 Found 149 hits of ki for UniProtKB: P30553
Found 149 hits of ki for UniProtKB: P30553 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50056101
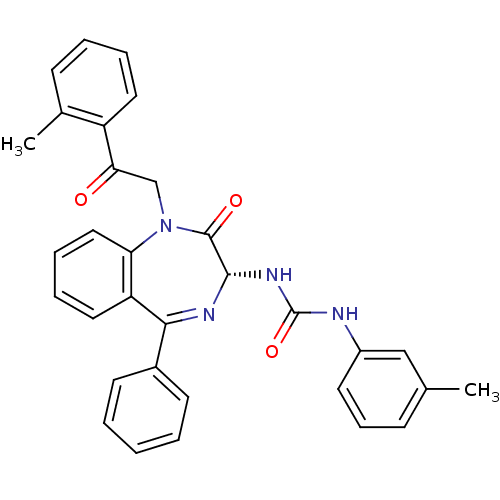
(1-[(R)-2-Oxo-1-(2-oxo-2-o-tolyl-ethyl)-5-phenyl-2,...)Show SMILES Cc1cccc(NC(=O)N[C@@H]2N=C(c3ccccc3)c3ccccc3N(CC(=O)c3ccccc3C)C2=O)c1 |t:11| Show InChI InChI=1S/C32H28N4O3/c1-21-11-10-15-24(19-21)33-32(39)35-30-31(38)36(20-28(37)25-16-7-6-12-22(25)2)27-18-9-8-17-26(27)29(34-30)23-13-4-3-5-14-23/h3-19,30H,20H2,1-2H3,(H2,33,35,39)/t30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 269: 725-31 (1994)
BindingDB Entry DOI: 10.7270/Q2WD3Z2F |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 269: 725-31 (1994)
BindingDB Entry DOI: 10.7270/Q2WD3Z2F |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Occupational and Environmental Health
Curated by PDSP Ki Database
| |
Br J Pharmacol 117: 1558-64 (1996)
Article DOI: 10.1111/j.1476-5381.1996.tb15321.x
BindingDB Entry DOI: 10.7270/Q28W3BT3 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50056101

(1-[(R)-2-Oxo-1-(2-oxo-2-o-tolyl-ethyl)-5-phenyl-2,...)Show SMILES Cc1cccc(NC(=O)N[C@@H]2N=C(c3ccccc3)c3ccccc3N(CC(=O)c3ccccc3C)C2=O)c1 |t:11| Show InChI InChI=1S/C32H28N4O3/c1-21-11-10-15-24(19-21)33-32(39)35-30-31(38)36(20-28(37)25-16-7-6-12-22(25)2)27-18-9-8-17-26(27)29(34-30)23-13-4-3-5-14-23/h3-19,30H,20H2,1-2H3,(H2,33,35,39)/t30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 269: 725-31 (1994)
BindingDB Entry DOI: 10.7270/Q2WD3Z2F |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50092393
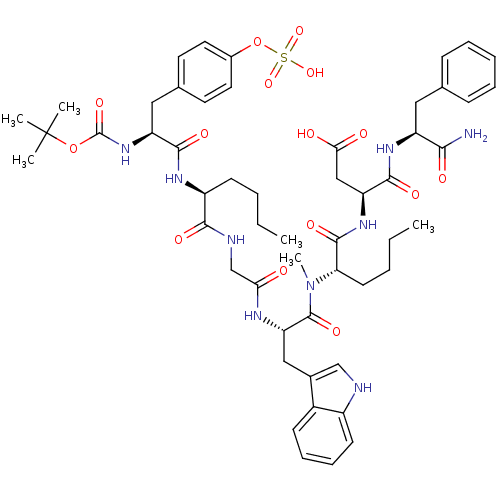
(3-(2-{[2-(2-{2-[2-tert-Butoxycarbonylamino-3-(4-su...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)OC(C)(C)C)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N(C)[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C53H71N9O15S/c1-7-9-19-38(58-48(68)40(61-52(72)76-53(3,4)5)27-33-22-24-35(25-23-33)77-78(73,74)75)47(67)56-31-44(63)57-42(28-34-30-55-37-20-15-14-18-36(34)37)51(71)62(6)43(21-10-8-2)50(70)60-41(29-45(64)65)49(69)59-39(46(54)66)26-32-16-12-11-13-17-32/h11-18,20,22-25,30,38-43,55H,7-10,19,21,26-29,31H2,1-6H3,(H2,54,66)(H,56,67)(H,57,63)(H,58,68)(H,59,69)(H,60,70)(H,61,72)(H,64,65)(H,73,74,75)/t38-,39-,40-,41-,42-,43-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against Cholecystokinin type B receptor expressed in CHO cells on the rat brain. |
J Med Chem 43: 3614-23 (2000)
BindingDB Entry DOI: 10.7270/Q2RN373C |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50005463

((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)c2cc3ccccc3[nH]2)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C25H20N4O2/c1-29-21-14-8-6-12-18(21)22(16-9-3-2-4-10-16)27-23(25(29)31)28-24(30)20-15-17-11-5-7-13-19(17)26-20/h2-15,23,26H,1H3,(H,28,30)/t23-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
Eur J Pharmacol 232: 13-9 (1993)
Article DOI: 10.1016/0014-2999(93)90722-t
BindingDB Entry DOI: 10.7270/Q2P26WN2 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50084033
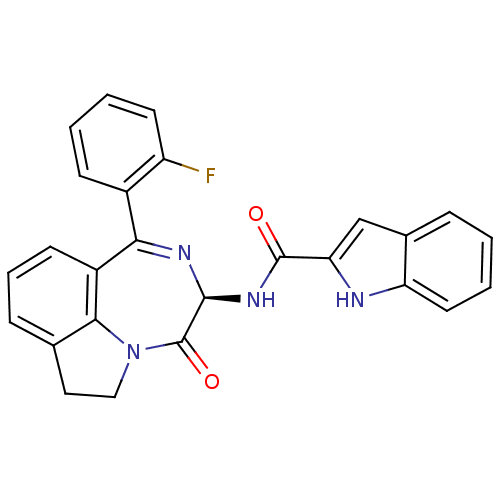
(1H-Indole-2-carboxylic acid [(R)-1-(2-fluoro-pheny...)Show SMILES Fc1ccccc1C1=N[C@@H](NC(=O)c2cc3ccccc3[nH]2)C(=O)N2CCc3cccc1c23 |t:8| Show InChI InChI=1S/C26H19FN4O2/c27-19-10-3-2-8-17(19)22-18-9-5-7-15-12-13-31(23(15)18)26(33)24(29-22)30-25(32)21-14-16-6-1-4-11-20(16)28-21/h1-11,14,24,28H,12-13H2,(H,30,32)/t24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co. Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 268: 571-5 (1994)
BindingDB Entry DOI: 10.7270/Q2WQ029S |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester
Curated by ChEMBL
| Assay Description
Binding affinity for Cholecystokinin type B receptor using [125I]-BH-CCK-8 in rat cortex synaptosomes |
J Med Chem 40: 4302-7 (1998)
Article DOI: 10.1021/jm970477u
BindingDB Entry DOI: 10.7270/Q2B858TF |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Binding affinity towards Cholecystokinin type B receptor in rat cortex synaptosomes using [125I]BH-CCK-8 as radioligand |
J Med Chem 43: 2350-5 (2000)
BindingDB Entry DOI: 10.7270/Q2FX7B4J |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50005463

((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)c2cc3ccccc3[nH]2)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C25H20N4O2/c1-29-21-14-8-6-12-18(21)22(16-9-3-2-4-10-16)27-23(25(29)31)28-24(30)20-15-17-11-5-7-13-19(17)26-20/h2-15,23,26H,1H3,(H,28,30)/t23-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Occupational and Environmental Health
Curated by PDSP Ki Database
| |
Br J Pharmacol 117: 1558-64 (1996)
Article DOI: 10.1111/j.1476-5381.1996.tb15321.x
BindingDB Entry DOI: 10.7270/Q28W3BT3 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50005463

((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)c2cc3ccccc3[nH]2)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C25H20N4O2/c1-29-21-14-8-6-12-18(21)22(16-9-3-2-4-10-16)27-23(25(29)31)28-24(30)20-15-17-11-5-7-13-19(17)26-20/h2-15,23,26H,1H3,(H,28,30)/t23-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 269: 725-31 (1994)
BindingDB Entry DOI: 10.7270/Q2WD3Z2F |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM82304

(CAS_122077 | NSC_122077 | SR 27897)Show SMILES OC(=O)Cn1c(cc2ccccc12)C(=O)Nc1nc(cs1)-c1ccccc1Cl Show InChI InChI=1S/C20H14ClN3O3S/c21-14-7-3-2-6-13(14)15-11-28-20(22-15)23-19(27)17-9-12-5-1-4-8-16(12)24(17)10-18(25)26/h1-9,11H,10H2,(H,25,26)(H,22,23,27) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
Eur J Pharmacol 232: 13-9 (1993)
Article DOI: 10.1016/0014-2999(93)90722-t
BindingDB Entry DOI: 10.7270/Q2P26WN2 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Affinity to inhibit [3H]pCCK-8 specific binding on rat brain Cholecystokinin type B receptor expressed in CHO cells |
J Med Chem 40: 647-58 (1997)
Article DOI: 10.1021/jm9603072
BindingDB Entry DOI: 10.7270/Q2PG1SDX |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against Cholecystokinin type B receptor expressed in CHO cells on the rat brain. |
J Med Chem 43: 3614-23 (2000)
BindingDB Entry DOI: 10.7270/Q2RN373C |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Ability to displace 1 nM [3H]pCCK-8 from rat Cholecystokinin type B receptor stably expressing in CHO cells |
Bioorg Med Chem Lett 14: 369-72 (2003)
BindingDB Entry DOI: 10.7270/Q2RJ4K1Z |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50092405
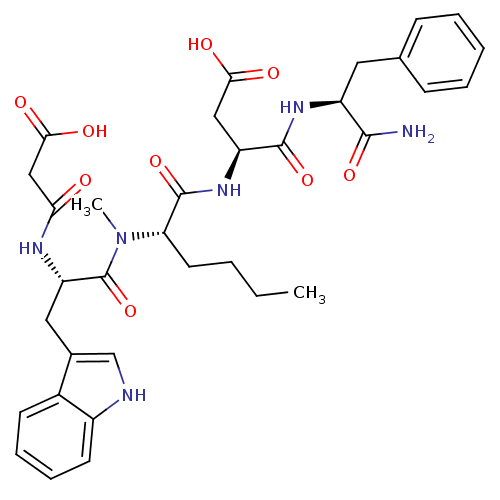
((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-((S)-2-{[...)Show SMILES CCCC[C@H](N(C)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C34H42N6O9/c1-3-4-14-27(33(48)39-25(17-29(42)43)32(47)38-24(31(35)46)15-20-10-6-5-7-11-20)40(2)34(49)26(37-28(41)18-30(44)45)16-21-19-36-23-13-9-8-12-22(21)23/h5-13,19,24-27,36H,3-4,14-18H2,1-2H3,(H2,35,46)(H,37,41)(H,38,47)(H,39,48)(H,42,43)(H,44,45)/t24-,25-,26-,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against Cholecystokinin type B receptor expressed in CHO cells on the rat brain. |
J Med Chem 43: 3614-23 (2000)
BindingDB Entry DOI: 10.7270/Q2RN373C |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM84958

(2-[[(R)-2-(1H-Indol-2-ylcarbonylamino)-3-(4-benzhy...)Show SMILES OC(=O)c1cccnc1SC[C@H](NC(=O)c1cc2ccccc2[nH]1)C(=O)N1CCN(CC1)C(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C35H33N5O4S/c41-32(29-22-26-14-7-8-16-28(26)37-29)38-30(23-45-33-27(35(43)44)15-9-17-36-33)34(42)40-20-18-39(19-21-40)31(24-10-3-1-4-11-24)25-12-5-2-6-13-25/h1-17,22,30-31,37H,18-21,23H2,(H,38,41)(H,43,44)/t30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Occupational and Environmental Health
Curated by PDSP Ki Database
| |
Br J Pharmacol 117: 1558-64 (1996)
Article DOI: 10.1111/j.1476-5381.1996.tb15321.x
BindingDB Entry DOI: 10.7270/Q28W3BT3 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50061832
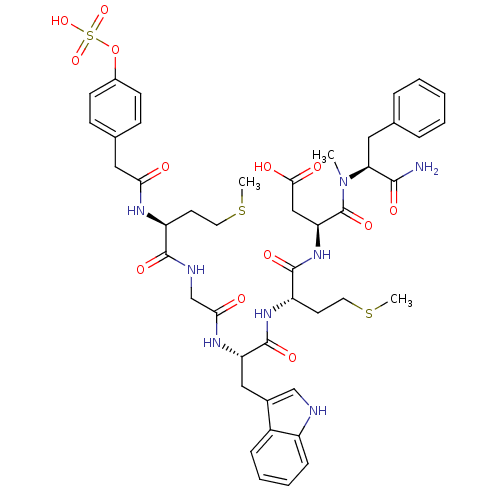
((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-{(S)-2-[(...)Show SMILES CSCC[C@H](NC(=O)Cc1ccc(OS(O)(=O)=O)cc1)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N(C)[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C45H56N8O13S3/c1-53(37(41(46)58)21-27-9-5-4-6-10-27)45(62)36(24-40(56)57)52-43(60)34(18-20-68-3)51-44(61)35(23-29-25-47-32-12-8-7-11-31(29)32)50-39(55)26-48-42(59)33(17-19-67-2)49-38(54)22-28-13-15-30(16-14-28)66-69(63,64)65/h4-16,25,33-37,47H,17-24,26H2,1-3H3,(H2,46,58)(H,48,59)(H,49,54)(H,50,55)(H,51,61)(H,52,60)(H,56,57)(H,63,64,65)/t33-,34-,35-,36-,37-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester
Curated by ChEMBL
| Assay Description
Binding affinity for Cholecystokinin type B receptor using [125I]-BH-CCK-8 in rat cortex synaptosomes |
J Med Chem 40: 4302-7 (1998)
Article DOI: 10.1021/jm970477u
BindingDB Entry DOI: 10.7270/Q2B858TF |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 124: 57-73 (1996)
Article DOI: 10.1007/bf02245606
BindingDB Entry DOI: 10.7270/Q2610XV6 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM82235
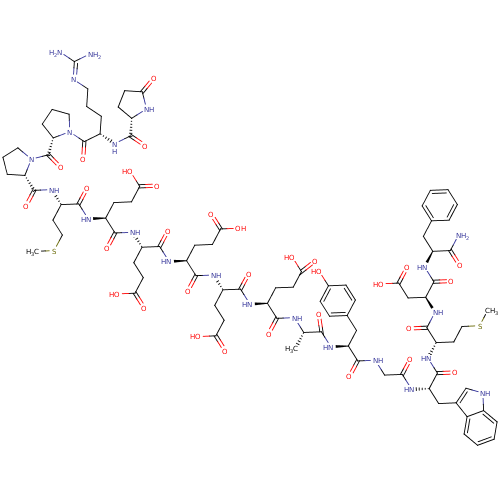
(Gastrin I | Gastrin-17 | Gastrin-I-(1-17))Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H]1CCC(=O)N1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,wU:4.4,138.143,38.41,52.60,70.78,88.95,96.98,wD:8.20,130.135,26.37,43.51,61.69,79.87,103.106,110.122,121.125,(26,6.14,;24.67,5.35,;24.67,3.8,;23.32,3.01,;23.32,1.49,;22,.73,;20.68,1.49,;20.68,3.04,;19.35,.73,;19.35,-.76,;20.68,-1.52,;21.94,-.7,;23.15,-1.69,;22.59,-3.1,;23.29,-4.48,;22.45,-5.77,;20.9,-5.71,;20.2,-4.31,;21.04,-2.98,;18,1.49,;16.68,.7,;16.68,-.76,;15.36,1.49,;14.03,.7,;12.68,1.46,;12.68,3.01,;11.36,.68,;11.36,-.79,;12.71,-1.55,;12.71,-3.1,;14.03,-3.86,;15.36,-3.07,;16.71,-3.86,;15.36,-1.55,;14.03,-.79,;10.01,1.44,;8.66,.68,;8.66,-.82,;7.33,1.44,;7.3,2.98,;6.01,.65,;4.66,1.44,;4.66,2.98,;3.34,.65,;3.34,-.84,;4.66,-1.6,;4.66,-3.15,;6.01,-3.91,;3.34,-3.91,;2.01,1.44,;.66,.65,;.66,-.84,;-.61,1.44,;-.61,2.96,;.66,3.74,;.66,5.32,;2.01,6.11,;-.61,6.11,;-1.93,.65,;-3.28,1.41,;-3.28,2.96,;-4.6,.65,;-4.6,-.84,;-3.28,-1.6,;-3.28,-3.15,;-1.93,-3.94,;-4.6,-3.94,;-5.93,1.41,;-7.28,.65,;-7.28,-.84,;-8.6,1.44,;-8.6,2.96,;-7.28,3.74,;-7.28,5.32,;-5.93,6.11,;-8.6,6.11,;-9.95,.65,;-11.33,1.44,;-11.33,2.98,;-12.65,.65,;-12.65,-.82,;-11.33,-1.6,;-11.33,-3.15,;-9.95,-3.94,;-12.65,-3.91,;-13.98,1.44,;-15.33,.68,;-15.33,-.79,;-16.65,1.44,;-16.65,2.96,;-15.3,3.77,;-15.3,5.29,;-13.98,6.08,;-17.97,.68,;-19.32,1.44,;-19.32,2.98,;-20.62,.68,;-19.69,1.83,;-20.39,3.26,;-21.91,2.96,;-21.91,1.44,;-23.24,.68,;-23.24,-.96,;-24.7,1.52,;-23.97,.34,;-24.56,-.98,;-26.16,-.7,;-26.16,.68,;-27.52,1.44,;-27.52,2.98,;-28.84,.68,;-28.84,-.82,;-27.52,-1.55,;-27.52,-3.1,;-26.14,-3.86,;-26.11,-5.4,;-27.52,-6.16,;-24.78,-6.16,;-30.19,1.44,;-31.51,.68,;-31.51,-.82,;-32.84,1.41,;-31.85,.28,;-32.55,-1.1,;-34.07,-.79,;-35.37,-1.58,;-34.07,.68,;24.67,.73,;24.67,-.73,;26,1.49,;27.35,.7,;27.35,-.76,;28.7,-1.55,;30.02,-.76,;28.7,-3.07,;28.7,1.49,;28.7,3.04,;30.02,.7,;31.34,1.49,;31.34,3.01,;32.7,3.83,;32.7,5.38,;34.02,6.16,;35.37,5.38,;35.37,3.83,;34.02,3.01,;32.7,.7,;34.02,1.49,;32.7,-.76,)| Show InChI InChI=1S/C94H128N22O31S2/c1-48(79(133)113-65(43-50-19-21-52(117)22-20-50)80(134)100-47-71(119)103-66(44-51-46-99-54-15-8-7-14-53(51)54)89(143)109-61(35-40-148-2)88(142)114-67(45-77(130)131)90(144)112-64(78(95)132)42-49-12-5-4-6-13-49)101-81(135)56(24-30-72(120)121)104-83(137)57(25-31-73(122)123)105-84(138)58(26-32-74(124)125)106-85(139)59(27-33-75(126)127)107-86(140)60(28-34-76(128)129)108-87(141)62(36-41-149-3)110-91(145)68-17-10-38-115(68)93(147)69-18-11-39-116(69)92(146)63(16-9-37-98-94(96)97)111-82(136)55-23-29-70(118)102-55/h4-8,12-15,19-22,46,48,55-69,99,117H,9-11,16-18,23-45,47H2,1-3H3,(H2,95,132)(H,100,134)(H,101,135)(H,102,118)(H,103,119)(H,104,137)(H,105,138)(H,106,139)(H,107,140)(H,108,141)(H,109,143)(H,110,145)(H,111,136)(H,112,144)(H,113,133)(H,114,142)(H,120,121)(H,122,123)(H,124,125)(H,126,127)(H,128,129)(H,130,131)(H4,96,97,98)/t48-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 269: 725-31 (1994)
BindingDB Entry DOI: 10.7270/Q2WD3Z2F |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50092398
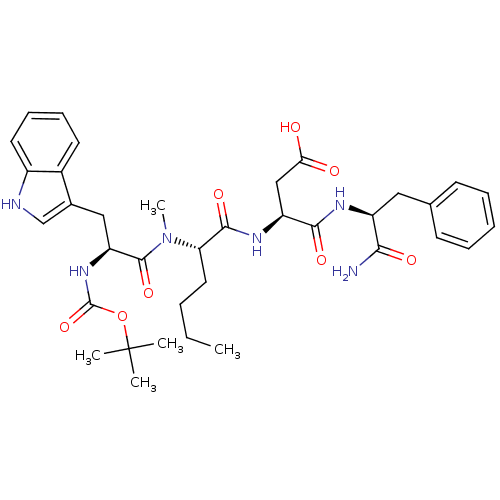
((S)-3-((S)-2-{[(S)-2-tert-Butoxycarbonylamino-3-(1...)Show SMILES CCCC[C@H](N(C)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C36H48N6O8/c1-6-7-17-29(33(47)40-27(20-30(43)44)32(46)39-26(31(37)45)18-22-13-9-8-10-14-22)42(5)34(48)28(41-35(49)50-36(2,3)4)19-23-21-38-25-16-12-11-15-24(23)25/h8-16,21,26-29,38H,6-7,17-20H2,1-5H3,(H2,37,45)(H,39,46)(H,40,47)(H,41,49)(H,43,44)/t26-,27-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against Cholecystokinin type B receptor expressed in CHO cells on the rat brain. |
J Med Chem 43: 3614-23 (2000)
BindingDB Entry DOI: 10.7270/Q2RN373C |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]- pCCK-8 binding to Cholecystokinin type B receptor of rat cerebral cortex membranes |
J Med Chem 40: 3402-7 (1997)
Article DOI: 10.1021/jm9703247
BindingDB Entry DOI: 10.7270/Q2B56HV4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50472854
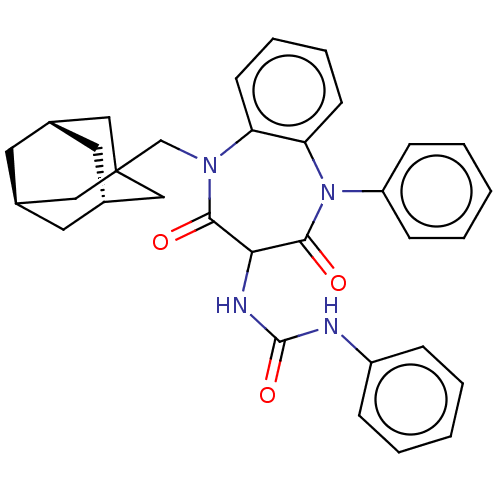
(CHEMBL330977)Show SMILES [H][C@]12C[C@]3([H])C[C@]([H])(C1)CC(CN1c4ccccc4N(c4ccccc4)C(=O)C(NC(=O)Nc4ccccc4)C1=O)(C2)C3 |TLB:8:1:42:6.9.5,8:6:42:1.41.2,THB:2:3:9:1.41.8,2:1:9:3.42.5| Show InChI InChI=1S/C33H34N4O3/c38-30-29(35-32(40)34-25-9-3-1-4-10-25)31(39)37(26-11-5-2-6-12-26)28-14-8-7-13-27(28)36(30)21-33-18-22-15-23(19-33)17-24(16-22)20-33/h1-14,22-24,29H,15-21H2,(H2,34,35,40)/t22-,23+,24-,29?,33? | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition by displacing [3H]CCK-8S against Cholecystokinin type B receptor of rat pancreatic membranes |
J Med Chem 43: 3596-613 (2000)
Article DOI: 10.1021/jm990967h
BindingDB Entry DOI: 10.7270/Q26H4M5V |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50061220
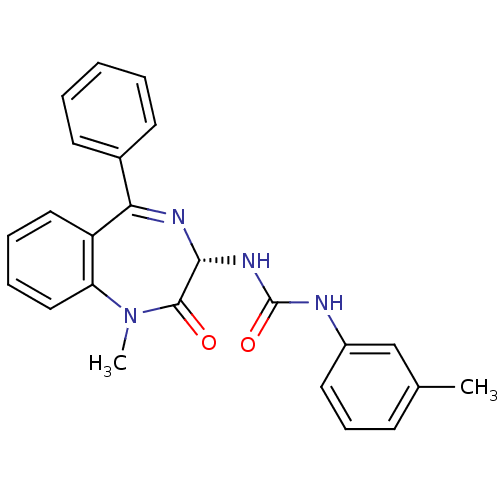
(1-((S)-1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)Nc2cccc(C)c2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C24H22N4O2/c1-16-9-8-12-18(15-16)25-24(30)27-22-23(29)28(2)20-14-7-6-13-19(20)21(26-22)17-10-4-3-5-11-17/h3-15,22H,1-2H3,(H2,25,27,30)/t22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 162: 273-80 (1989)
Article DOI: 10.1016/0014-2999(89)90290-2
BindingDB Entry DOI: 10.7270/Q2QF8RB7 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM82403
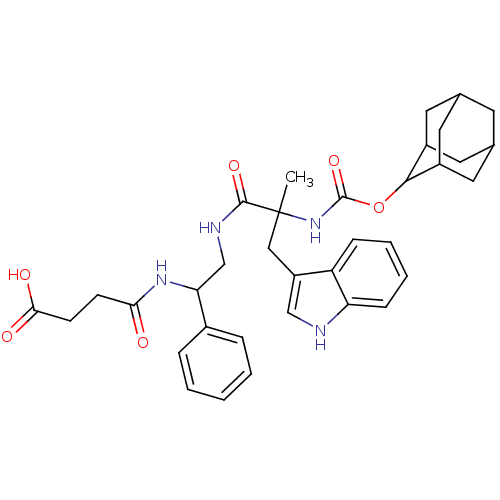
(CAS_108186 | CI-988 | NSC_108186)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NCC(NC(=O)CCC(O)=O)c1ccccc1 |TLB:25:24:18.19.20:22,THB:15:16:18.19.20:22,20:19:16:21.22.23,20:21:16:18.19.25,25:19:22:16.23.24,(.76,2.29,;.32,.82,;-.11,-.66,;.95,-1.78,;.68,-3.29,;2.03,-4.02,;3.15,-2.96,;4.68,-3.07,;5.55,-1.8,;4.88,-.42,;3.35,-.3,;2.48,-1.57,;1.8,.38,;2.92,1.45,;2.55,2.94,;4.39,1.01,;5.51,2.08,;6.88,2.77,;6.88,4.17,;7.72,5.64,;9.09,4.95,;9.09,3.55,;8.25,2.08,;7.72,2.85,;6.35,3.55,;6.35,4.95,;-1.15,1.25,;-1.52,2.75,;-2.27,.19,;-3.75,.62,;-4.86,-.44,;-6.34,-.01,;-7.45,-1.07,;-7.09,-2.57,;-8.93,-.64,;-10.04,-1.7,;-11.52,-1.27,;-11.89,.23,;-12.64,-2.33,;-4.5,-1.94,;-3.02,-2.37,;-2.65,-3.87,;-3.77,-4.93,;-5.25,-4.5,;-5.61,-3,)| Show InChI InChI=1S/C35H42N4O6/c1-35(18-26-19-36-28-10-6-5-9-27(26)28,39-34(44)45-32-24-14-21-13-22(16-24)17-25(32)15-21)33(43)37-20-29(23-7-3-2-4-8-23)38-30(40)11-12-31(41)42/h2-10,19,21-22,24-25,29,32,36H,11-18,20H2,1H3,(H,37,43)(H,38,40)(H,39,44)(H,41,42) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 269: 725-31 (1994)
BindingDB Entry DOI: 10.7270/Q2WD3Z2F |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50449787
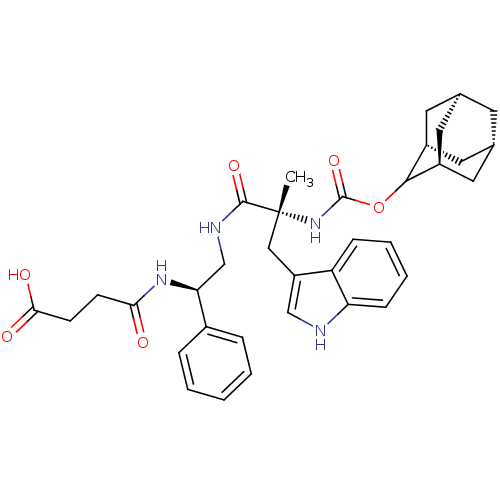
(CHEMBL2062154 | PD-134308)Show SMILES [H][C@@]12C[C@@]3([H])C[C@@]([H])(C1)C(OC(=O)N[C@](C)(Cc1c[nH]c4ccccc14)C(=O)NC[C@H](NC(=O)CCC(O)=O)c1ccccc1)[C@@]([H])(C2)C3 |wU:30.41,14.15,45.49,3.3,wD:6.6,1.0,TLB:5:3:47:9.6.8,10:9:47:3.48.2,THB:5:6:47:3.48.2,10:9:1.47.8:3.5.48,2:3:9:1.47.8,2:1:9:3.5.48,(-14.99,-2.1,;-13.56,-2.66,;-14.77,-3.94,;-13.26,-3.53,;-13.35,-5.06,;-11.86,-4.09,;-10.83,-2.82,;-9.38,-3.33,;-12.24,-3.16,;-10.83,-1.28,;-9.29,-1.31,;-8.5,.01,;-9.25,1.36,;-6.96,-.01,;-6.19,1.3,;-5.42,-.02,;-7.44,2.2,;-7.28,3.74,;-8.44,4.76,;-7.81,6.18,;-6.29,6.03,;-5.14,7.07,;-3.69,6.6,;-3.34,5.08,;-4.49,4.06,;-5.94,4.52,;-4.66,1.42,;-3.99,2.8,;-3.79,.15,;-2.27,.27,;-1.4,-1.02,;-2.08,-2.4,;-1.22,-3.69,;.32,-3.58,;-1.9,-5.07,;-3.43,-5.16,;-4.1,-6.57,;-5.64,-6.67,;-3.25,-7.83,;.14,-.91,;.99,-2.2,;2.51,-2.08,;3.19,-.7,;2.32,.59,;.8,.47,;-12.23,-.7,;-12.2,.82,;-13.58,-1.18,;-13.27,-1.94,)| Show InChI InChI=1S/C35H42N4O6/c1-35(18-26-19-36-28-10-6-5-9-27(26)28,39-34(44)45-32-24-14-21-13-22(16-24)17-25(32)15-21)33(43)37-20-29(23-7-3-2-4-8-23)38-30(40)11-12-31(41)42/h2-10,19,21-22,24-25,29,32,36H,11-18,20H2,1H3,(H,37,43)(H,38,40)(H,39,44)(H,41,42)/t21-,22+,24-,25+,29-,32?,35+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against rat brain Cholecystokinin type B receptor expressed in CHO cells. |
J Med Chem 40: 3947-56 (1998)
Article DOI: 10.1021/jm970439a
BindingDB Entry DOI: 10.7270/Q27H1K8Z |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50002477

((S)-3-{(S)-2-[(S)-2-tert-Butoxycarbonylamino-3-(1H...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C34H44N6O8S/c1-34(2,3)48-33(47)40-26(17-21-19-36-23-13-9-8-12-22(21)23)31(45)37-24(14-15-49-4)30(44)39-27(18-28(41)42)32(46)38-25(29(35)43)16-20-10-6-5-7-11-20/h5-13,19,24-27,36H,14-18H2,1-4H3,(H2,35,43)(H,37,45)(H,38,46)(H,39,44)(H,40,47)(H,41,42)/t24-,25-,26-,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against Cholecystokinin type B receptor expressed in CHO cells on the rat brain. |
J Med Chem 43: 3614-23 (2000)
BindingDB Entry DOI: 10.7270/Q2RN373C |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50281669

(Boc-Trp-Phg-Asp-1Nal-NH2 | CHEMBL353862)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1cccc2ccccc12)C(N)=O)c1ccccc1 Show InChI InChI=1S/C41H44N6O8/c1-41(2,3)55-40(54)46-32(21-27-23-43-30-19-10-9-18-29(27)30)38(52)47-35(25-13-5-4-6-14-25)39(53)45-33(22-34(48)49)37(51)44-31(36(42)50)20-26-16-11-15-24-12-7-8-17-28(24)26/h4-19,23,31-33,35,43H,20-22H2,1-3H3,(H2,42,50)(H,44,51)(H,45,53)(H,46,54)(H,47,52)(H,48,49)/t31-,32-,33-,35-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for the inhibition of [3H]-pCCK-8 binding to Merk CCK-B antagonist L365,260 receptor in rat brain membranes |
Bioorg Med Chem Lett 3: 847-850 (1993)
Article DOI: 10.1016/S0960-894X(00)80678-2
BindingDB Entry DOI: 10.7270/Q2P26Z28 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM81962

(S-L-365,260)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(C)c2)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C24H22N4O2/c1-16-9-8-12-18(15-16)25-24(30)27-22-23(29)28(2)20-14-7-6-13-19(20)21(26-22)17-10-4-3-5-11-17/h3-15,22H,1-2H3,(H2,25,27,30)/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Affinity of compound on binding of [3H]pCCK-8 to the cholecystokinin type B receptor in rat brain membrane |
J Med Chem 36: 166-72 (1993)
BindingDB Entry DOI: 10.7270/Q26M37FJ |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50061276
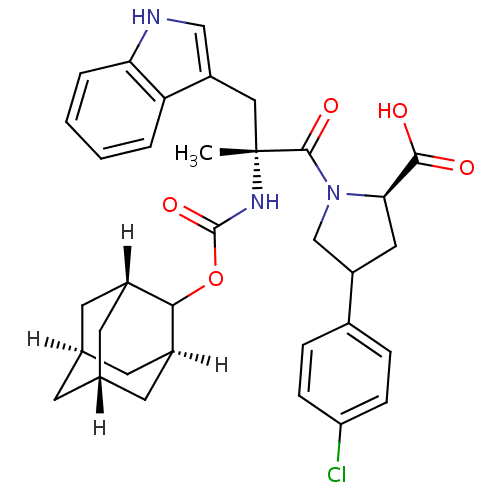
((R)-1-[(R)-2-(Adamantan-2-yloxycarbonylamino)-3-(1...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N1CC(C[C@@H]1C(O)=O)c1ccc(Cl)cc1 |TLB:15:16:22:19.24.20,THB:18:17:22:19.24.20,18:19:22:16.17.25| Show InChI InChI=1S/C34H38ClN3O5/c1-34(16-25-17-36-28-5-3-2-4-27(25)28,37-33(42)43-30-22-11-19-10-20(13-22)14-23(30)12-19)32(41)38-18-24(15-29(38)31(39)40)21-6-8-26(35)9-7-21/h2-9,17,19-20,22-24,29-30,36H,10-16,18H2,1H3,(H,37,42)(H,39,40)/t19-,20+,22-,23+,24?,29-,30?,34-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Compounds were tested for binding affinity against rat brain Cholecystokinin type B receptor expressed in CHO cells. |
J Med Chem 40: 3947-56 (1998)
Article DOI: 10.1021/jm970439a
BindingDB Entry DOI: 10.7270/Q27H1K8Z |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50070414
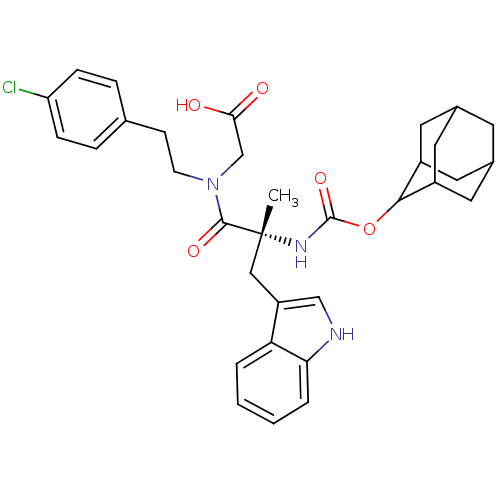
(CHEMBL286247 | {[(R)-2-(Adamantan-2-yloxycarbonyla...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N(CCc1ccc(Cl)cc1)CC(O)=O |wU:1.13,wD:1.0,TLB:15:16:19.18.25:21.23.22,THB:23:24:18:21.22.20,23:21:18:16.24.25,20:19:16:21.23.22,20:21:16:19.18.25,15:16:18:21.22.20,(7.91,-7.33,;7.94,-8.88,;7.94,-10.43,;7.17,-11.75,;5.62,-11.9,;5.27,-13.39,;6.62,-14.19,;6.91,-15.68,;8.36,-16.2,;9.52,-15.17,;9.23,-13.68,;7.78,-13.17,;6.59,-8.13,;5.23,-8.91,;5.27,-10.46,;3.91,-8.17,;2.56,-8.97,;1.07,-8.54,;-.34,-9.1,;-1.38,-7.84,;-1.38,-6.3,;.01,-5.72,;1.07,-6.94,;1.37,-6.2,;1.37,-7.69,;.04,-8.17,;9.27,-8.07,;9.23,-6.52,;10.81,-8.07,;11.55,-6.74,;13.09,-6.72,;13.84,-5.38,;13.06,-4.07,;13.8,-2.75,;15.35,-2.72,;16.1,-1.4,;16.12,-4.04,;15.36,-5.38,;11.55,-9.42,;13.1,-9.39,;13.9,-10.72,;13.87,-8.04,)| Show InChI InChI=1S/C33H38ClN3O5/c1-33(17-25-18-35-28-5-3-2-4-27(25)28,31(40)37(19-29(38)39)11-10-20-6-8-26(34)9-7-20)36-32(41)42-30-23-13-21-12-22(15-23)16-24(30)14-21/h2-9,18,21-24,30,35H,10-17,19H2,1H3,(H,36,41)(H,38,39)/t21?,22?,23?,24?,30?,33-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Capacity to inhibit [3H]-p CCK 8 binding to membrane preparations of CHO cells transfected with the rat CCK-B receptor |
Bioorg Med Chem Lett 8: 1419-24 (1999)
BindingDB Entry DOI: 10.7270/Q2NZ86S0 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50070420
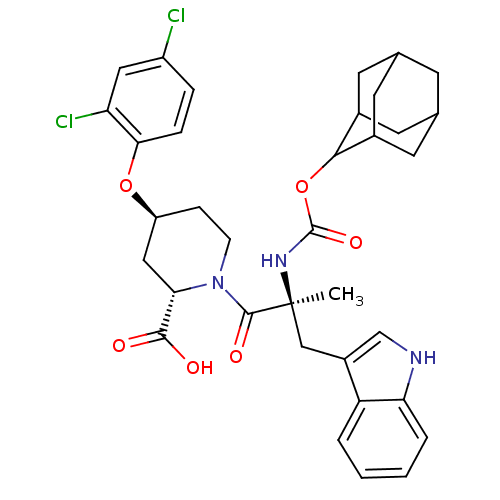
((2S,4S)-1-[(S)-2-(Adamantan-2-yloxycarbonylamino)-...)Show SMILES C[C@@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N1CC[C@@H](C[C@H]1C(O)=O)Oc1ccc(Cl)cc1Cl |wU:33.39,1.0,wD:1.13,31.42,TLB:22:17:25:21.23.20,22:21:16.17.18:25,THB:20:19:16:21.22.23,20:21:16:19.18.25,15:16:19.18.25:21.22.23,15:16:25:21.23.20,(10.79,-4.05,;10.79,-5.59,;10.82,-7.13,;10.02,-8.45,;8.48,-8.58,;8.16,-10.09,;9.48,-10.86,;9.76,-12.36,;11.21,-12.88,;12.36,-11.85,;12.07,-10.37,;10.63,-9.86,;9.44,-4.82,;8.13,-5.63,;8.16,-7.17,;6.78,-4.89,;5.47,-5.69,;3.96,-5.24,;3.96,-3.67,;2.93,-2.42,;1.52,-3.03,;1.55,-4.53,;2.55,-5.82,;2.93,-4.89,;4.25,-4.41,;4.28,-2.93,;12.11,-4.79,;12.07,-3.25,;13.65,-4.79,;14.42,-3.48,;15.96,-3.48,;16.73,-4.79,;15.96,-6.14,;14.42,-6.14,;14.42,-7.68,;15.73,-8.45,;13.07,-8.42,;18.27,-4.82,;19.81,-4.82,;20.58,-6.14,;22.08,-6.14,;22.85,-4.82,;24.39,-4.82,;22.08,-3.48,;20.54,-3.48,;19.77,-2.16,)| Show InChI InChI=1S/C35H39Cl2N3O6/c1-35(17-23-18-38-28-5-3-2-4-26(23)28,39-34(44)46-31-21-11-19-10-20(13-21)14-22(31)12-19)33(43)40-9-8-25(16-29(40)32(41)42)45-30-7-6-24(36)15-27(30)37/h2-7,15,18-22,25,29,31,38H,8-14,16-17H2,1H3,(H,39,44)(H,41,42)/t19?,20?,21?,22?,25-,29-,31?,35-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Capacity to inhibit [3H]-p CCK 8 binding to membrane preparations of CHO cells transfected with the rat CCK-B receptor |
Bioorg Med Chem Lett 8: 1419-24 (1999)
BindingDB Entry DOI: 10.7270/Q2NZ86S0 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM81962

(S-L-365,260)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(C)c2)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C24H22N4O2/c1-16-9-8-12-18(15-16)25-24(30)27-22-23(29)28(2)20-14-7-6-13-19(20)21(26-22)17-10-4-3-5-11-17/h3-15,22H,1-2H3,(H2,25,27,30)/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against rat brain Cholecystokinin type B receptor expressed in CHO cells. |
J Med Chem 40: 3947-56 (1998)
Article DOI: 10.1021/jm970439a
BindingDB Entry DOI: 10.7270/Q27H1K8Z |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50061270
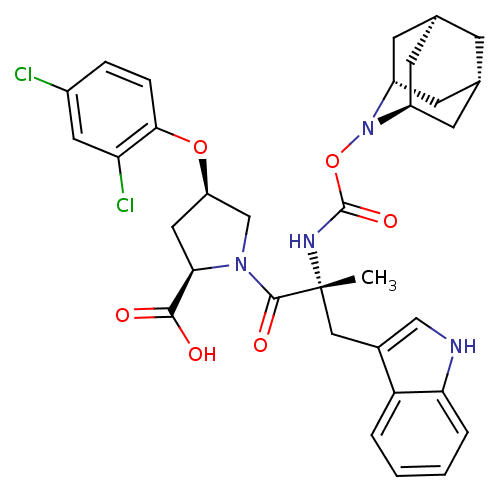
((2R,4R)-1-[(R)-2-(2-Aza-tricyclo[3.3.1.1*3,7*]dec-...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)ON1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N1C[C@@H](C[C@@H]1C(O)=O)Oc1ccc(Cl)cc1Cl |TLB:24:23:25:19.18.20,15:16:18:21.25.20,THB:22:23:18:21.25.20,22:21:18:16.23.24,15:16:25:19.18.20| Show InChI InChI=1S/C33H36Cl2N4O6/c1-33(15-20-16-36-27-5-3-2-4-25(20)27,37-32(43)45-39-22-9-18-8-19(11-22)12-23(39)10-18)31(42)38-17-24(14-28(38)30(40)41)44-29-7-6-21(34)13-26(29)35/h2-7,13,16,18-19,22-24,28,36H,8-12,14-15,17H2,1H3,(H,37,43)(H,40,41)/t18-,19+,22-,23+,24-,28-,33-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against rat brain Cholecystokinin type B receptor expressed in CHO cells. |
J Med Chem 40: 3947-56 (1998)
Article DOI: 10.1021/jm970439a
BindingDB Entry DOI: 10.7270/Q27H1K8Z |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50070412
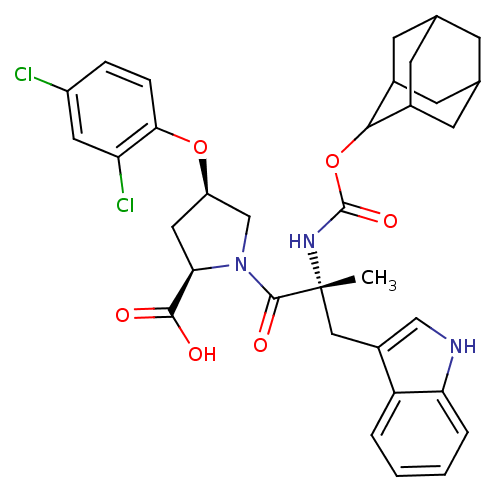
((2R,4R)-1-[(R)-2-(Adamantan-2-yloxycarbonylamino)-...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N1C[C@@H](C[C@@H]1C(O)=O)Oc1ccc(Cl)cc1Cl |wU:1.13,wD:32.38,30.41,1.0,TLB:22:17:25:21.23.20,22:21:16.17.18:25,THB:20:19:16:21.22.23,20:21:16:19.18.25,15:16:19.18.25:21.22.23,15:16:25:21.23.20,(22.06,-1.42,;22.08,-2.96,;22.09,-4.51,;21.3,-5.84,;19.77,-5.96,;19.42,-7.48,;20.76,-8.25,;21.05,-9.76,;22.49,-10.27,;23.65,-9.25,;23.36,-7.76,;21.92,-7.25,;20.72,-2.2,;19.38,-3,;19.42,-4.55,;18.05,-2.26,;16.71,-3.06,;15.22,-2.62,;15.23,-1.04,;14.17,.19,;12.79,-.39,;12.79,-1.91,;13.82,-3.2,;14.2,-2.26,;15.52,-1.77,;15.52,-.29,;23.4,-2.17,;23.37,-.62,;24.94,-2.17,;25.84,-.91,;27.3,-1.38,;27.3,-2.91,;25.85,-3.39,;25.37,-4.87,;26.42,-6,;23.88,-5.19,;28.56,-.46,;29.96,-1.09,;30.13,-2.62,;31.54,-3.25,;32.79,-2.33,;34.19,-2.96,;32.61,-.78,;31.19,-.17,;31.03,1.35,)| Show InChI InChI=1S/C34H37Cl2N3O6/c1-34(15-22-16-37-27-5-3-2-4-25(22)27,38-33(43)45-30-20-9-18-8-19(11-20)12-21(30)10-18)32(42)39-17-24(14-28(39)31(40)41)44-29-7-6-23(35)13-26(29)36/h2-7,13,16,18-21,24,28,30,37H,8-12,14-15,17H2,1H3,(H,38,43)(H,40,41)/t18?,19?,20?,21?,24-,28-,30?,34-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Capacity to inhibit [3H]-p CCK 8 binding to membrane preparations of CHO cells transfected with the rat CCK-B receptor |
Bioorg Med Chem Lett 8: 1419-24 (1999)
BindingDB Entry DOI: 10.7270/Q2NZ86S0 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50006878

((R)-1-(1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2ccc(C)cc2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C24H22N4O2/c1-16-12-14-18(15-13-16)25-24(30)27-22-23(29)28(2)20-11-7-6-10-19(20)21(26-22)17-8-4-3-5-9-17/h3-15,22H,1-2H3,(H2,25,27,30)/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Capacity to inhibit [3H]-p CCK 8 binding to membrane preparations of CHO cells transfected with the rat CCK-B receptor |
Bioorg Med Chem Lett 8: 1419-24 (1999)
BindingDB Entry DOI: 10.7270/Q2NZ86S0 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50045796

(CHEMBL99939 | {[2-(Adamantan-2-yloxycarbonylamino)...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N(CCc1ccc(Cl)cc1)CC(O)=O |wU:23.24,wD:21.23,19.27,17.29,TLB:15:16:18:21.25.20,THB:24:19:16.23.22:25,22:23:18:21.25.20,22:21:16.23.24:18,(1.85,-13.1,;.54,-13.87,;-.79,-14.65,;-.79,-16.19,;.12,-17.43,;-.77,-18.69,;-2.24,-18.22,;-3.59,-18.99,;-4.92,-18.22,;-4.92,-16.68,;-3.59,-15.9,;-2.26,-16.67,;-.97,-13.47,;-2.3,-12.7,;-3.4,-13.77,;-2.7,-11.21,;-4.18,-10.81,;-5.51,-10.01,;-6.08,-8.59,;-7.72,-8.75,;-8.82,-9.8,;-8.28,-11.21,;-6.99,-11.85,;-5.93,-10.81,;-6.49,-9.52,;-6.67,-11.11,;1.87,-14.64,;1.85,-16.18,;3.41,-14.64,;4.18,-13.3,;5.72,-13.3,;6.49,-11.95,;8.03,-11.97,;8.8,-10.64,;8.01,-9.29,;8.77,-7.96,;6.47,-9.31,;5.7,-10.64,;4.18,-15.97,;5.72,-15.97,;6.49,-17.3,;6.49,-14.63,)| Show InChI InChI=1S/C33H38ClN3O5/c1-33(17-25-18-35-28-5-3-2-4-27(25)28,31(40)37(19-29(38)39)11-10-20-6-8-26(34)9-7-20)36-32(41)42-30-23-13-21-12-22(15-23)16-24(30)14-21/h2-9,18,21-24,30,35H,10-17,19H2,1H3,(H,36,41)(H,38,39)/t21-,22+,23-,24+,30?,33? | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for binding affinity towards CCK-B in rat cortex by displacement of [3H]-pBC264 radioligand |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50056449
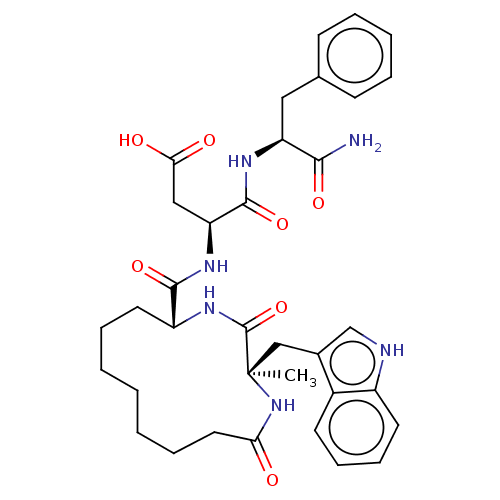
(CHEMBL2371222 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{...)Show SMILES C[C@]1(Cc2c[nH]c3ccccc23)NC(=O)CCCCCCC[C@@H](NC1=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C35H44N6O7/c1-35(20-23-21-37-25-15-11-10-14-24(23)25)34(48)40-26(16-8-3-2-4-9-17-29(42)41-35)32(46)39-28(19-30(43)44)33(47)38-27(31(36)45)18-22-12-6-5-7-13-22/h5-7,10-15,21,26-28,37H,2-4,8-9,16-20H2,1H3,(H2,36,45)(H,38,47)(H,39,46)(H,40,48)(H,41,42)(H,43,44)/t26-,27+,28+,35-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Affinity to inhibit [3H]pCCK-8 specific binding on rat brain Cholecystokinin type B receptor expressed in CHO cells |
J Med Chem 40: 647-58 (1997)
Article DOI: 10.1021/jm9603072
BindingDB Entry DOI: 10.7270/Q2PG1SDX |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50092401
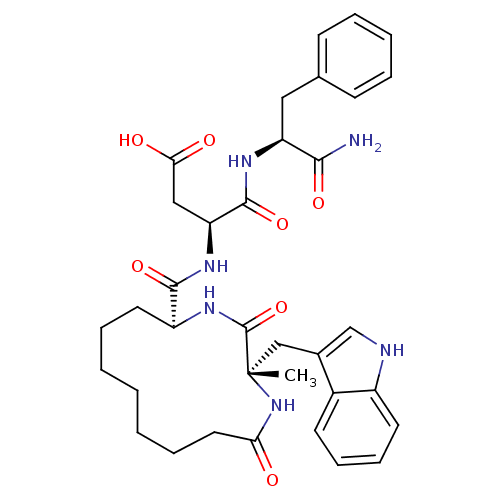
(CHEMBL177799 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{[...)Show SMILES C[C@@]1(Cc2c[nH]c3ccccc23)NC(=O)CCCCCCC[C@H](NC1=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C35H44N6O7/c1-35(20-23-21-37-25-15-11-10-14-24(23)25)34(48)40-26(16-8-3-2-4-9-17-29(42)41-35)32(46)39-28(19-30(43)44)33(47)38-27(31(36)45)18-22-12-6-5-7-13-22/h5-7,10-15,21,26-28,37H,2-4,8-9,16-20H2,1H3,(H2,36,45)(H,38,47)(H,39,46)(H,40,48)(H,41,42)(H,43,44)/t26-,27-,28-,35-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against Cholecystokinin type B receptor expressed in CHO cells on the rat brain. |
J Med Chem 43: 3614-23 (2000)
BindingDB Entry DOI: 10.7270/Q2RN373C |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50061220

(1-((S)-1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)Nc2cccc(C)c2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C24H22N4O2/c1-16-9-8-12-18(15-16)25-24(30)27-22-23(29)28(2)20-14-7-6-13-19(20)21(26-22)17-10-4-3-5-11-17/h3-15,22H,1-2H3,(H2,25,27,30)/t22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 269: 725-31 (1994)
BindingDB Entry DOI: 10.7270/Q2WD3Z2F |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50070415

((2R,4R)-1-[(R)-2-(Adamantan-2-yloxycarbonylamino)-...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N1C[C@@H](C[C@@H]1C(O)=O)Oc1ccc(F)cc1F |wU:1.13,wD:32.38,30.41,1.0,TLB:22:17:25:21.23.20,22:21:16.17.18:25,THB:20:19:16:21.22.23,20:21:16:19.18.25,15:16:19.18.25:21.22.23,15:16:25:21.23.20,(18.68,-9.94,;18.7,-11.49,;18.71,-13.03,;17.93,-14.36,;16.39,-14.49,;16.04,-16,;17.38,-16.78,;17.67,-18.29,;19.12,-18.79,;20.27,-17.77,;19.98,-16.28,;18.54,-15.78,;17.35,-10.73,;16,-11.53,;16.04,-13.08,;14.67,-10.79,;13.33,-11.59,;11.84,-11.15,;11.85,-9.57,;10.79,-8.33,;9.41,-8.91,;9.41,-10.44,;10.44,-11.72,;10.82,-10.79,;12.14,-10.3,;12.14,-8.82,;20.02,-10.7,;20,-9.15,;21.56,-10.7,;22.46,-9.44,;23.92,-9.91,;23.92,-11.44,;22.47,-11.92,;21.99,-13.4,;23.04,-14.52,;20.5,-13.72,;25.18,-8.99,;26.58,-9.62,;26.75,-11.15,;28.16,-11.78,;29.41,-10.86,;30.81,-11.49,;29.23,-9.31,;27.82,-8.7,;27.65,-7.18,)| Show InChI InChI=1S/C34H37F2N3O6/c1-34(15-22-16-37-27-5-3-2-4-25(22)27,38-33(43)45-30-20-9-18-8-19(11-20)12-21(30)10-18)32(42)39-17-24(14-28(39)31(40)41)44-29-7-6-23(35)13-26(29)36/h2-7,13,16,18-21,24,28,30,37H,8-12,14-15,17H2,1H3,(H,38,43)(H,40,41)/t18?,19?,20?,21?,24-,28-,30?,34-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity against CCK-B receptor |
Bioorg Med Chem Lett 8: 1419-24 (1999)
BindingDB Entry DOI: 10.7270/Q2NZ86S0 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50061267
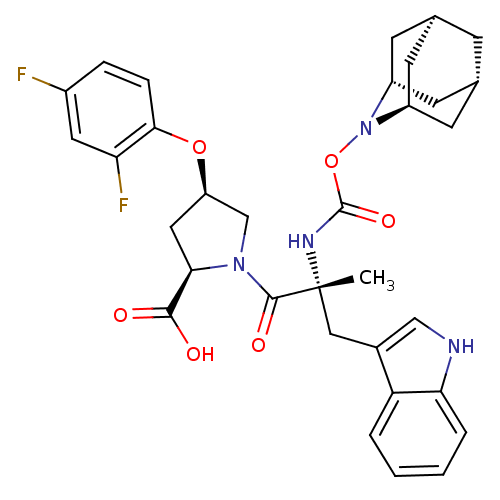
((2R,4R)-1-[(R)-2-(2-Aza-tricyclo[3.3.1.1*3,7*]dec-...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)ON1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N1C[C@@H](C[C@@H]1C(O)=O)Oc1ccc(F)cc1F |TLB:24:23:25:19.18.20,15:16:18:21.25.20,THB:22:23:18:21.25.20,22:21:18:16.23.24,15:16:25:19.18.20| Show InChI InChI=1S/C33H36F2N4O6/c1-33(15-20-16-36-27-5-3-2-4-25(20)27,37-32(43)45-39-22-9-18-8-19(11-22)12-23(39)10-18)31(42)38-17-24(14-28(38)30(40)41)44-29-7-6-21(34)13-26(29)35/h2-7,13,16,18-19,22-24,28,36H,8-12,14-15,17H2,1H3,(H,37,43)(H,40,41)/t18-,19+,22-,23+,24-,28-,33-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against rat brain Cholecystokinin type B receptor expressed in CHO cells. |
J Med Chem 40: 3947-56 (1998)
Article DOI: 10.1021/jm970439a
BindingDB Entry DOI: 10.7270/Q27H1K8Z |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50061273

((2R,4R)-1-[(R)-2-(2-Aza-tricyclo[3.3.1.1*3,7*]dec-...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)ON1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N1C[C@@H](C[C@@H]1C(O)=O)Oc1ccc(F)cc1 |TLB:25:17:24:21.22.20,15:16:22:19.24.20,THB:18:17:22:19.24.20,18:19:22:16.17.25,15:16:24:21.22.20| Show InChI InChI=1S/C33H37FN4O6/c1-33(16-21-17-35-28-5-3-2-4-27(21)28,36-32(42)44-38-23-11-19-10-20(13-23)14-24(38)12-19)31(41)37-18-26(15-29(37)30(39)40)43-25-8-6-22(34)7-9-25/h2-9,17,19-20,23-24,26,29,35H,10-16,18H2,1H3,(H,36,42)(H,39,40)/t19-,20+,23-,24+,26-,29-,33-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against rat brain Cholecystokinin type B receptor expressed in CHO cells. |
J Med Chem 40: 3947-56 (1998)
Article DOI: 10.1021/jm970439a
BindingDB Entry DOI: 10.7270/Q27H1K8Z |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50045796

(CHEMBL99939 | {[2-(Adamantan-2-yloxycarbonylamino)...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N(CCc1ccc(Cl)cc1)CC(O)=O |wU:23.24,wD:21.23,19.27,17.29,TLB:15:16:18:21.25.20,THB:24:19:16.23.22:25,22:23:18:21.25.20,22:21:16.23.24:18,(1.85,-13.1,;.54,-13.87,;-.79,-14.65,;-.79,-16.19,;.12,-17.43,;-.77,-18.69,;-2.24,-18.22,;-3.59,-18.99,;-4.92,-18.22,;-4.92,-16.68,;-3.59,-15.9,;-2.26,-16.67,;-.97,-13.47,;-2.3,-12.7,;-3.4,-13.77,;-2.7,-11.21,;-4.18,-10.81,;-5.51,-10.01,;-6.08,-8.59,;-7.72,-8.75,;-8.82,-9.8,;-8.28,-11.21,;-6.99,-11.85,;-5.93,-10.81,;-6.49,-9.52,;-6.67,-11.11,;1.87,-14.64,;1.85,-16.18,;3.41,-14.64,;4.18,-13.3,;5.72,-13.3,;6.49,-11.95,;8.03,-11.97,;8.8,-10.64,;8.01,-9.29,;8.77,-7.96,;6.47,-9.31,;5.7,-10.64,;4.18,-15.97,;5.72,-15.97,;6.49,-17.3,;6.49,-14.63,)| Show InChI InChI=1S/C33H38ClN3O5/c1-33(17-25-18-35-28-5-3-2-4-27(25)28,31(40)37(19-29(38)39)11-10-20-6-8-26(34)9-7-20)36-32(41)42-30-23-13-21-12-22(15-23)16-24(30)14-21/h2-9,18,21-24,30,35H,10-17,19H2,1H3,(H,36,41)(H,38,39)/t21-,22+,23-,24+,30?,33? | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for binding affinity towards cholecystokinin type B receptor in rat cortex by displacement of [3H]-pBC264 radioligand |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50213845
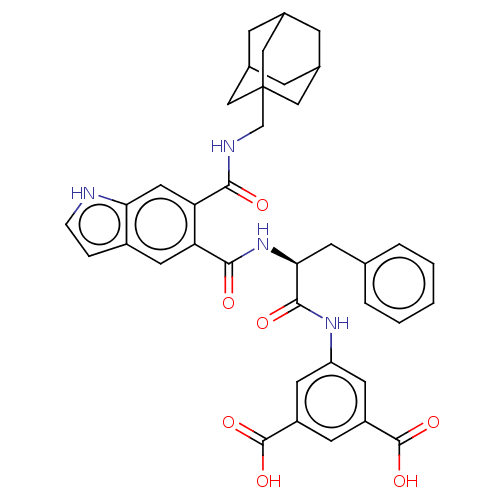
(CHEMBL14557)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2)NC(=O)c2cc3cc[nH]c3cc2C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |TLB:40:35:42:39.41.38,40:39:42:35.34.36,THB:38:37:34:39.41.40,38:39:34:37.42.36| Show InChI InChI=1S/C38H38N4O7/c43-33(40-20-38-17-22-8-23(18-38)10-24(9-22)19-38)30-16-31-25(6-7-39-31)15-29(30)34(44)42-32(11-21-4-2-1-3-5-21)35(45)41-28-13-26(36(46)47)12-27(14-28)37(48)49/h1-7,12-16,22-24,32,39H,8-11,17-20H2,(H,40,43)(H,41,45)(H,42,44)(H,46,47)(H,48,49)/t22?,23?,24?,32-,38?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compound was tested for binding affinity against Cholecystokinin type B receptor in rat cortical membranes using L-365260 as the radioligand. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q29W0HN3 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50056448

(CHEMBL2371221 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{...)Show SMILES C[C@]1(Cc2c[nH]c3ccccc23)NC(=O)CCCCCCCC[C@@H](NC1=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C36H46N6O7/c1-36(21-24-22-38-26-16-12-11-15-25(24)26)35(49)41-27(17-9-4-2-3-5-10-18-30(43)42-36)33(47)40-29(20-31(44)45)34(48)39-28(32(37)46)19-23-13-7-6-8-14-23/h6-8,11-16,22,27-29,38H,2-5,9-10,17-21H2,1H3,(H2,37,46)(H,39,48)(H,40,47)(H,41,49)(H,42,43)(H,44,45)/t27-,28+,29+,36-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Affinity to inhibit [3H]pCCK-8 specific binding on rat brain Cholecystokinin type B receptor expressed in CHO cells |
J Med Chem 40: 647-58 (1997)
Article DOI: 10.1021/jm9603072
BindingDB Entry DOI: 10.7270/Q2PG1SDX |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50070413
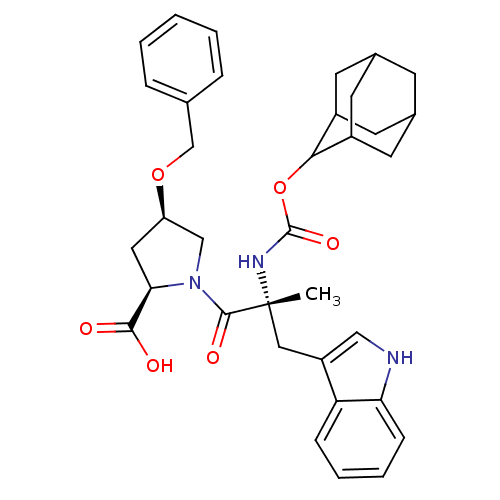
((2R,4R)-1-[(R)-2-(Adamantan-2-yloxycarbonylamino)-...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N1C[C@@H](C[C@@H]1C(O)=O)OCc1ccccc1 |wU:1.13,wD:32.38,30.41,1.0,TLB:25:24:22:19.18.20,15:16:19.18.25:21.23.22,THB:25:19:16.24.23:22,20:21:16:19.18.25,20:19:16:21.23.22,15:16:22:19.18.20,(18.68,-9.94,;18.7,-11.49,;18.71,-13.03,;17.93,-14.36,;16.39,-14.49,;16.04,-16,;17.38,-16.78,;17.67,-18.29,;19.12,-18.79,;20.27,-17.77,;19.98,-16.28,;18.54,-15.78,;17.35,-10.73,;16,-11.53,;16.04,-13.08,;14.67,-10.79,;13.33,-11.59,;12.14,-10.3,;10.82,-10.79,;9.41,-10.44,;9.41,-8.91,;10.79,-8.33,;12.14,-8.82,;11.85,-9.57,;11.84,-11.15,;10.44,-11.72,;20.02,-10.7,;20,-9.15,;21.56,-10.7,;22.46,-9.44,;23.92,-9.91,;23.92,-11.44,;22.47,-11.92,;21.99,-13.4,;23.04,-14.52,;20.5,-13.72,;25.18,-8.99,;26.58,-9.62,;27.82,-8.7,;27.65,-7.18,;28.9,-6.25,;30.31,-6.89,;30.49,-8.41,;29.23,-9.33,)| Show InChI InChI=1S/C35H41N3O6/c1-35(17-26-18-36-29-10-6-5-9-28(26)29,37-34(42)44-31-24-12-22-11-23(14-24)15-25(31)13-22)33(41)38-19-27(16-30(38)32(39)40)43-20-21-7-3-2-4-8-21/h2-10,18,22-25,27,30-31,36H,11-17,19-20H2,1H3,(H,37,42)(H,39,40)/t22?,23?,24?,25?,27-,30-,31?,35-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Capacity to inhibit [3H]-p CCK 8 binding to membrane preparations of CHO cells transfected with the rat CCK-B receptor |
Bioorg Med Chem Lett 8: 1419-24 (1999)
BindingDB Entry DOI: 10.7270/Q2NZ86S0 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50061271

((2R,4R)-1-[(R)-2-(2-Aza-tricyclo[3.3.1.1*3,7*]dec-...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)ON1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N1C[C@@H](C[C@@H]1C(O)=O)Oc1ccc(Cl)cc1 |TLB:25:17:24:21.22.20,15:16:22:19.24.20,THB:18:17:22:19.24.20,18:19:22:16.17.25,15:16:24:21.22.20| Show InChI InChI=1S/C33H37ClN4O6/c1-33(16-21-17-35-28-5-3-2-4-27(21)28,36-32(42)44-38-23-11-19-10-20(13-23)14-24(38)12-19)31(41)37-18-26(15-29(37)30(39)40)43-25-8-6-22(34)7-9-25/h2-9,17,19-20,23-24,26,29,35H,10-16,18H2,1H3,(H,36,42)(H,39,40)/t19-,20+,23-,24+,26-,29-,33-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Compound was tested for the affinity against Cholecystokinin type B receptor on guinea pig cortex. |
J Med Chem 40: 3947-56 (1998)
Article DOI: 10.1021/jm970439a
BindingDB Entry DOI: 10.7270/Q27H1K8Z |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50045805
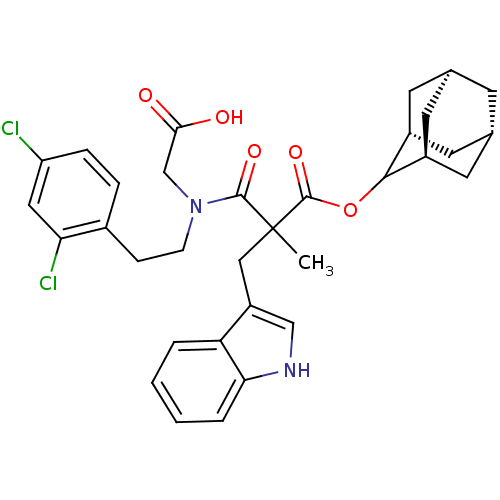
(CHEMBL318852 | N-Carboxymethyl-N-[2-(2,4-dichloro-...)Show SMILES CC(Cc1c[nH]c2ccccc12)(C(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N(CCc1ccc(Cl)cc1Cl)CC(O)=O |wU:20.27,18.19,22.23,wD:16.28,TLB:14:15:21:18.23.19,THB:17:16:21:18.23.19,17:18:21:15.16.24,(15.87,-6.38,;14.54,-7.15,;13.21,-7.93,;13.23,-9.47,;14.14,-10.71,;13.24,-11.97,;11.77,-11.5,;10.41,-12.27,;9.08,-11.5,;9.08,-9.96,;10.41,-9.18,;11.76,-9.95,;13.04,-6.75,;11.95,-7.82,;12.65,-5.26,;11.16,-4.86,;9.83,-4.06,;9.27,-2.64,;7.64,-2.8,;6.52,-3.85,;7.08,-5.26,;8.35,-5.9,;9.41,-4.86,;8.85,-3.57,;8.69,-5.16,;15.87,-7.92,;15.87,-9.46,;17.41,-7.92,;18.18,-6.58,;19.72,-6.58,;20.49,-5.23,;19.72,-3.92,;20.47,-2.59,;22.03,-2.57,;22.78,-1.24,;22.8,-3.92,;22.03,-5.25,;22.8,-6.58,;18.18,-9.25,;19.72,-9.25,;20.49,-10.58,;20.49,-7.91,)| Show InChI InChI=1S/C33H36Cl2N2O5/c1-33(16-24-17-36-28-5-3-2-4-26(24)28,32(41)42-30-22-11-19-10-20(13-22)14-23(30)12-19)31(40)37(18-29(38)39)9-8-21-6-7-25(34)15-27(21)35/h2-7,15,17,19-20,22-23,30,36H,8-14,16,18H2,1H3,(H,38,39)/t19-,20+,22-,23+,30?,33? | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for binding affinity towards cholecystokinin type B receptor in rat pancreas by displacement of [3H]-pCCK-8 radioligand |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50070419
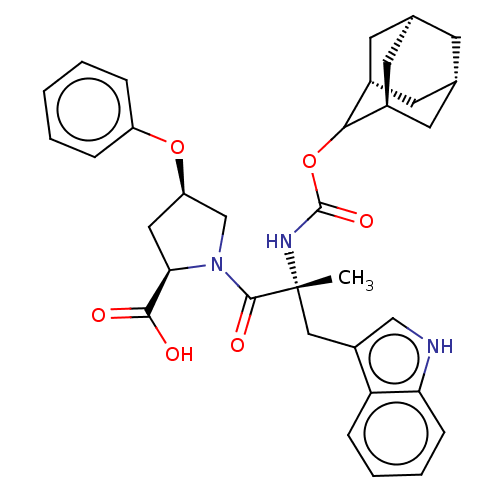
((2R,4R)-1-[(R)-2-(Adamantan-2-yloxycarbonylamino)-...)Show SMILES [H][C@@]12C[C@@]3([H])C[C@@]([H])(C1)C(OC(=O)N[C@](C)(Cc1c[nH]c4ccccc14)C(=O)N1C[C@@H](C[C@@H]1C(O)=O)Oc1ccccc1)[C@@]([H])(C2)C3 |wU:14.28,43.48,3.3,wD:32.36,30.39,6.6,1.0,TLB:5:3:45:9.6.8,10:9:45:3.46.2,THB:5:6:45:3.46.2,2:3:9:1.45.8,2:1:9:3.5.46,(5.34,-8.16,;6.78,-8.71,;5.58,-10,;7.08,-9.56,;6.98,-11.1,;8.47,-10.13,;9.49,-8.85,;10.92,-9.37,;8.08,-9.21,;9.5,-7.34,;10.82,-6.56,;12.14,-7.3,;12.56,-5.72,;12.17,-8.85,;13.69,-8.85,;13.69,-7.3,;13.69,-10.37,;12.82,-11.81,;13.47,-13.37,;12.21,-14.47,;10.78,-13.56,;9.18,-14.05,;7.95,-12.89,;8.34,-11.26,;9.95,-10.79,;11.17,-11.95,;15.23,-8.85,;15.98,-10.18,;15.98,-7.52,;15.37,-6.11,;16.5,-5.1,;17.84,-5.85,;17.52,-7.36,;18.53,-8.5,;20.04,-8.17,;18.07,-9.95,;16.34,-3.56,;17.6,-2.66,;17.43,-1.14,;18.65,-.24,;20.07,-.87,;20.23,-2.4,;18.99,-3.29,;8.11,-6.76,;8.14,-5.24,;6.76,-7.24,;7.07,-8,)| Show InChI InChI=1S/C34H39N3O6/c1-34(17-24-18-35-28-10-6-5-9-27(24)28,36-33(41)43-30-22-12-20-11-21(14-22)15-23(30)13-20)32(40)37-19-26(16-29(37)31(38)39)42-25-7-3-2-4-8-25/h2-10,18,20-23,26,29-30,35H,11-17,19H2,1H3,(H,36,41)(H,38,39)/t20-,21+,22-,23+,26-,29-,30?,34-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Capacity to inhibit [3H]-p CCK 8 binding to membrane preparations of CHO cells transfected with the rat CCK-B receptor |
Bioorg Med Chem Lett 8: 1419-24 (1999)
BindingDB Entry DOI: 10.7270/Q2NZ86S0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data