

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance-K receptor (Homo sapiens (Human)) | BDBM50071484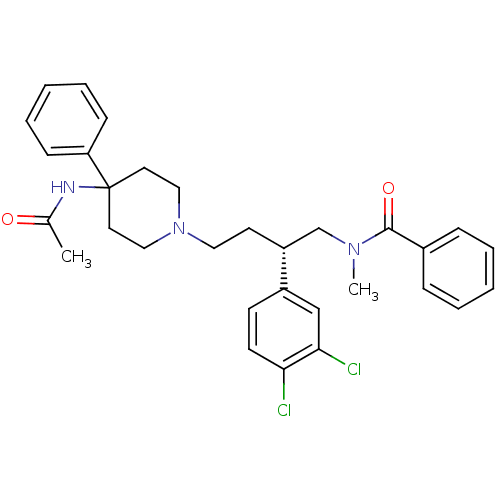 (CHEMBL308148 | N-[(R)-4-(4-Acetylamino-4-phenyl-pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of cloned human NK2 (Neurokinin 2) receptor, stably expressed in chinese hamster ovary (CHO) cells was determined | Bioorg Med Chem Lett 8: 2259-62 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97485 (US8470816, 458) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97489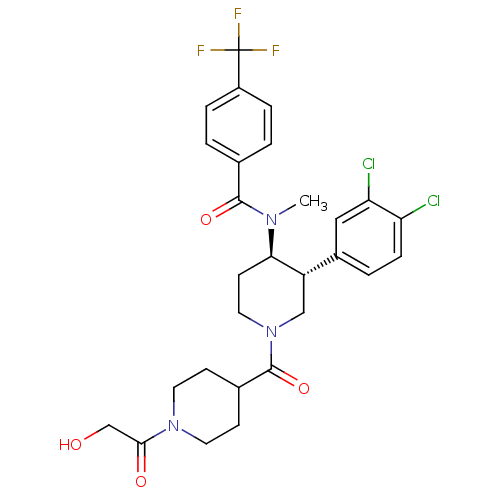 (US8470816, 632) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97486 (US8470816, 464) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97481 (US8470816, 265) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97490 (US8470816, 633) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97483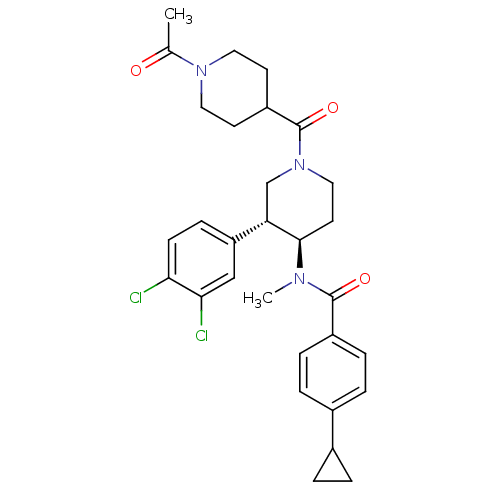 (US8470816, 292) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97479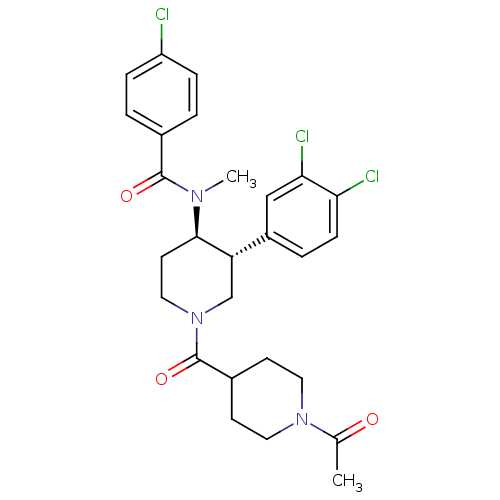 (US8470816, 251a) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97487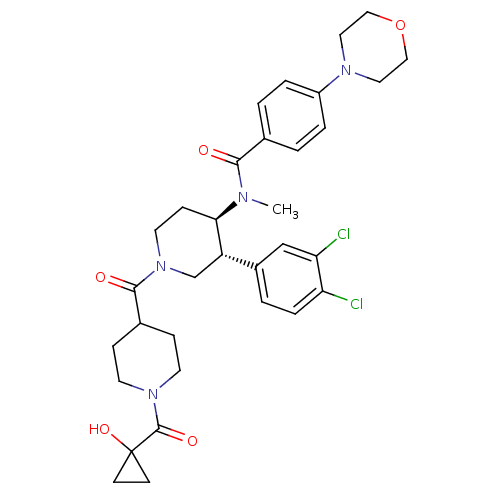 (US8470816, 623) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97491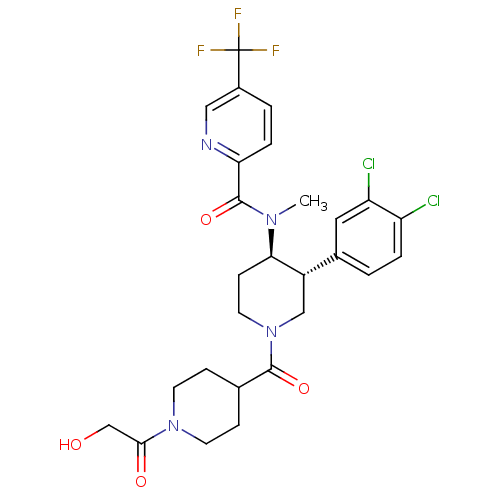 (US8470816, 635) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50170968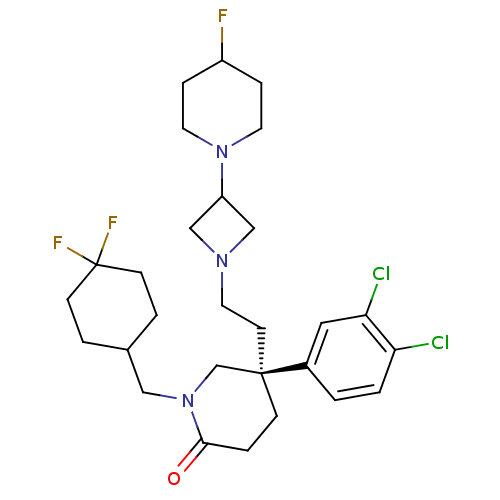 ((S)-5-(3,4-Dichloro-phenyl)-1-(4,4-difluoro-cycloh...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human Neurokinin 2 receptor | Bioorg Med Chem Lett 15: 3957-61 (2005) Article DOI: 10.1016/j.bmcl.2005.05.134 BindingDB Entry DOI: 10.7270/Q20G3JQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97482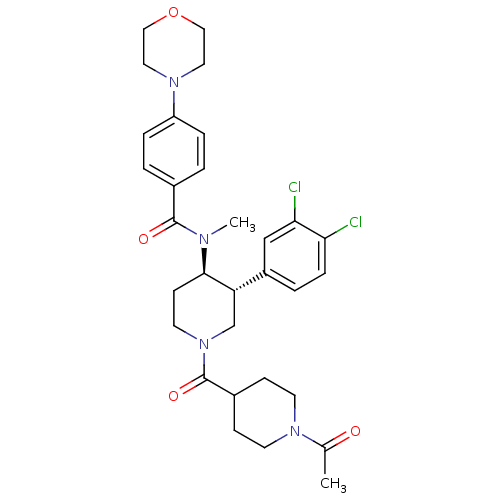 (US8470816, 268) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50071484 (CHEMBL308148 | N-[(R)-4-(4-Acetylamino-4-phenyl-pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against human NK2 receptors from HSKR-1 cells using [125I]-Iodohistidyl NKA | Bioorg Med Chem Lett 6: 951-956 (1996) Article DOI: 10.1016/0960-894X(96)00148-5 BindingDB Entry DOI: 10.7270/Q2GM8794 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97472 (US8470816, 18) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97488 (US8470816, 629) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97480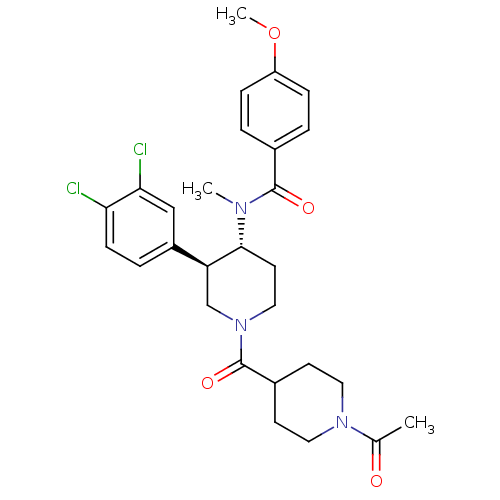 (US8470816, 252) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97478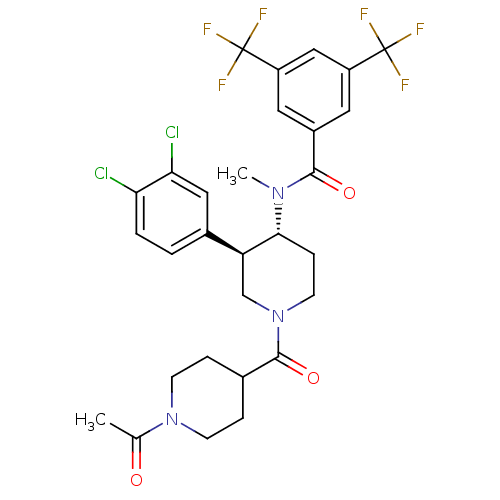 (US8470816, 91) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50409627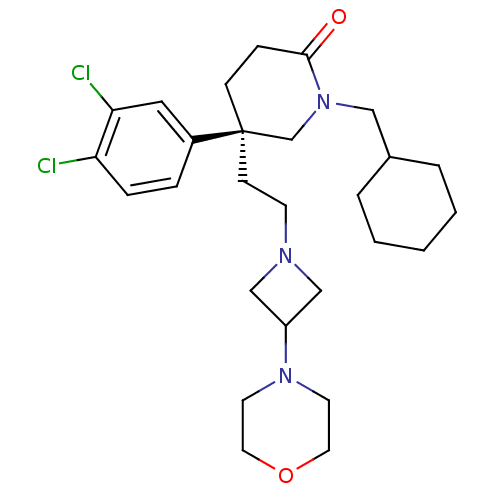 (CHEMBL185572) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human Neurokinin 2 receptor | Bioorg Med Chem Lett 15: 3957-61 (2005) Article DOI: 10.1016/j.bmcl.2005.05.134 BindingDB Entry DOI: 10.7270/Q20G3JQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50071484 (CHEMBL308148 | N-[(R)-4-(4-Acetylamino-4-phenyl-pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity was determined against human NK2 receptor in CHO cells using [125I]-neurokinin A as radioligand | Bioorg Med Chem Lett 6: 605-608 (1996) Article DOI: 10.1016/0960-894X(96)00075-3 BindingDB Entry DOI: 10.7270/Q2B27V80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97484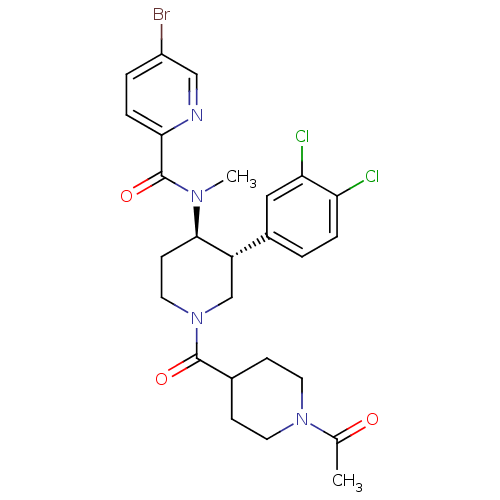 (US8470816, 318) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50410049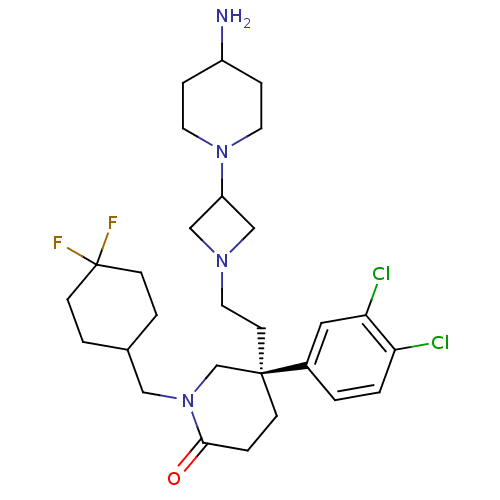 (CHEMBL434142) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human Neurokinin 2 receptor | Bioorg Med Chem Lett 15: 3957-61 (2005) Article DOI: 10.1016/j.bmcl.2005.05.134 BindingDB Entry DOI: 10.7270/Q20G3JQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97475 (US8470816, 30) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM392076 (US10301272, Example 7/9) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Displacement of [125I]NKA from human recombinant NK2 receptor after 60 mins by scintillation counting analysis | Bioorg Med Chem Lett 28: 1446-1455 (2018) Article DOI: 10.1016/j.bmcl.2018.03.093 BindingDB Entry DOI: 10.7270/Q27W6FPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50410051 (CHEMBL185182) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human Neurokinin 2 receptor | Bioorg Med Chem Lett 15: 3957-61 (2005) Article DOI: 10.1016/j.bmcl.2005.05.134 BindingDB Entry DOI: 10.7270/Q20G3JQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50410048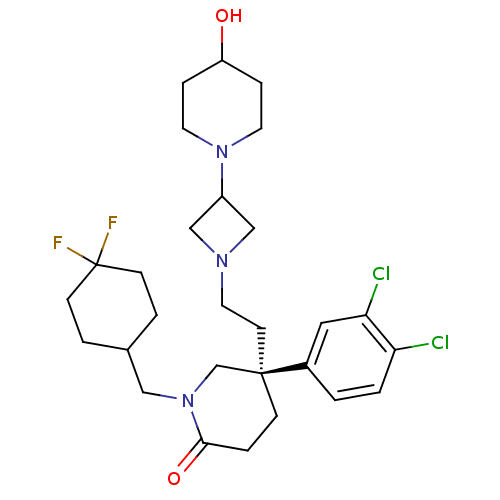 (CHEMBL434548) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human Neurokinin 2 receptor | Bioorg Med Chem Lett 15: 3957-61 (2005) Article DOI: 10.1016/j.bmcl.2005.05.134 BindingDB Entry DOI: 10.7270/Q20G3JQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97476 (US8470816, 31) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50001447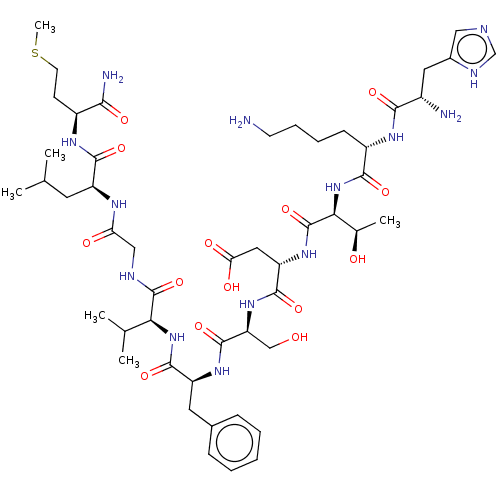 (CHEMBL217406 | His-Lys-Thr-Asp-Ser-Phe-Val-Gly-Leu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Creighton University School of Medicine Curated by ChEMBL | Assay Description Inhibitory activity against human Tachykinin receptor 2 | J Med Chem 42: 3004-7 (1999) Article DOI: 10.1021/jm9807151 BindingDB Entry DOI: 10.7270/Q2154HQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97474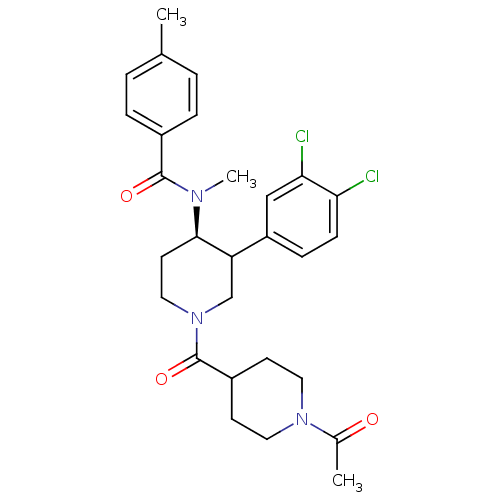 (US8470816, 24) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM106976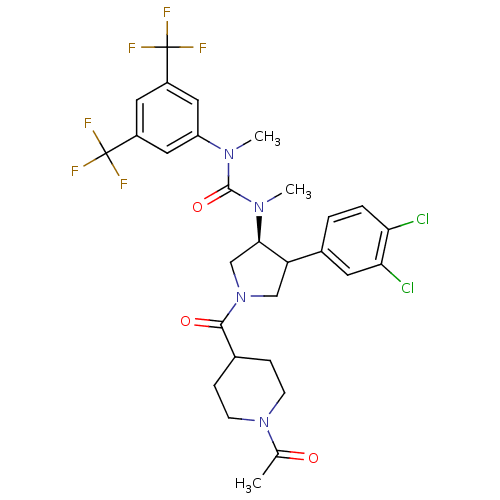 (US8592454, 66) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligand receptor binding inhibitory assay using human NK receptor. | US Patent US8592454 (2013) BindingDB Entry DOI: 10.7270/Q2XD10B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50070352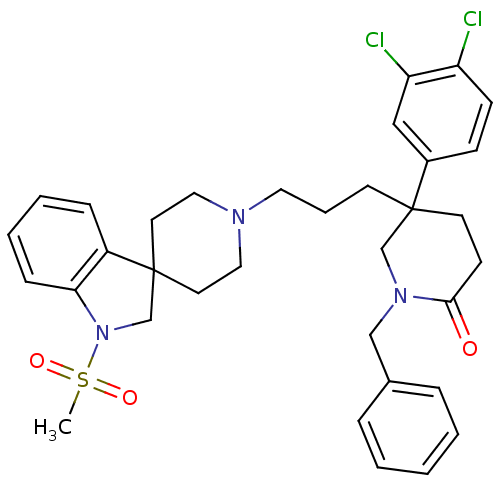 (1-benzyl-5-(3,4-dichlorophenyl)-5-{3-[1-methylsulf...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp Laboratory Curated by ChEMBL | Assay Description Compound was evaluated for affinity towards human Neurokinin NK2 receptor | Bioorg Med Chem Lett 8: 1343-8 (1999) BindingDB Entry DOI: 10.7270/Q2QV3N16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97477 (US8470816, 82) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50175505 (1-{2-[1-Benzoyl-3-((S)-3,4-dichloro-phenyl)-pyrrol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against tachykinin receptor 2 | J Med Chem 48: 6523-43 (2005) Article DOI: 10.1021/jm058225d BindingDB Entry DOI: 10.7270/Q2SF2WZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97473 (US8470816, 21) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM106981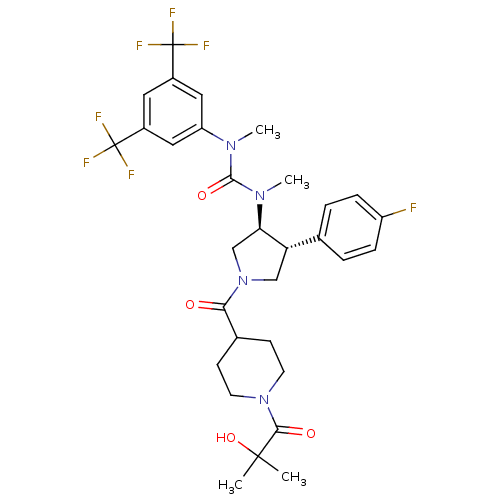 (US8592454, 308) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligand receptor binding inhibitory assay using human NK receptor. | US Patent US8592454 (2013) BindingDB Entry DOI: 10.7270/Q2XD10B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50410054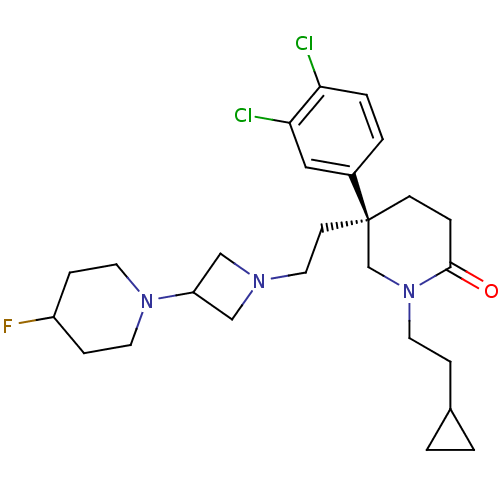 (CHEMBL185133) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human Neurokinin 2 receptor | Bioorg Med Chem Lett 15: 3957-61 (2005) Article DOI: 10.1016/j.bmcl.2005.05.134 BindingDB Entry DOI: 10.7270/Q20G3JQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50435131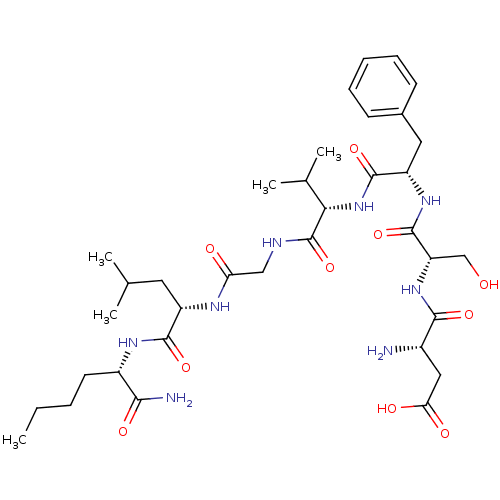 (CHEMBL2390989) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis Curated by ChEMBL | Assay Description Binding affinity to human NK2 receptor by radioligand displacement assay | Bioorg Med Chem 21: 2764-71 (2013) Article DOI: 10.1016/j.bmc.2013.03.016 BindingDB Entry DOI: 10.7270/Q2N87C5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50070358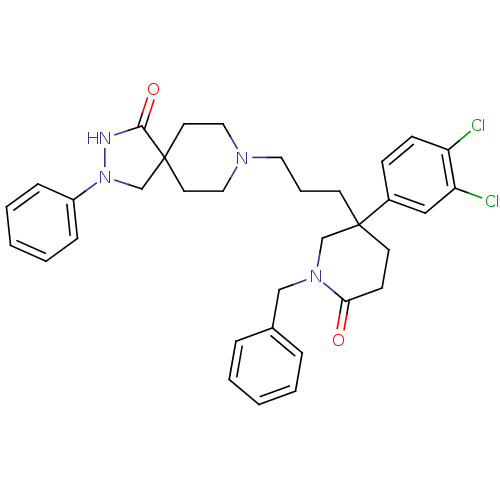 (8-{3-[1-Benzyl-3-(3,4-dichloro-phenyl)-6-oxo-piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp Laboratory Curated by ChEMBL | Assay Description Compound was evaluated for affinity towards human Neurokinin NK2 receptor | Bioorg Med Chem Lett 8: 1343-8 (1999) BindingDB Entry DOI: 10.7270/Q2QV3N16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50097911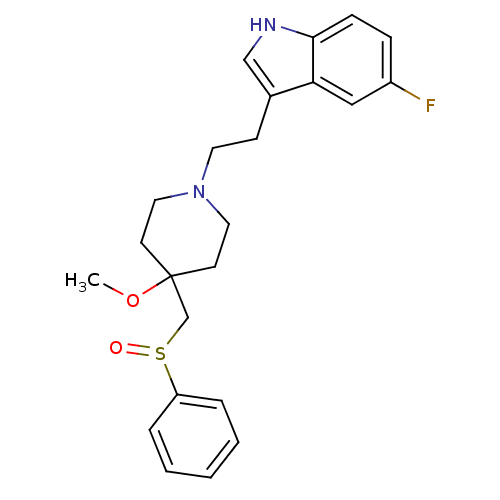 (3-[2-(4-Benzenesulfinylmethyl-4-methoxy-piperidin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds Curated by ChEMBL | Assay Description Binding affinity of the compound for heterologously expressed human Tachykinin receptor 2 using [3H]-SR- 48968 as radioligand | Bioorg Med Chem Lett 11: 819-22 (2001) BindingDB Entry DOI: 10.7270/Q2PV6JMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50410052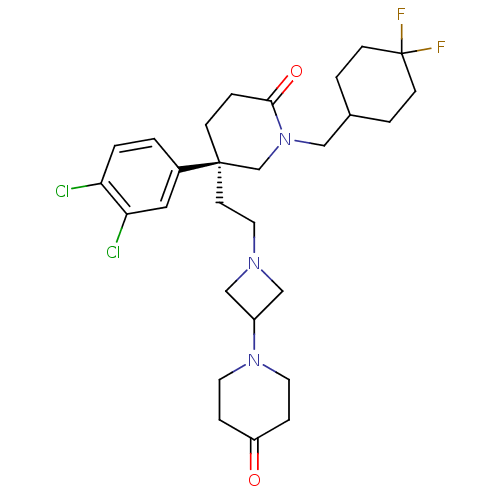 (CHEMBL367815) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human Neurokinin 2 receptor | Bioorg Med Chem Lett 15: 3957-61 (2005) Article DOI: 10.1016/j.bmcl.2005.05.134 BindingDB Entry DOI: 10.7270/Q20G3JQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50435131 (CHEMBL2390989) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [125I]NKA from human recombinant NK2 receptor expressed in CHO cells measured after 60 mins by scintillation counting method | Bioorg Med Chem 25: 471-482 (2017) Article DOI: 10.1016/j.bmc.2016.11.014 BindingDB Entry DOI: 10.7270/Q2CF9S3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50155253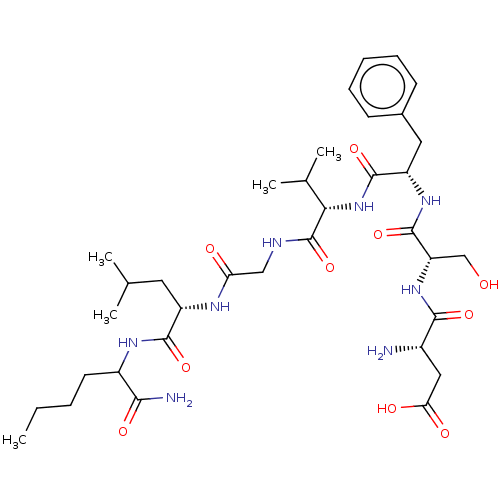 (CHEMBL3775108) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [125I]NKA from human recombinant NK2 receptor expressed in CHO cells | Bioorg Med Chem 24: 1793-810 (2016) Article DOI: 10.1016/j.bmc.2016.03.006 BindingDB Entry DOI: 10.7270/Q2J67JS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50233962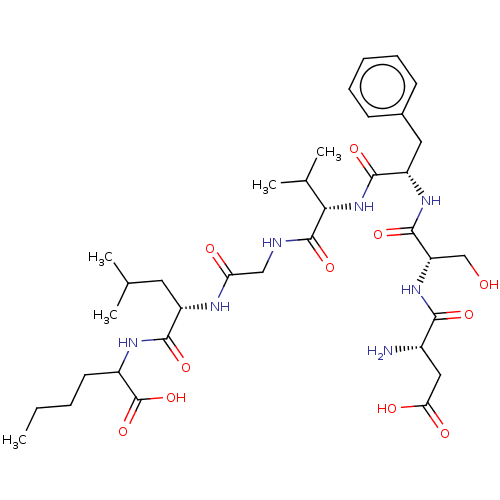 (CHEMBL4068783) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle Curated by ChEMBL | Assay Description Displacement of [125I]NKA from human recombinant tachykinin NK2 receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 60: 349-361 (2017) Article DOI: 10.1021/acs.jmedchem.6b01422 BindingDB Entry DOI: 10.7270/Q27M0B6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50070356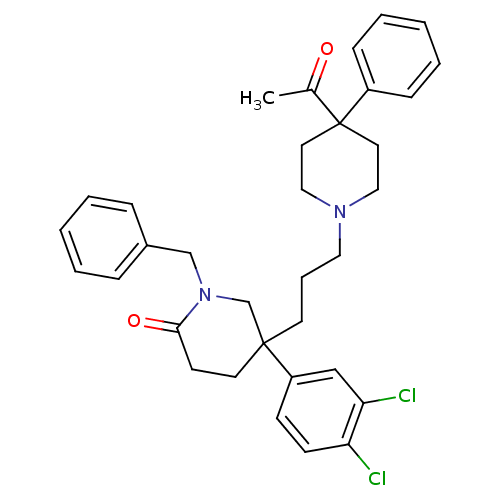 (5-[3-(4-Acetyl-4-phenyl-piperidin-1-yl)-propyl]-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp Laboratory Curated by ChEMBL | Assay Description Compound was evaluated for affinity towards human Neurokinin NK2 receptor | Bioorg Med Chem Lett 8: 1343-8 (1999) BindingDB Entry DOI: 10.7270/Q2QV3N16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50410047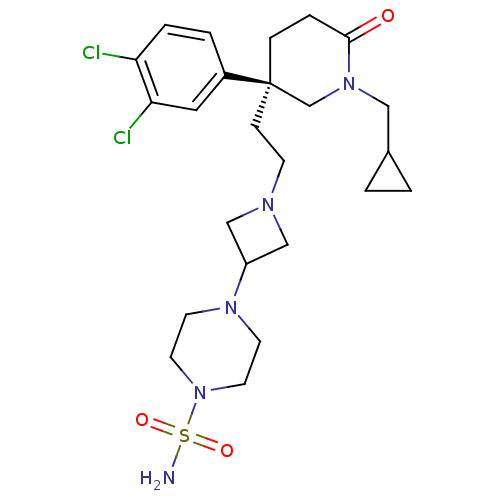 (CHEMBL365613 | UK-224671) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human Neurokinin 2 receptor | Bioorg Med Chem Lett 15: 3957-61 (2005) Article DOI: 10.1016/j.bmcl.2005.05.134 BindingDB Entry DOI: 10.7270/Q20G3JQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50289182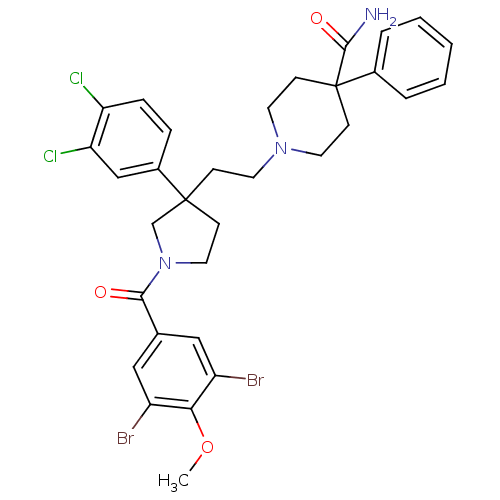 (1-{2-[1-(3,5-Dibromo-4-methoxy-benzoyl)-3-(3,4-dic...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against human NK2 receptors from HSKR-1 cells using [125I]-Iodohistidyl NKA | Bioorg Med Chem Lett 6: 951-956 (1996) Article DOI: 10.1016/0960-894X(96)00148-5 BindingDB Entry DOI: 10.7270/Q2GM8794 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50106892 (3-[4-(3,4-dichlorophenyl)-3-[3,5-di(trifluoromethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity for Tachykinin receptor 2 of human-CHO cells using 125 I-NKA radioligand | Bioorg Med Chem Lett 11: 3081-4 (2001) BindingDB Entry DOI: 10.7270/Q2V40THD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50106891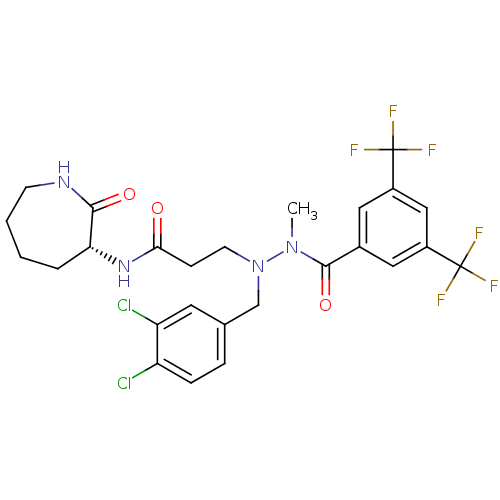 (3-[N'-(3,5-Bis-trifluoromethyl-benzoyl)-N-(3,4-dic...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity for Tachykinin receptor 2 of human-CHO cells using 125 I-NKA radioligand | Bioorg Med Chem Lett 11: 3081-4 (2001) BindingDB Entry DOI: 10.7270/Q2V40THD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50097912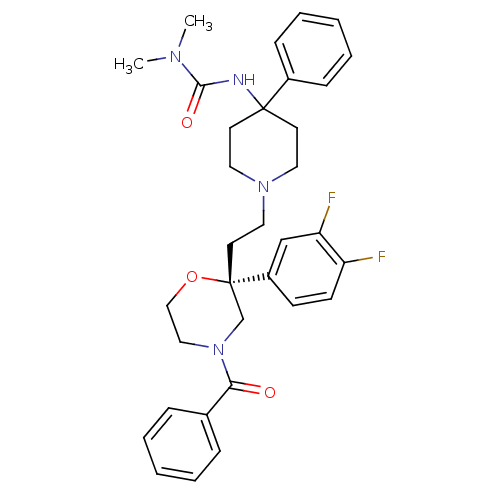 (3-(1-{2-[(R)-4-Benzoyl-2-(3,4-difluoro-phenyl)-mor...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds Curated by ChEMBL | Assay Description Binding affinity of the compound for heterologously expressed human Tachykinin receptor 2 using [3H]-SR- 48968 as radioligand | Bioorg Med Chem Lett 11: 819-22 (2001) BindingDB Entry DOI: 10.7270/Q2PV6JMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50129511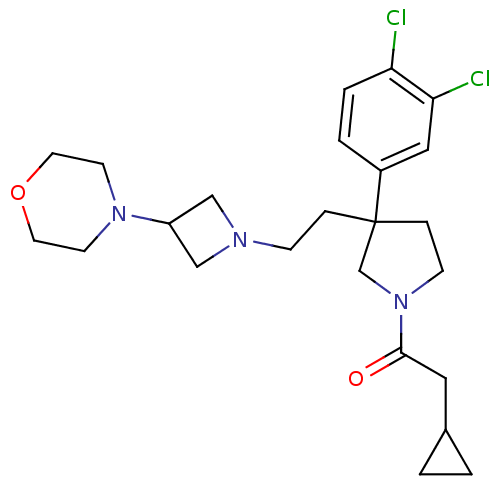 (2-Cyclopropyl-1-{3-(3,4-dichloro-phenyl)-3-[2-(3-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity for human tachykinin receptor 2 expressed in CHO cells using [125I]- NKA radioligand | Bioorg Med Chem Lett 13: 2211-5 (2003) BindingDB Entry DOI: 10.7270/Q2D799TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50129516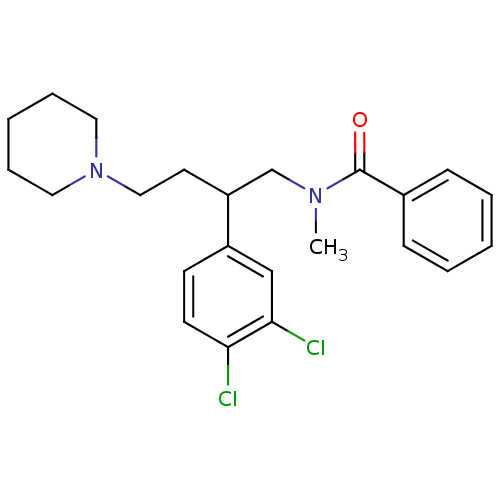 (CHEMBL71918 | N-[2-(3,4-Dichloro-phenyl)-4-piperid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity for human tachykinin receptor 2 expressed in CHO cells using [125I]- NKA radioligand | Bioorg Med Chem Lett 13: 2211-5 (2003) BindingDB Entry DOI: 10.7270/Q2D799TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 297 total ) | Next | Last >> |