)(H2,76,96)(H2,77,97)(H2,78,108)(H,80,110)(H,81,111)(H,82,99)(H,83,98)(H,84,113)(H,85,112)(H,86,115)(H,87,116)(H,88,109)(H,89,114)(H,100,101)(H,102,103)(H,104,105)(H,106,107)/t42-,49+,50+,51+,52-,53-,54-,55-,56-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a |
University Medical Centre Ljubljana
Curated by ChEMBL
| Assay Description
Agonist activity at gastrin receptor in rat AR4-2J cells assessed as intracellular calcium mobilization by fluorescence assay |
J Med Chem 54: 2602-9 (2011)
Article DOI: 10.1021/jm101279a
BindingDB Entry DOI: 10.7270/Q2668DK5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50348752
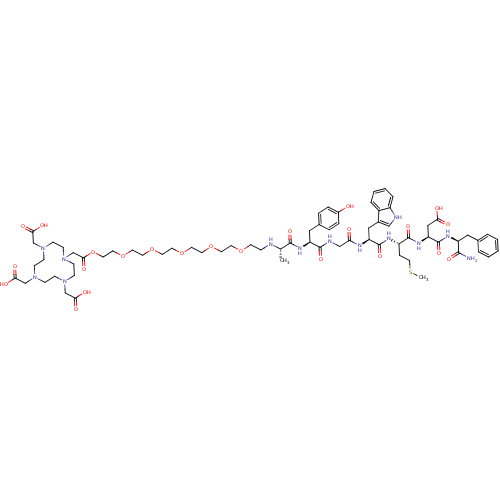
(CHEMBL1807150)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](C)NCCOCCOCCOCCOCCOCCOC(=O)CN1CCN(CC(O)=O)CCN(CC(O)=O)CCN(CC(O)=O)CC1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C71H103N13O23S/c1-48(73-17-26-102-27-28-103-29-30-104-31-32-105-33-34-106-35-36-107-65(95)47-84-24-22-82(45-63(91)92)20-18-81(44-62(89)90)19-21-83(23-25-84)46-64(93)94)67(97)79-57(39-50-12-14-52(85)15-13-50)68(98)75-43-60(86)76-58(40-51-42-74-54-11-7-6-10-53(51)54)70(100)77-55(16-37-108-2)69(99)80-59(41-61(87)88)71(101)78-56(66(72)96)38-49-8-4-3-5-9-49/h3-15,42,48,55-59,73-74,85H,16-41,43-47H2,1-2H3,(H2,72,96)(H,75,98)(H,76,86)(H,77,100)(H,78,101)(H,79,97)(H,80,99)(H,87,88)(H,89,90)(H,91,92)(H,93,94)/t48-,55-,56-,57-,58-,59-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a |
University Medical Centre Ljubljana
Curated by ChEMBL
| Assay Description
Agonist activity at gastrin receptor in rat AR4-2J cells assessed as intracellular calcium mobilization by fluorescence assay |
J Med Chem 54: 2602-9 (2011)
Article DOI: 10.1021/jm101279a
BindingDB Entry DOI: 10.7270/Q2668DK5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50092405
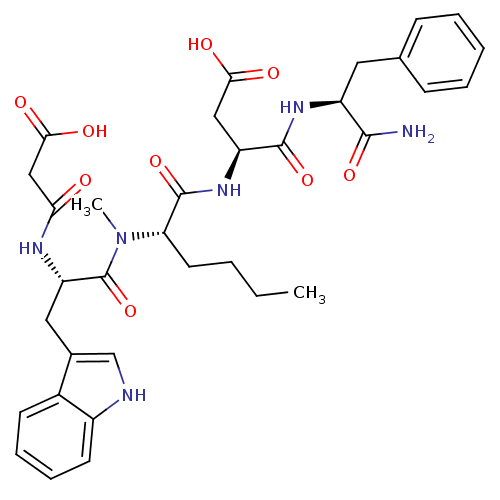
((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-((S)-2-{[...)Show SMILES CCCC[C@H](N(C)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C34H42N6O9/c1-3-4-14-27(33(48)39-25(17-29(42)43)32(47)38-24(31(35)46)15-20-10-6-5-7-11-20)40(2)34(49)26(37-28(41)18-30(44)45)16-21-19-36-23-13-9-8-12-22(21)23/h5-13,19,24-27,36H,3-4,14-18H2,1-2H3,(H2,35,46)(H,37,41)(H,38,47)(H,39,48)(H,42,43)(H,44,45)/t24-,25-,26-,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Affinity against Cholecystokinin type B receptor expressed in CHO cells on rat brain. |
J Med Chem 43: 3614-23 (2000)
BindingDB Entry DOI: 10.7270/Q2RN373C |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50348756
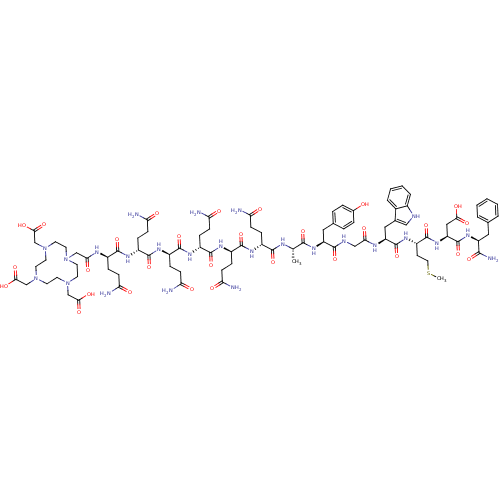
(CHEMBL1806532)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](C)NC(=O)[C@@H](CCC(N)=O)NC(=O)[C@@H](CCC(N)=O)NC(=O)[C@@H](CCC(N)=O)NC(=O)[C@@H](CCC(N)=O)NC(=O)[C@@H](CCC(N)=O)NC(=O)[C@@H](CCC(N)=O)NC(=O)CN1CCN(CC(O)=O)CCN(CC(O)=O)CCN(CC(O)=O)CC1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C89H127N25O29S/c1-48(79(133)109-63(39-50-12-14-52(115)15-13-50)80(134)98-43-72(122)101-64(40-51-42-97-54-11-7-6-10-53(51)54)88(142)107-61(28-37-144-2)87(141)110-65(41-74(124)125)89(143)108-62(78(96)132)38-49-8-4-3-5-9-49)99-81(135)56(17-23-67(91)117)102-83(137)58(19-25-69(93)119)104-85(139)60(21-27-71(95)121)106-86(140)59(20-26-70(94)120)105-84(138)57(18-24-68(92)118)103-82(136)55(16-22-66(90)116)100-73(123)44-111-29-31-112(45-75(126)127)33-35-114(47-77(130)131)36-34-113(32-30-111)46-76(128)129/h3-15,42,48,55-65,97,115H,16-41,43-47H2,1-2H3,(H2,90,116)(H2,91,117)(H2,92,118)(H2,93,119)(H2,94,120)(H2,95,121)(H2,96,132)(H,98,134)(H,99,135)(H,100,123)(H,101,122)(H,102,137)(H,103,136)(H,104,139)(H,105,138)(H,106,140)(H,107,142)(H,108,143)(H,109,133)(H,110,141)(H,124,125)(H,126,127)(H,128,129)(H,130,131)/t48-,55+,56+,57+,58+,59+,60+,61-,62-,63-,64-,65-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a |
University Medical Centre Ljubljana
Curated by ChEMBL
| Assay Description
Agonist activity at gastrin receptor in rat AR4-2J cells assessed as intracellular calcium mobilization by fluorescence assay |
J Med Chem 54: 2602-9 (2011)
Article DOI: 10.1021/jm101279a
BindingDB Entry DOI: 10.7270/Q2668DK5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50024321
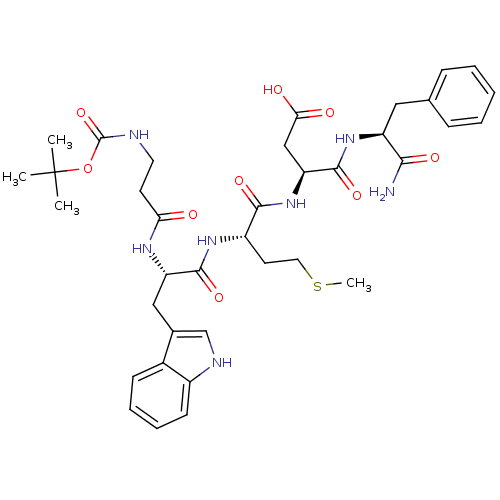
(3-{2-[2-(3-tert-Butoxycarbonylamino-propionylamino...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CCNC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C37H49N7O9S/c1-37(2,3)53-36(52)39-16-14-30(45)41-28(19-23-21-40-25-13-9-8-12-24(23)25)34(50)42-26(15-17-54-4)33(49)44-29(20-31(46)47)35(51)43-27(32(38)48)18-22-10-6-5-7-11-22/h5-13,21,26-29,40H,14-20H2,1-4H3,(H2,38,48)(H,39,52)(H,41,45)(H,42,50)(H,43,51)(H,44,49)(H,46,47)/t26-,27-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at rat CCK2R expressed in rat AR42J cells assessed as intracellular calcium mobilization measured after 24 hrs by Fluo-4AM dye based... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01233
BindingDB Entry DOI: 10.7270/Q2P84GJ2 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50092408
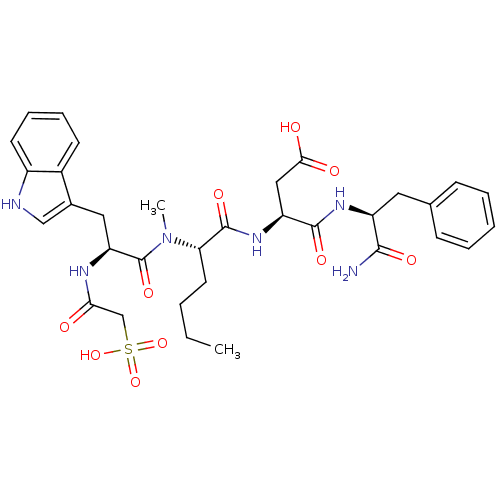
(CHEMBL120150 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-(2...)Show SMILES CCCC[C@H](N(C)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CS(O)(=O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C33H42N6O10S/c1-3-4-14-27(32(45)38-25(17-29(41)42)31(44)37-24(30(34)43)15-20-10-6-5-7-11-20)39(2)33(46)26(36-28(40)19-50(47,48)49)16-21-18-35-23-13-9-8-12-22(21)23/h5-13,18,24-27,35H,3-4,14-17,19H2,1-2H3,(H2,34,43)(H,36,40)(H,37,44)(H,38,45)(H,41,42)(H,47,48,49)/t24-,25-,26-,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Affinity against Cholecystokinin type B receptor expressed in CHO cells on rat brain. |
J Med Chem 43: 3614-23 (2000)
BindingDB Entry DOI: 10.7270/Q2RN373C |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Effective concentration for the stimulation of Inositol Phosphate accumulation in CHO cells expressing wild-type Cholecystokinin type B receptor |
J Med Chem 40: 647-58 (1997)
Article DOI: 10.1021/jm9603072
BindingDB Entry DOI: 10.7270/Q2PG1SDX |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Affinity against Cholecystokinin type B receptor expressed in CHO cells on rat brain. |
J Med Chem 43: 3614-23 (2000)
BindingDB Entry DOI: 10.7270/Q2RN373C |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50002477

((S)-3-{(S)-2-[(S)-2-tert-Butoxycarbonylamino-3-(1H...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C34H44N6O8S/c1-34(2,3)48-33(47)40-26(17-21-19-36-23-13-9-8-12-22(21)23)31(45)37-24(14-15-49-4)30(44)39-27(18-28(41)42)32(46)38-25(29(35)43)16-20-10-6-5-7-11-20/h5-13,19,24-27,36H,14-18H2,1-4H3,(H2,35,43)(H,37,45)(H,38,46)(H,39,44)(H,40,47)(H,41,42)/t24-,25-,26-,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Affinity against Cholecystokinin type B receptor expressed in CHO cells on rat brain. |
J Med Chem 43: 3614-23 (2000)
BindingDB Entry DOI: 10.7270/Q2RN373C |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50549228
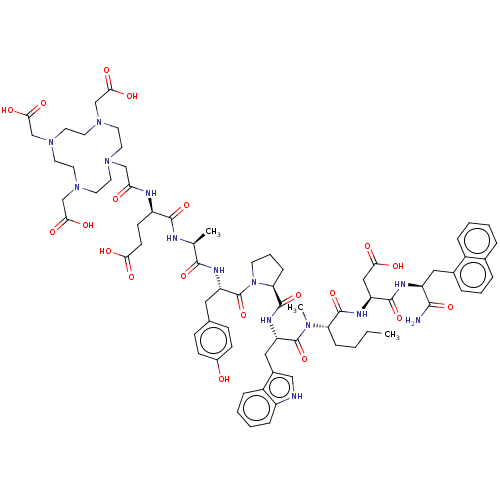
(CHEMBL4758706)Show SMILES CCCC[C@H](N(C)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](C)NC(=O)[C@@H](CCC(O)=O)NC(=O)CN1CCN(CC(O)=O)CCN(CC(O)=O)CCN(CC(O)=O)CC1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1cccc2ccccc12)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at rat CCK2R expressed in rat AR42J cells assessed as intracellular calcium mobilization measured after 24 hrs by Fluo-4AM dye based... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01233
BindingDB Entry DOI: 10.7270/Q2P84GJ2 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50024317
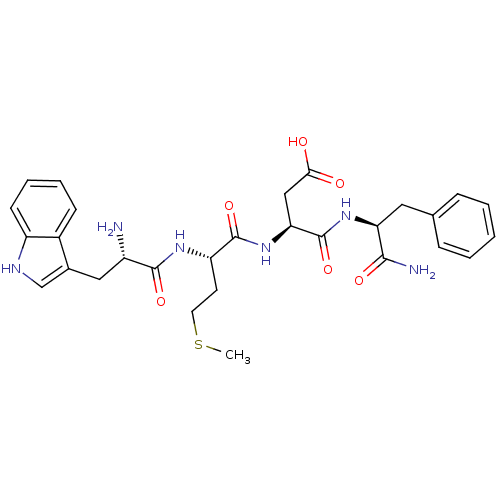
(3-{2-[2-Amino-3-(1H-indol-3-yl)-propionylamino]-4-...)Show SMILES CSCC[C@H](NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C29H36N6O6S/c1-42-12-11-22(33-27(39)20(30)14-18-16-32-21-10-6-5-9-19(18)21)28(40)35-24(15-25(36)37)29(41)34-23(26(31)38)13-17-7-3-2-4-8-17/h2-10,16,20,22-24,32H,11-15,30H2,1H3,(H2,31,38)(H,33,39)(H,34,41)(H,35,40)(H,36,37)/t20-,22-,23-,24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Affinity against Cholecystokinin type B receptor expressed in CHO cells on rat brain. |
J Med Chem 43: 3614-23 (2000)
BindingDB Entry DOI: 10.7270/Q2RN373C |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50092384
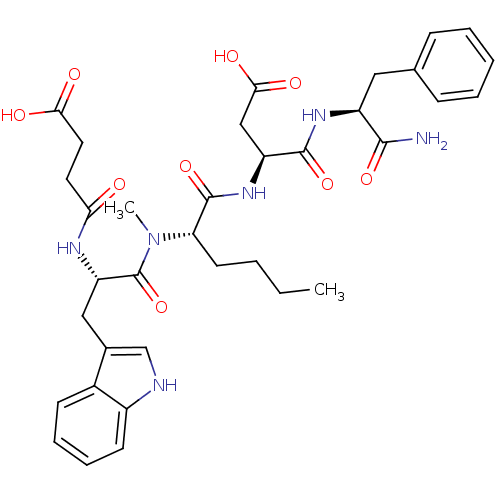
(CHEMBL100538 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-(2...)Show SMILES CCCC[C@H](N(C)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C35H44N6O9/c1-3-4-14-28(34(49)40-26(19-31(45)46)33(48)39-25(32(36)47)17-21-10-6-5-7-11-21)41(2)35(50)27(38-29(42)15-16-30(43)44)18-22-20-37-24-13-9-8-12-23(22)24/h5-13,20,25-28,37H,3-4,14-19H2,1-2H3,(H2,36,47)(H,38,42)(H,39,48)(H,40,49)(H,43,44)(H,45,46)/t25-,26-,27-,28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Affinity against Cholecystokinin type B receptor expressed in CHO cells on rat brain. |
J Med Chem 43: 3614-23 (2000)
BindingDB Entry DOI: 10.7270/Q2RN373C |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50092398
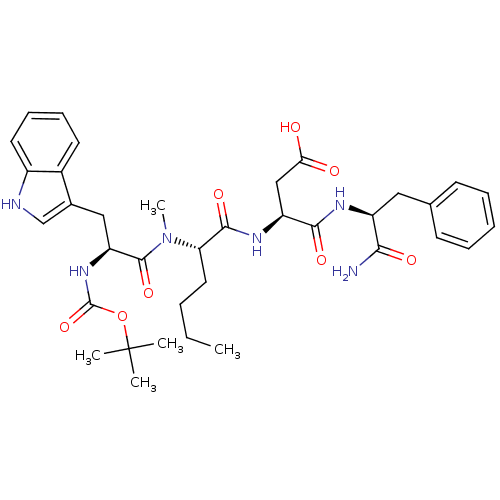
((S)-3-((S)-2-{[(S)-2-tert-Butoxycarbonylamino-3-(1...)Show SMILES CCCC[C@H](N(C)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C36H48N6O8/c1-6-7-17-29(33(47)40-27(20-30(43)44)32(46)39-26(31(37)45)18-22-13-9-8-10-14-22)42(5)34(48)28(41-35(49)50-36(2,3)4)19-23-21-38-25-16-12-11-15-24(23)25/h8-16,21,26-29,38H,6-7,17-20H2,1-5H3,(H2,37,45)(H,39,46)(H,40,47)(H,41,49)(H,43,44)/t26-,27-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Affinity against Cholecystokinin type B receptor expressed in CHO cells on rat brain. |
J Med Chem 43: 3614-23 (2000)
BindingDB Entry DOI: 10.7270/Q2RN373C |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50056453
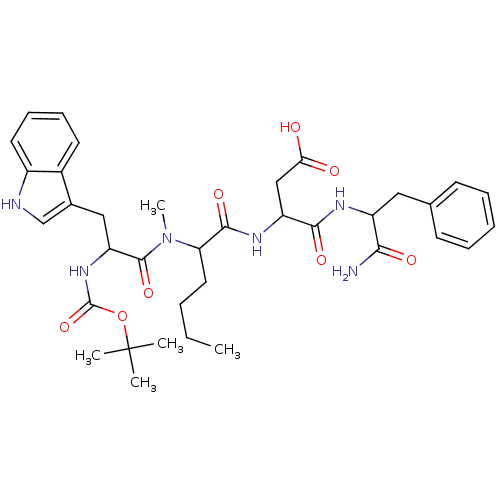
(3-(2-{[2-tert-Butoxycarbonylamino-3-(1H-indol-3-yl...)Show SMILES CCCCC(N(C)C(=O)C(Cc1c[nH]c2ccccc12)NC(=O)OC(C)(C)C)C(=O)NC(CC(O)=O)C(=O)NC(Cc1ccccc1)C(N)=O Show InChI InChI=1S/C36H48N6O8/c1-6-7-17-29(33(47)40-27(20-30(43)44)32(46)39-26(31(37)45)18-22-13-9-8-10-14-22)42(5)34(48)28(41-35(49)50-36(2,3)4)19-23-21-38-25-16-12-11-15-24(23)25/h8-16,21,26-29,38H,6-7,17-20H2,1-5H3,(H2,37,45)(H,39,46)(H,40,47)(H,41,49)(H,43,44) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Effective concentration for the stimulation of Inositol Phosphate accumulation in CHO cells expressing wild-type Cholecystokinin type B receptor |
J Med Chem 40: 647-58 (1997)
Article DOI: 10.1021/jm9603072
BindingDB Entry DOI: 10.7270/Q2PG1SDX |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50092391
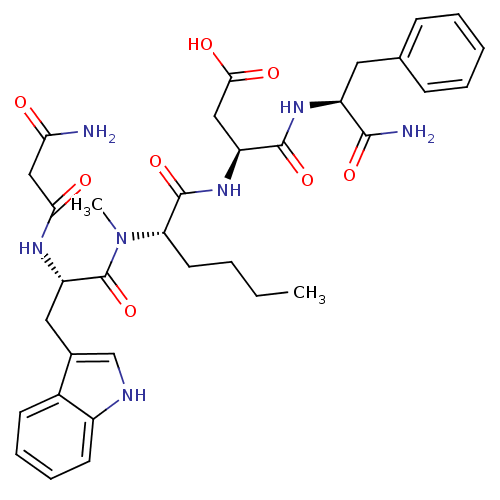
(3-(2-{[2-(2-Carbamoyl-acetylamino)-3-(1H-indol-3-y...)Show SMILES CCCC[C@H](N(C)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C34H43N7O8/c1-3-4-14-27(33(48)40-25(17-30(44)45)32(47)39-24(31(36)46)15-20-10-6-5-7-11-20)41(2)34(49)26(38-29(43)18-28(35)42)16-21-19-37-23-13-9-8-12-22(21)23/h5-13,19,24-27,37H,3-4,14-18H2,1-2H3,(H2,35,42)(H2,36,46)(H,38,43)(H,39,47)(H,40,48)(H,44,45)/t24-,25-,26-,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.64 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Affinity against Cholecystokinin type B receptor expressed in CHO cells on rat brain. |
J Med Chem 43: 3614-23 (2000)
BindingDB Entry DOI: 10.7270/Q2RN373C |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50549226
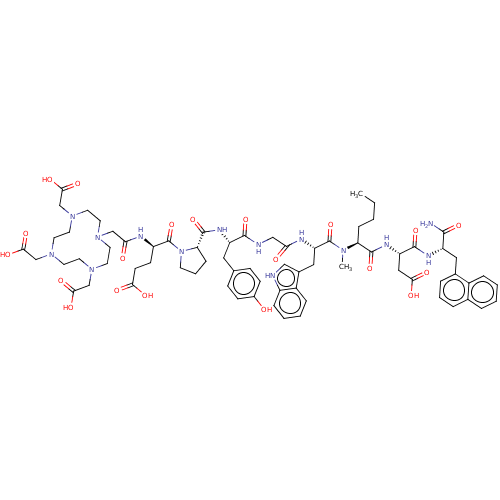
(CHEMBL4795913)Show SMILES CCCC[C@H](N(C)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](CCC(O)=O)NC(=O)CN1CCN(CC(O)=O)CCN(CC(O)=O)CCN(CC(O)=O)CC1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1cccc2ccccc12)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at rat CCK2R expressed in rat AR42J cells assessed as intracellular calcium mobilization measured after 24 hrs by Fluo-4AM dye based... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01233
BindingDB Entry DOI: 10.7270/Q2P84GJ2 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50549227
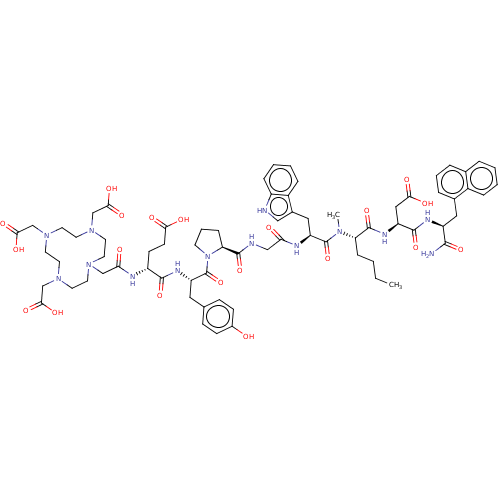
(CHEMBL4759105)Show SMILES CCCC[C@H](N(C)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](CCC(O)=O)NC(=O)CN1CCN(CC(O)=O)CCN(CC(O)=O)CCN(CC(O)=O)CC1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1cccc2ccccc12)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at rat CCK2R expressed in rat AR42J cells assessed as intracellular calcium mobilization measured after 24 hrs by Fluo-4AM dye based... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01233
BindingDB Entry DOI: 10.7270/Q2P84GJ2 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50092389

(CHEMBL321456 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-(2...)Show SMILES CCCC[C@H](N(C)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CC(=O)OC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C35H44N6O9/c1-4-5-15-28(34(48)40-26(18-30(43)44)33(47)39-25(32(36)46)16-21-11-7-6-8-12-21)41(2)35(49)27(38-29(42)19-31(45)50-3)17-22-20-37-24-14-10-9-13-23(22)24/h6-14,20,25-28,37H,4-5,15-19H2,1-3H3,(H2,36,46)(H,38,42)(H,39,47)(H,40,48)(H,43,44)/t25-,26-,27-,28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Affinity against Cholecystokinin type B receptor expressed in CHO cells on rat brain. |
J Med Chem 43: 3614-23 (2000)
BindingDB Entry DOI: 10.7270/Q2RN373C |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50092399
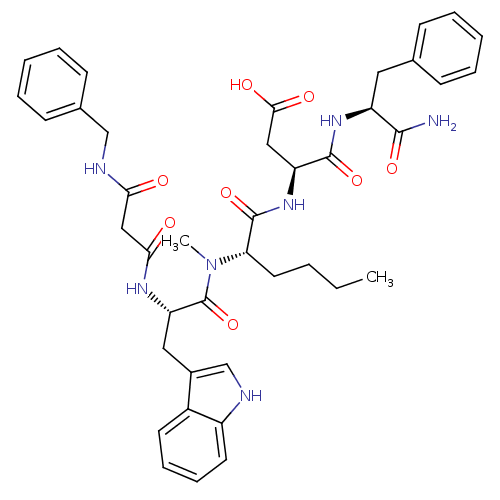
(3-(2-{[2-(2-Benzylcarbamoyl-acetylamino)-3-(1H-ind...)Show SMILES CCCC[C@H](N(C)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CC(=O)NCc1ccccc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C41H49N7O8/c1-3-4-19-34(40(55)47-32(22-37(51)52)39(54)46-31(38(42)53)20-26-13-7-5-8-14-26)48(2)41(56)33(21-28-25-43-30-18-12-11-17-29(28)30)45-36(50)23-35(49)44-24-27-15-9-6-10-16-27/h5-18,25,31-34,43H,3-4,19-24H2,1-2H3,(H2,42,53)(H,44,49)(H,45,50)(H,46,54)(H,47,55)(H,51,52)/t31-,32-,33-,34-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Affinity against Cholecystokinin type B receptor expressed in CHO cells on rat brain. |
J Med Chem 43: 3614-23 (2000)
BindingDB Entry DOI: 10.7270/Q2RN373C |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50092393
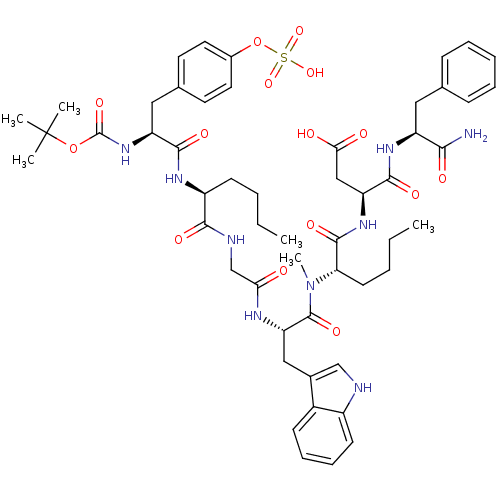
(3-(2-{[2-(2-{2-[2-tert-Butoxycarbonylamino-3-(4-su...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)OC(C)(C)C)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N(C)[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C53H71N9O15S/c1-7-9-19-38(58-48(68)40(61-52(72)76-53(3,4)5)27-33-22-24-35(25-23-33)77-78(73,74)75)47(67)56-31-44(63)57-42(28-34-30-55-37-20-15-14-18-36(34)37)51(71)62(6)43(21-10-8-2)50(70)60-41(29-45(64)65)49(69)59-39(46(54)66)26-32-16-12-11-13-17-32/h11-18,20,22-25,30,38-43,55H,7-10,19,21,26-29,31H2,1-6H3,(H2,54,66)(H,56,67)(H,57,63)(H,58,68)(H,59,69)(H,60,70)(H,61,72)(H,64,65)(H,73,74,75)/t38-,39-,40-,41-,42-,43-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Affinity against Cholecystokinin type B receptor expressed in CHO cells on rat brain. |
J Med Chem 43: 3614-23 (2000)
BindingDB Entry DOI: 10.7270/Q2RN373C |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50056448

(CHEMBL2371221 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{...)Show SMILES C[C@]1(Cc2c[nH]c3ccccc23)NC(=O)CCCCCCCC[C@@H](NC1=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C36H46N6O7/c1-36(21-24-22-38-26-16-12-11-15-25(24)26)35(49)41-27(17-9-4-2-3-5-10-18-30(43)42-36)33(47)40-29(20-31(44)45)34(48)39-28(32(37)46)19-23-13-7-6-8-14-23/h6-8,11-16,22,27-29,38H,2-5,9-10,17-21H2,1H3,(H2,37,46)(H,39,48)(H,40,47)(H,41,49)(H,42,43)(H,44,45)/t27-,28+,29+,36-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Effective concentration for the stimulation of Inositol Phosphate accumulation in CHO cells expressing wild-type Cholecystokinin type B receptor |
J Med Chem 40: 647-58 (1997)
Article DOI: 10.1021/jm9603072
BindingDB Entry DOI: 10.7270/Q2PG1SDX |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50056455
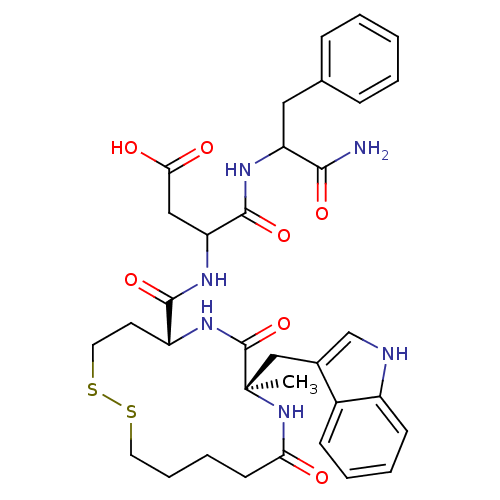
(CHEMBL348344 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{[...)Show SMILES C[C@]1(Cc2c[nH]c3ccccc23)NC(=O)CCCCSSCC[C@@H](NC1=O)C(=O)NC(CC(O)=O)C(=O)NC(Cc1ccccc1)C(N)=O Show InChI InChI=1S/C34H42N6O7S2/c1-34(19-22-20-36-24-12-6-5-11-23(22)24)33(47)39-25(14-16-49-48-15-8-7-13-28(41)40-34)31(45)38-27(18-29(42)43)32(46)37-26(30(35)44)17-21-9-3-2-4-10-21/h2-6,9-12,20,25-27,36H,7-8,13-19H2,1H3,(H2,35,44)(H,37,46)(H,38,45)(H,39,47)(H,40,41)(H,42,43)/t25-,26?,27?,34-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Effective concentration for the stimulation of Inositol Phosphate accumulation in CHO cells expressing wild-type Cholecystokinin type B receptor |
J Med Chem 40: 647-58 (1997)
Article DOI: 10.1021/jm9603072
BindingDB Entry DOI: 10.7270/Q2PG1SDX |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50056449
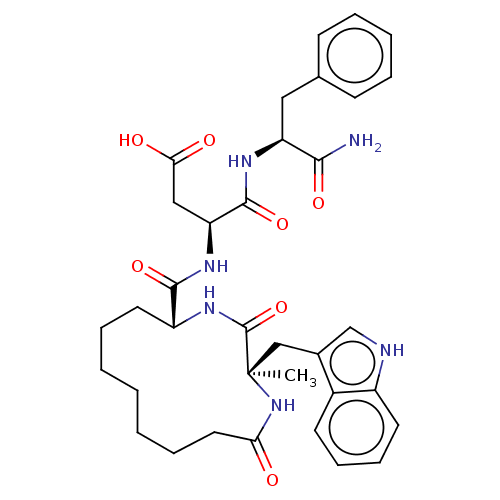
(CHEMBL2371222 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{...)Show SMILES C[C@]1(Cc2c[nH]c3ccccc23)NC(=O)CCCCCCC[C@@H](NC1=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C35H44N6O7/c1-35(20-23-21-37-25-15-11-10-14-24(23)25)34(48)40-26(16-8-3-2-4-9-17-29(42)41-35)32(46)39-28(19-30(43)44)33(47)38-27(31(36)45)18-22-12-6-5-7-13-22/h5-7,10-15,21,26-28,37H,2-4,8-9,16-20H2,1H3,(H2,36,45)(H,38,47)(H,39,46)(H,40,48)(H,41,42)(H,43,44)/t26-,27+,28+,35-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Effective concentration for the stimulation of Inositol Phosphate accumulation in CHO cells expressing wild-type Cholecystokinin type B receptor |
J Med Chem 40: 647-58 (1997)
Article DOI: 10.1021/jm9603072
BindingDB Entry DOI: 10.7270/Q2PG1SDX |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50092401
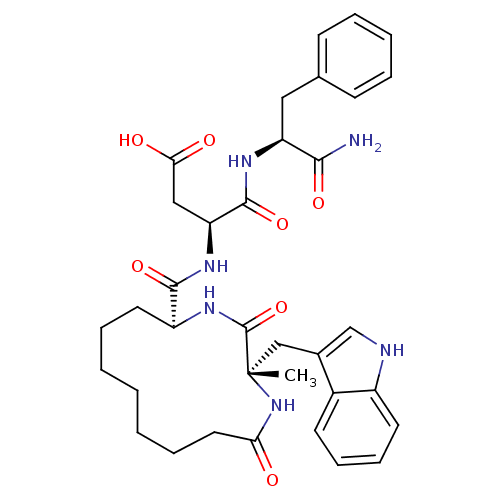
(CHEMBL177799 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{[...)Show SMILES C[C@@]1(Cc2c[nH]c3ccccc23)NC(=O)CCCCCCC[C@H](NC1=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C35H44N6O7/c1-35(20-23-21-37-25-15-11-10-14-24(23)25)34(48)40-26(16-8-3-2-4-9-17-29(42)41-35)32(46)39-28(19-30(43)44)33(47)38-27(31(36)45)18-22-12-6-5-7-13-22/h5-7,10-15,21,26-28,37H,2-4,8-9,16-20H2,1H3,(H2,36,45)(H,38,47)(H,39,46)(H,40,48)(H,41,42)(H,43,44)/t26-,27-,28-,35-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Affinity against Cholecystokinin type B receptor expressed in CHO cells on rat brain. |
J Med Chem 43: 3614-23 (2000)
BindingDB Entry DOI: 10.7270/Q2RN373C |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50092395
(3-{2-[2-{[9-tert-Butoxycarbonylamino-3-butyl-2,5,8...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H]1CCCCNC(=O)C[C@H](NC(=O)OC(C)(C)C)C(=O)N[C@H](Cc2ccc(OS(O)(=O)=O)cc2)C(=O)N[C@@H](CCCC)C(=O)N1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C60H81N11O17S/c1-6-8-20-41-52(76)64-43(23-15-16-28-62-49(72)32-47(71-59(83)87-60(3,4)5)57(81)68-45(55(79)65-41)30-36-24-26-38(27-25-36)88-89(84,85)86)54(78)69-46(31-37-34-63-40-22-14-13-19-39(37)40)56(80)66-42(21-9-7-2)53(77)70-48(33-50(73)74)58(82)67-44(51(61)75)29-35-17-11-10-12-18-35/h10-14,17-19,22,24-27,34,41-48,63H,6-9,15-16,20-21,23,28-33H2,1-5H3,(H2,61,75)(H,62,72)(H,64,76)(H,65,79)(H,66,80)(H,67,82)(H,68,81)(H,69,78)(H,70,77)(H,71,83)(H,73,74)(H,84,85,86)/t41-,42-,43+,44-,45+,46-,47-,48-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Affinity against Cholecystokinin type B receptor expressed in CHO cells on rat brain. |
J Med Chem 43: 3614-23 (2000)
BindingDB Entry DOI: 10.7270/Q2RN373C |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50026690
(2-[2-(2-{2-[2-{2-[2-(2-Amino-3-phenyl-propionylami...)Show SMILES CSCC[C@@H](NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)[C@@H](Cc1ccc(O)cc1)NC(=O)[C@@H](C)NC(=O)[C@@H](CCC(O)=O)NC(=O)[C@@H](CCC(O)=O)NC(=O)[C@@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H]1CCCN1C(=O)CNC(=O)[C@@H](N)CCC(O)=O)C(=O)N[C@H](CC(O)=O)C(=O)N[C@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C97H126N20O32S/c1-49(2)39-68(114-95(147)71(43-54-46-101-60-18-11-9-16-57(54)60)116-97(149)73-19-12-37-117(73)75(120)48-103-85(137)58(98)24-30-76(121)122)93(145)110-65(29-35-81(131)132)91(143)109-64(28-34-80(129)130)90(142)108-63(27-33-79(127)128)89(141)107-62(26-32-78(125)126)88(140)106-61(25-31-77(123)124)87(139)104-50(3)84(136)113-69(41-52-20-22-55(118)23-21-52)86(138)102-47-74(119)105-70(42-53-45-100-59-17-10-8-15-56(53)59)94(146)111-66(36-38-150-4)92(144)115-72(44-82(133)134)96(148)112-67(83(99)135)40-51-13-6-5-7-14-51/h5-11,13-18,20-23,45-46,49-50,58,61-73,100-101,118H,12,19,24-44,47-48,98H2,1-4H3,(H2,99,135)(H,102,138)(H,103,137)(H,104,139)(H,105,119)(H,106,140)(H,107,141)(H,108,142)(H,109,143)(H,110,145)(H,111,146)(H,112,148)(H,113,136)(H,114,147)(H,115,144)(H,116,149)(H,121,122)(H,123,124)(H,125,126)(H,127,128)(H,129,130)(H,131,132)(H,133,134)/t50-,58+,61-,62-,63-,64+,65+,66-,67-,68+,69-,70-,71+,72-,73+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
NCI-Frederick Cancer Research and Development Center
Curated by ChEMBL
| Assay Description
Tested for the 50% inhibition level against [125I]- gastrin binding in AR42J cells |
J Med Chem 37: 3812-8 (1994)
BindingDB Entry DOI: 10.7270/Q2TM7BRX |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50037873
(CBS-2 | CHEMBL341130)Show SMILES OC(=O)C(Cc1c[nH]c2ccccc12)NC(=O)CCNC(=O)CCC(=O)N(Cc1ccccc1)N=NCCCl |w:35.38| Show InChI InChI=1S/C27H31ClN6O5/c28-13-15-31-33-34(18-19-6-2-1-3-7-19)26(37)11-10-24(35)29-14-12-25(36)32-23(27(38)39)16-20-17-30-22-9-5-4-8-21(20)22/h1-9,17,23,30H,10-16,18H2,(H,29,35)(H,32,36)(H,38,39) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a |
NCI-Frederick Cancer Research and Development Center
Curated by ChEMBL
| Assay Description
Tested for the 50% inhibition level against [125I]- gastrin binding in AR42J cells |
J Med Chem 37: 3812-8 (1994)
BindingDB Entry DOI: 10.7270/Q2TM7BRX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data



























