 Found 67 hits of kd data for polymerid = 49000892,49000894,49000895
Found 67 hits of kd data for polymerid = 49000892,49000894,49000895 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50103475

(Pmp-Tyr-Ile-Thr-Asn-Cys-Pro-Orn-phe(I,N3)-NH2 | Pm...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(OC)cc1)NC(=O)CC1(CCCCC1)SCCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CS)C(=O)N1CCCC1C(=O)NC(CCCN)C(=O)NC(Cc1ccc(N=[N+]=[N-])c(I)c1)C(N)=O Show InChI InChI=1S/C57H84IN15O13S2/c1-5-31(2)47(69-52(81)40(26-33-13-16-35(86-4)17-14-33)64-46(77)29-57(20-7-6-8-21-57)88-24-19-44(60)75)54(83)70-48(32(3)74)55(84)67-41(28-45(61)76)51(80)68-42(30-87)56(85)73-23-10-12-43(73)53(82)65-38(11-9-22-59)50(79)66-39(49(62)78)27-34-15-18-37(71-72-63)36(58)25-34/h13-18,25,31-32,38-43,47-48,74,87H,5-12,19-24,26-30,59H2,1-4H3,(H2,60,75)(H2,61,76)(H2,62,78)(H,64,77)(H,65,82)(H,66,79)(H,67,84)(H,68,80)(H,69,81)(H,70,83)/t31-,32+,38?,39?,40-,41-,42-,43?,47-,48-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Dissociation constant value of the compound was determined for human OT-receptor |
J Med Chem 44: 3022-30 (2001)
BindingDB Entry DOI: 10.7270/Q29S1Q9F |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50103475

(Pmp-Tyr-Ile-Thr-Asn-Cys-Pro-Orn-phe(I,N3)-NH2 | Pm...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(OC)cc1)NC(=O)CC1(CCCCC1)SCCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CS)C(=O)N1CCCC1C(=O)NC(CCCN)C(=O)NC(Cc1ccc(N=[N+]=[N-])c(I)c1)C(N)=O Show InChI InChI=1S/C57H84IN15O13S2/c1-5-31(2)47(69-52(81)40(26-33-13-16-35(86-4)17-14-33)64-46(77)29-57(20-7-6-8-21-57)88-24-19-44(60)75)54(83)70-48(32(3)74)55(84)67-41(28-45(61)76)51(80)68-42(30-87)56(85)73-23-10-12-43(73)53(82)65-38(11-9-22-59)50(79)66-39(49(62)78)27-34-15-18-37(71-72-63)36(58)25-34/h13-18,25,31-32,38-43,47-48,74,87H,5-12,19-24,26-30,59H2,1-4H3,(H2,60,75)(H2,61,76)(H2,62,78)(H,64,77)(H,65,82)(H,66,79)(H,67,84)(H,68,80)(H,69,81)(H,70,83)/t31-,32+,38?,39?,40-,41-,42-,43?,47-,48-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Dissociation constant value of the compound was determined for human OT-receptor |
J Med Chem 44: 3022-30 (2001)
BindingDB Entry DOI: 10.7270/Q29S1Q9F |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50077135

(CHEMBL3416750)Show SMILES CCN1\C(=C/C=C/C=C/C2=[N+](CC)c3cc(ccc3C2(C)C)S([O-])(=O)=O)C(C)(CCCC(=O)NCCNC(=O)CCc2ccc(cc2)S(=O)(=O)N(CC(=O)N\N=C2/C(=O)Nc3ccccc23)c2ccc(Cl)cc2)c2cc(ccc12)S(O)(=O)=O |c:9| Show InChI InChI=1S/C59H63ClN8O12S3/c1-6-66-49-31-29-43(82(75,76)77)36-47(49)59(5,52(66)17-10-8-9-16-51-58(3,4)46-30-28-44(83(78,79)80)37-50(46)67(51)7-2)33-13-18-53(69)61-34-35-62-54(70)32-21-39-19-26-42(27-20-39)81(73,74)68(41-24-22-40(60)23-25-41)38-55(71)64-65-56-45-14-11-12-15-48(45)63-57(56)72/h8-12,14-17,19-20,22-31,36-37H,6-7,13,18,21,32-35,38H2,1-5H3,(H5-,61,62,63,64,65,69,70,71,72,75,76,77,78,79,80) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Binding affinity to SNAP-tagged oxytocin receptor (unknown origin) expressed in HEK293 cells by TR-FRET assay |
J Med Chem 58: 2547-52 (2015)
Article DOI: 10.1021/jm501395b
BindingDB Entry DOI: 10.7270/Q2TB18KT |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50077134
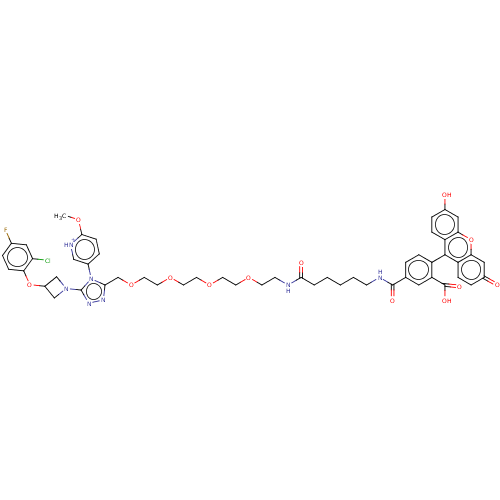
(CHEMBL3416751)Show SMILES [O-]C(=O)C(F)(F)F.COc1ccc(c[nH+]1)-n1c(COCCOCCOCCOCCNC(=O)CCCCCNC(=O)c2ccc(c(c2)C(O)=O)-c2c3ccc(O)cc3oc3cc(=O)ccc23)nnc1N1CC(C1)Oc1ccc(F)cc1Cl |(1.33,2,;1.33,.77,;.27,.15,;2.67,,;3.74,.62,;3.74,-.62,;2.67,-1.23,;6.78,11.83,;7.64,10.94,;9.13,11.31,;10.2,10.2,;11.69,10.57,;12.12,12.04,;11.06,13.16,;9.56,12.79,;13.58,12.53,;14.83,11.64,;14.83,10.1,;16.17,9.34,;16.17,7.79,;17.51,7.03,;17.51,5.49,;18.85,4.72,;18.86,3.18,;20.19,2.41,;20.2,.87,;21.53,.1,;21.54,-1.44,;22.87,-2.21,;22.88,-3.75,;24.21,-4.51,;24.22,-6.06,;23.15,-6.67,;25.55,-6.82,;25.56,-8.36,;26.89,-9.13,;26.9,-10.67,;28.23,-11.44,;28.24,-12.98,;29.57,-13.75,;30.64,-13.13,;29.58,-15.29,;28.25,-16.06,;28.25,-17.6,;29.58,-18.37,;30.91,-17.6,;30.91,-16.06,;32.25,-18.36,;32.25,-19.39,;33.32,-17.75,;29.58,-19.91,;28.24,-20.68,;26.91,-19.91,;25.58,-20.68,;25.58,-22.22,;24.51,-22.84,;26.91,-22.99,;28.24,-22.22,;29.58,-22.99,;30.89,-22.22,;32.24,-22.99,;33.58,-22.22,;34.64,-22.84,;33.58,-20.68,;32.24,-19.91,;30.89,-20.68,;16.08,12.53,;15.6,13.99,;14.06,13.99,;13.15,15.24,;11.69,15.55,;12.05,17.05,;13.55,16.69,;11.26,18.36,;12,19.71,;13.54,19.74,;14.28,21.09,;13.49,22.41,;14.08,23.49,;11.95,22.38,;11.2,21.03,;9.97,21.01,)| Show InChI InChI=1S/C53H55ClFN7O13/c1-69-49-15-8-35(29-58-49)62-47(59-60-53(62)61-30-38(31-61)74-44-14-7-34(55)26-43(44)54)32-73-24-23-72-22-21-71-20-19-70-18-17-56-48(65)5-3-2-4-16-57-51(66)33-6-11-39(42(25-33)52(67)68)50-40-12-9-36(63)27-45(40)75-46-28-37(64)10-13-41(46)50/h6-15,25-29,38,63H,2-5,16-24,30-32H2,1H3,(H,56,65)(H,57,66)(H,67,68)/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Binding affinity to SNAP-tagged oxytocin receptor (unknown origin) expressed in HEK293 cells by TR-FRET assay |
J Med Chem 58: 2547-52 (2015)
Article DOI: 10.1021/jm501395b
BindingDB Entry DOI: 10.7270/Q2TB18KT |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50077138

(CHEMBL3416753)Show SMILES [O-]C(=O)C(F)(F)F.CCN1\C(=C/C=C/C=C/C2=[N+](CC)c3cc(ccc3C2(C)C)S([O-])(=O)=O)C(C)(CCCC(=O)NCCOCCOCCOCCOCc2nnc(N3CC(C3)Oc3ccc(F)cc3Cl)n2-c2ccc(OC)[nH+]c2)c2cc(ccc12)S(O)(=O)=O |c:15| Show InChI InChI=1S/C58H70ClFN8O13S2/c1-7-66-48-21-19-43(82(70,71)72)34-46(48)58(5,52(66)14-11-9-10-13-51-57(3,4)45-20-18-44(83(73,74)75)35-49(45)67(51)8-2)24-12-15-54(69)61-25-26-77-27-28-78-29-30-79-31-32-80-39-53-63-64-56(68(53)41-17-23-55(76-6)62-36-41)65-37-42(38-65)81-50-22-16-40(60)33-47(50)59/h9-11,13-14,16-23,33-36,42H,7-8,12,15,24-32,37-39H2,1-6H3,(H2-,61,69,70,71,72,73,74,75)/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Binding affinity to SNAP-tagged oxytocin receptor (unknown origin) expressed in HEK293 cells by TR-FRET assay |
J Med Chem 58: 2547-52 (2015)
Article DOI: 10.1021/jm501395b
BindingDB Entry DOI: 10.7270/Q2TB18KT |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50077136
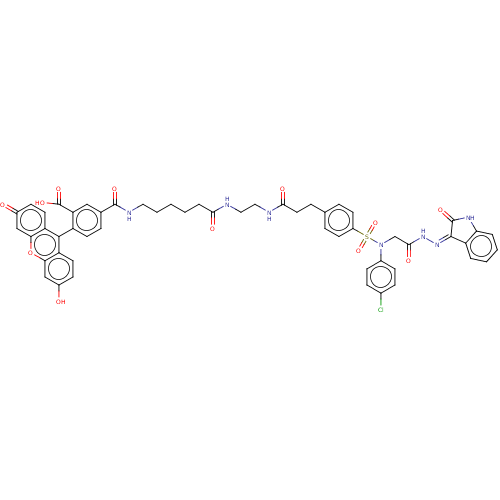
(CHEMBL3416749)Show SMILES OC(=O)c1cc(ccc1-c1c2ccc(O)cc2oc2cc(=O)ccc12)C(=O)NCCCCCC(=O)NCCNC(=O)CCc1ccc(cc1)S(=O)(=O)N(CC(=O)N\N=C1/C(=O)Nc2ccccc12)c1ccc(Cl)cc1 |(2.68,2.06,;2.67,3.09,;3.74,3.7,;1.34,3.85,;1.34,5.39,;0,6.16,;-1.33,5.39,;-1.33,3.85,;0,3.08,;,1.54,;-1.33,.77,;-2.67,1.54,;-4,.77,;-4,-.77,;-5.07,-1.39,;-2.67,-1.54,;-1.33,-.77,;,-1.54,;1.31,-.77,;2.67,-1.54,;4,-.77,;5.07,-1.39,;4,.77,;2.67,1.54,;1.31,.77,;-0,7.7,;1.06,8.32,;-1.34,8.47,;-1.34,10.01,;-2.68,10.78,;-2.68,12.32,;-4.02,13.09,;-4.02,14.63,;-5.36,15.39,;-6.42,14.78,;-5.36,16.93,;-6.7,17.7,;-6.7,19.24,;-8.04,20.01,;-8.04,21.55,;-6.98,22.17,;-9.38,22.32,;-9.38,23.86,;-10.72,24.63,;-10.73,26.17,;-12.06,26.93,;-13.39,26.16,;-13.39,24.62,;-12.05,23.85,;-14.73,26.92,;-14.74,28.15,;-15.8,27.53,;-16.06,26.15,;-17.4,26.91,;-18.73,26.14,;-18.73,24.9,;-20.07,26.9,;-21.4,26.12,;-22.74,26.89,;-22.89,28.41,;-21.97,29.23,;-24.37,28.72,;-25.14,27.4,;-26.66,27.09,;-27.14,25.61,;-26.11,24.47,;-24.58,24.78,;-24.12,26.25,;-16.06,24.6,;-14.72,23.84,;-14.72,22.3,;-16.05,21.52,;-16.05,20.29,;-17.39,22.29,;-17.39,23.83,)| Show InChI InChI=1S/C54H48ClN7O12S/c55-34-13-15-35(16-14-34)62(31-49(67)60-61-51-40-6-3-4-7-44(40)59-53(51)69)75(72,73)38-19-9-32(10-20-38)11-24-48(66)57-27-26-56-47(65)8-2-1-5-25-58-52(68)33-12-21-39(43(28-33)54(70)71)50-41-22-17-36(63)29-45(41)74-46-30-37(64)18-23-42(46)50/h3-4,6-7,9-10,12-23,28-30,63H,1-2,5,8,11,24-27,31H2,(H,56,65)(H,57,66)(H,58,68)(H,60,67)(H,70,71)(H,59,61,69) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Binding affinity to SNAP-tagged oxytocin receptor (unknown origin) expressed in HEK293 cells by TR-FRET assay |
J Med Chem 58: 2547-52 (2015)
Article DOI: 10.1021/jm501395b
BindingDB Entry DOI: 10.7270/Q2TB18KT |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50077137

(CHEMBL3416754)Show SMILES [O-]C(=O)C(F)(F)F.CCN1\C(=C/C=C/C=C/C2=[N+](CC)c3cc(ccc3C2(C)C)S([O-])(=O)=O)C(C)(CCCC(=O)NCCOCCOCCOCCOCCOCCOCCOCCOCc2nnc(N3CC(C3)Oc3ccc(F)cc3Cl)n2-c2ccc(OC)[nH+]c2)c2cc(ccc12)S(O)(=O)=O |c:15| Show InChI InChI=1S/C66H86ClFN8O17S2/c1-7-74-56-21-19-51(94(78,79)80)42-54(56)66(5,60(74)14-11-9-10-13-59-65(3,4)53-20-18-52(95(81,82)83)43-57(53)75(59)8-2)24-12-15-62(77)69-25-26-85-27-28-86-29-30-87-31-32-88-33-34-89-35-36-90-37-38-91-39-40-92-47-61-71-72-64(76(61)49-17-23-63(84-6)70-44-49)73-45-50(46-73)93-58-22-16-48(68)41-55(58)67/h9-11,13-14,16-23,41-44,50H,7-8,12,15,24-40,45-47H2,1-6H3,(H2-,69,77,78,79,80,81,82,83)/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Binding affinity to SNAP-tagged oxytocin receptor (unknown origin) expressed in HEK293 cells by TR-FRET assay |
J Med Chem 58: 2547-52 (2015)
Article DOI: 10.1021/jm501395b
BindingDB Entry DOI: 10.7270/Q2TB18KT |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50077139
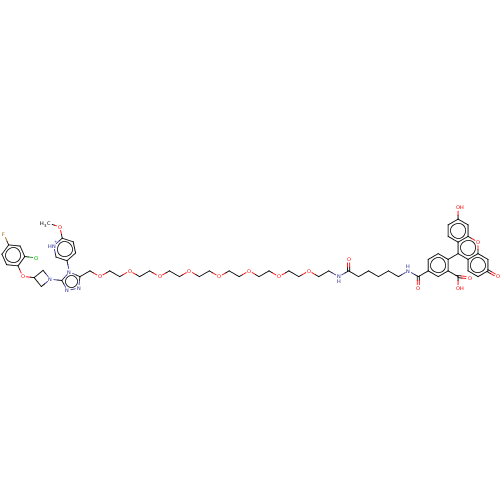
(CHEMBL3416752)Show SMILES [O-]C(=O)C(F)(F)F.COc1ccc(c[nH+]1)-n1c(COCCOCCOCCOCCOCCOCCOCCOCCNC(=O)CCCCCNC(=O)c2ccc(c(c2)C(O)=O)-c2c3ccc(O)cc3oc3cc(=O)ccc23)nnc1N1CC(C1)Oc1ccc(F)cc1Cl |(9.24,30.38,;9.24,29.14,;8.17,28.53,;10.57,28.37,;11.64,28.99,;11.64,27.76,;10.57,27.14,;-30.83,47.13,;-29.98,46.24,;-28.48,46.61,;-27.42,45.5,;-25.92,45.86,;-25.5,47.34,;-26.56,48.45,;-28.05,48.09,;-24.03,47.82,;-22.79,46.94,;-22.78,45.4,;-21.45,44.63,;-21.44,43.09,;-20.11,42.32,;-20.1,40.78,;-18.77,40.02,;-18.76,38.48,;-17.43,37.71,;-17.42,36.17,;-16.09,35.4,;-16.08,33.86,;-14.75,33.09,;-14.74,31.55,;-13.41,30.78,;-13.4,29.24,;-12.06,28.48,;-12.06,26.93,;-10.72,26.17,;-10.72,24.63,;-9.38,23.86,;-9.38,22.32,;-8.04,21.55,;-8.04,20.01,;-6.7,19.24,;-6.7,17.7,;-5.36,16.93,;-5.36,15.39,;-6.42,14.77,;-4.02,14.63,;-4.02,13.09,;-2.68,12.32,;-2.68,10.78,;-1.34,10.01,;-1.34,8.47,;-0,7.7,;1.06,8.32,;0,6.16,;-1.33,5.39,;-1.33,3.85,;0,3.08,;1.34,3.85,;1.34,5.39,;2.67,3.09,;2.68,2.06,;3.74,3.7,;,1.54,;-1.33,.77,;-2.67,1.54,;-4,.77,;-4,-.77,;-5.07,-1.39,;-2.67,-1.54,;-1.33,-.77,;,-1.54,;1.31,-.77,;2.67,-1.54,;4,-.77,;5.07,-1.39,;4,.77,;2.67,1.54,;1.31,.77,;-21.54,47.83,;-22.02,49.29,;-23.56,49.29,;-24.47,50.53,;-25.93,50.85,;-25.57,52.35,;-24.07,51.98,;-26.36,53.66,;-25.62,55.01,;-24.08,55.04,;-23.33,56.39,;-24.13,57.71,;-23.54,58.79,;-25.67,57.68,;-26.41,56.33,;-27.65,56.31,)| Show InChI InChI=1S/C61H71ClFN7O17/c1-77-57-15-8-43(37-66-57)70-55(67-68-61(70)69-38-46(39-69)86-52-14-7-42(63)34-51(52)62)40-85-32-31-84-30-29-83-28-27-82-26-25-81-24-23-80-22-21-79-20-19-78-18-17-64-56(73)5-3-2-4-16-65-59(74)41-6-11-47(50(33-41)60(75)76)58-48-12-9-44(71)35-53(48)87-54-36-45(72)10-13-49(54)58/h6-15,33-37,46,71H,2-5,16-32,38-40H2,1H3,(H,64,73)(H,65,74)(H,75,76)/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Binding affinity to SNAP-tagged oxytocin receptor (unknown origin) expressed in HEK293 cells by TR-FRET assay |
J Med Chem 58: 2547-52 (2015)
Article DOI: 10.1021/jm501395b
BindingDB Entry DOI: 10.7270/Q2TB18KT |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50407350

(CHEMBL263521)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H](Cc2ccc(O)cc2)N(C)C(=O)CC2(CCCCC2)SSC[C@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN)C(=O)NCC(N)=O Show InChI InChI=1S/C48H74N12O12S2/c1-4-27(2)40-46(71)55-31(16-17-36(50)62)42(67)56-32(23-37(51)63)43(68)57-33(47(72)60-21-9-11-34(60)44(69)54-30(10-8-20-49)41(66)53-25-38(52)64)26-73-74-48(18-6-5-7-19-48)24-39(65)59(3)35(45(70)58-40)22-28-12-14-29(61)15-13-28/h12-15,27,30-35,40,61H,4-11,16-26,49H2,1-3H3,(H2,50,62)(H2,51,63)(H2,52,64)(H,53,66)(H,54,69)(H,55,71)(H,56,67)(H,57,68)(H,58,70)/t27-,30-,31-,32+,33-,34-,35-,40+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
In vitro antioxycic activity with out Mg2+. |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50407333
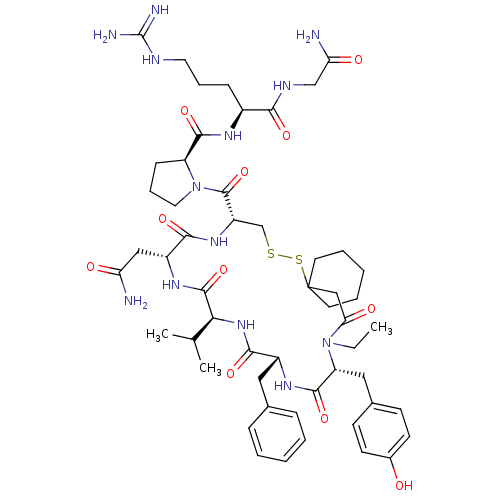
(CHEMBL412942)Show SMILES CCN1[C@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](CC(N)=O)C(=O)N[C@@H](CSSC2(CCCCC2)CC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C53H77N13O11S2/c1-4-65-40(26-33-17-19-34(67)20-18-33)49(75)61-36(25-32-13-7-5-8-14-32)47(73)64-44(31(2)3)50(76)62-37(27-41(54)68)46(72)63-38(30-78-79-53(28-43(65)70)21-9-6-10-22-53)51(77)66-24-12-16-39(66)48(74)60-35(15-11-23-58-52(56)57)45(71)59-29-42(55)69/h5,7-8,13-14,17-20,31,35-40,44,67H,4,6,9-12,15-16,21-30H2,1-3H3,(H2,54,68)(H2,55,69)(H,59,71)(H,60,74)(H,61,75)(H,62,76)(H,63,72)(H,64,73)(H4,56,57,58)/t35-,36+,37+,38-,39-,40+,44-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 4.17 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
In vitro activity was determinedfor the anti-oxytocic activity with 0.5 mM Mg2+. |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50407333

(CHEMBL412942)Show SMILES CCN1[C@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](CC(N)=O)C(=O)N[C@@H](CSSC2(CCCCC2)CC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C53H77N13O11S2/c1-4-65-40(26-33-17-19-34(67)20-18-33)49(75)61-36(25-32-13-7-5-8-14-32)47(73)64-44(31(2)3)50(76)62-37(27-41(54)68)46(72)63-38(30-78-79-53(28-43(65)70)21-9-6-10-22-53)51(77)66-24-12-16-39(66)48(74)60-35(15-11-23-58-52(56)57)45(71)59-29-42(55)69/h5,7-8,13-14,17-20,31,35-40,44,67H,4,6,9-12,15-16,21-30H2,1-3H3,(H2,54,68)(H2,55,69)(H,59,71)(H,60,74)(H,61,75)(H,62,76)(H,63,72)(H,64,73)(H4,56,57,58)/t35-,36+,37+,38-,39-,40+,44-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
In vitro activity for the anti-oxytocic activity with out Mg2+. |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50407343
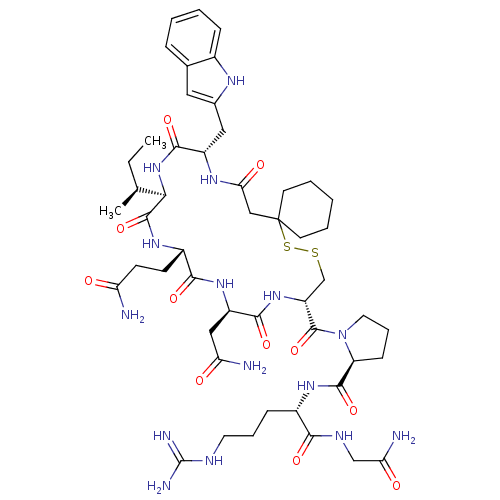
(CHEMBL412806)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H](Cc2cc3ccccc3[nH]2)NC(=O)CC2(CCCCC2)SSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C50H75N15O11S2/c1-3-27(2)41-47(75)61-32(15-16-37(51)66)43(71)62-34(23-38(52)67)44(72)63-35(48(76)65-20-10-14-36(65)46(74)60-31(13-9-19-56-49(54)55)42(70)57-25-39(53)68)26-77-78-50(17-7-4-8-18-50)24-40(69)59-33(45(73)64-41)22-29-21-28-11-5-6-12-30(28)58-29/h5-6,11-12,21,27,31-36,41,58H,3-4,7-10,13-20,22-26H2,1-2H3,(H2,51,66)(H2,52,67)(H2,53,68)(H,57,70)(H,59,69)(H,60,74)(H,61,75)(H,62,71)(H,63,72)(H,64,73)(H4,54,55,56)/t27-,31-,32-,33-,34+,35+,36-,41+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
In vitro anti-oxytocic activity with out Mg2+. |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50407345

(CHEMBL1790315)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H](Cc2ccc(O)cc2)N(C)C(=O)CC2(CCCCC2)SSC[C@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC1=O)[C@@H](C)O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C54H79N11O13S2/c1-5-30(2)44-51(76)63-45(31(3)66)52(77)60-38(27-42(56)69)48(73)61-39(29-79-80-54(21-7-6-8-22-54)28-43(70)64(4)41(50(75)62-44)26-33-15-19-35(68)20-16-33)53(78)65-24-10-12-40(65)49(74)58-36(11-9-23-55)47(72)59-37(46(57)71)25-32-13-17-34(67)18-14-32/h13-20,30-31,36-41,44-45,66-68H,5-12,21-29,55H2,1-4H3,(H2,56,69)(H2,57,71)(H,58,74)(H,59,72)(H,60,77)(H,61,73)(H,62,75)(H,63,76)/t30-,31+,36-,37-,38+,39-,40-,41-,44+,45-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 5.25 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
In vitro anti-oxytocic activity with 0.5 mM Mg2+. |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50407337
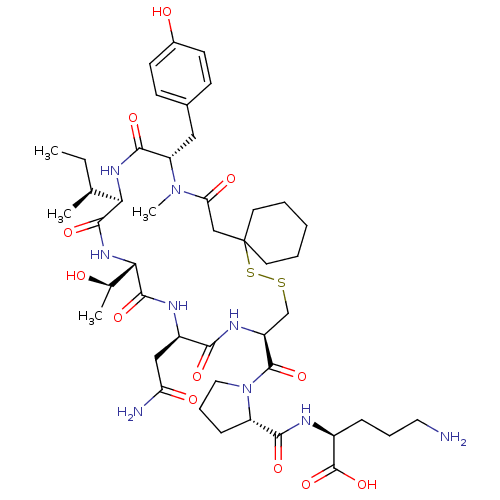
(CHEMBL1790314)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H](Cc2ccc(O)cc2)N(C)C(=O)CC2(CCCCC2)SSC[C@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC1=O)[C@@H](C)O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN)C(O)=O Show InChI InChI=1S/C45H69N9O12S2/c1-5-25(2)36-41(62)52-37(26(3)55)42(63)49-30(22-34(47)57)38(59)50-31(43(64)54-20-10-12-32(54)39(60)48-29(44(65)66)11-9-19-46)24-67-68-45(17-7-6-8-18-45)23-35(58)53(4)33(40(61)51-36)21-27-13-15-28(56)16-14-27/h13-16,25-26,29-33,36-37,55-56H,5-12,17-24,46H2,1-4H3,(H2,47,57)(H,48,60)(H,49,63)(H,50,59)(H,51,61)(H,52,62)(H,65,66)/t25-,26+,29-,30+,31-,32-,33-,36+,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 5.75 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
In vitro anti-oxytocic activity with 0.5 mM Mg2+. |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50030087
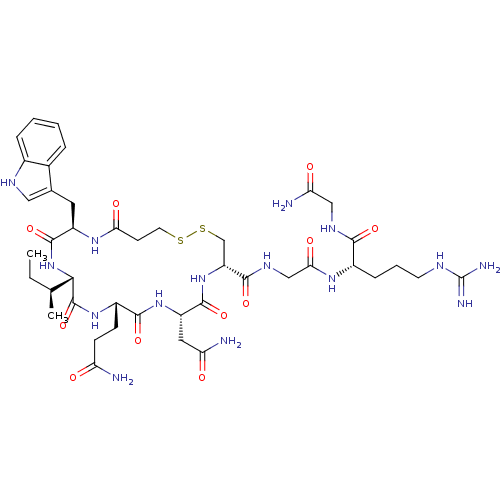
(CHEMBL274397 | [Mca1,D-Trp2,Sar7]AVT)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)CCSSC[C@@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C42H63N15O11S2/c1-3-21(2)35-41(68)54-26(10-11-30(43)58)38(65)55-28(16-31(44)59)39(66)56-29(37(64)51-19-34(62)52-25(9-6-13-48-42(46)47)36(63)50-18-32(45)60)20-70-69-14-12-33(61)53-27(40(67)57-35)15-22-17-49-24-8-5-4-7-23(22)24/h4-5,7-8,17,21,25-29,35,49H,3,6,9-16,18-20H2,1-2H3,(H2,43,58)(H2,44,59)(H2,45,60)(H,50,63)(H,51,64)(H,52,62)(H,53,61)(H,54,68)(H,55,65)(H,56,66)(H,57,67)(H4,46,47,48)/t21-,25-,26-,27+,28-,29+,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institut f£r Biophysik
Curated by ChEMBL
| Assay Description
Inhibition of radioligand [3H]OT binding to oxytocin receptor (OT) in guinea pig myometrium membrane |
J Med Chem 37: 255-9 (1994)
BindingDB Entry DOI: 10.7270/Q2H995TM |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50075816

(CHEMBL264208 | Mca1,D-Trp2,Sar7AVT)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)c1cc(=O)oc2cc(OC)ccc12)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CS)C(=O)N(C)CC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(O)=O Show InChI InChI=1S/C51H68N14O15S/c1-5-25(2)43(64-48(76)34(17-26-21-57-31-10-7-6-9-28(26)31)61-44(72)30-19-42(71)80-37-18-27(79-4)12-13-29(30)37)49(77)60-33(14-15-38(52)66)46(74)62-35(20-39(53)67)47(75)63-36(24-81)50(78)65(3)23-40(68)59-32(11-8-16-56-51(54)55)45(73)58-22-41(69)70/h6-7,9-10,12-13,18-19,21,25,32-36,43,57,81H,5,8,11,14-17,20,22-24H2,1-4H3,(H2,52,66)(H2,53,67)(H,58,73)(H,59,68)(H,60,77)(H,61,72)(H,62,74)(H,63,75)(H,64,76)(H,69,70)(H4,54,55,56)/t25-,32-,33-,34+,35-,36-,43-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a |
Albert Szent-Gy£rgyi Medical University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its dissociation constant (Kd) to guinea pig myometrial Oxytocin receptor |
Bioorg Med Chem Lett 9: 667-72 (1999)
BindingDB Entry DOI: 10.7270/Q2QC040K |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50407349

(CHEMBL265357)Show SMILES CCN1[C@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](CC(N)=O)C(=O)N[C@H](CSSC2(CCCCC2)CC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C53H77N13O11S2/c1-4-65-40(26-33-17-19-34(67)20-18-33)49(75)61-36(25-32-13-7-5-8-14-32)47(73)64-44(31(2)3)50(76)62-37(27-41(54)68)46(72)63-38(30-78-79-53(28-43(65)70)21-9-6-10-22-53)51(77)66-24-12-16-39(66)48(74)60-35(15-11-23-58-52(56)57)45(71)59-29-42(55)69/h5,7-8,13-14,17-20,31,35-40,44,67H,4,6,9-12,15-16,21-30H2,1-3H3,(H2,54,68)(H2,55,69)(H,59,71)(H,60,74)(H,61,75)(H,62,76)(H,63,72)(H,64,73)(H4,56,57,58)/t35-,36+,37+,38+,39-,40+,44-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
In vitro activity was determinedfor the anti-oxytocic activity with 0.5 mM Mg2+. |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50205990

(CHEMBL395429 | OXYTOCIN)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C43H66N12O12S2/c1-5-22(4)35-42(66)49-26(12-13-32(45)57)38(62)51-29(17-33(46)58)39(63)53-30(20-69-68-19-25(44)36(60)50-28(40(64)54-35)16-23-8-10-24(56)11-9-23)43(67)55-14-6-7-31(55)41(65)52-27(15-21(2)3)37(61)48-18-34(47)59/h8-11,21-22,25-31,35,56H,5-7,12-20,44H2,1-4H3,(H2,45,57)(H2,46,58)(H2,47,59)(H,48,61)(H,49,66)(H,50,60)(H,51,62)(H,52,65)(H,53,63)(H,54,64)/t22-,25-,26-,27-,28-,29-,30-,31-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institut f£r Biophysik
Curated by ChEMBL
| Assay Description
Inhibition of radioligand [3H]OT binding to oxytocin receptor (OT) in guinea pig myometrium membrane |
J Med Chem 37: 255-9 (1994)
BindingDB Entry DOI: 10.7270/Q2H995TM |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50205990

(CHEMBL395429 | OXYTOCIN)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C43H66N12O12S2/c1-5-22(4)35-42(66)49-26(12-13-32(45)57)38(62)51-29(17-33(46)58)39(63)53-30(20-69-68-19-25(44)36(60)50-28(40(64)54-35)16-23-8-10-24(56)11-9-23)43(67)55-14-6-7-31(55)41(65)52-27(15-21(2)3)37(61)48-18-34(47)59/h8-11,21-22,25-31,35,56H,5-7,12-20,44H2,1-4H3,(H2,45,57)(H2,46,58)(H2,47,59)(H,48,61)(H,49,66)(H,50,60)(H,51,62)(H,52,65)(H,53,63)(H,54,64)/t22-,25-,26-,27-,28-,29-,30-,31-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a |
Albert Szent-Gy£rgyi Medical University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its dissociation constant (Kd) to guinea pig myometrial Oxytocin receptor |
Bioorg Med Chem Lett 9: 667-72 (1999)
BindingDB Entry DOI: 10.7270/Q2QC040K |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50030081

(CHEMBL265017 | [Mca1,D-Phe2,Sar7]AVP)Show SMILES CN(CC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O)C(=O)[C@@H]1CSSC2(CCCCC2)CC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1 Show InChI InChI=1S/C49H70N14O11S2/c1-63(27-41(68)57-31(16-11-21-55-48(53)54)42(69)56-26-39(52)66)47(74)36-28-75-76-49(19-9-4-10-20-49)25-40(67)58-33(22-29-12-5-2-6-13-29)44(71)60-34(23-30-14-7-3-8-15-30)45(72)59-32(17-18-37(50)64)43(70)61-35(24-38(51)65)46(73)62-36/h2-3,5-8,12-15,31-36H,4,9-11,16-28H2,1H3,(H2,50,64)(H2,51,65)(H2,52,66)(H,56,69)(H,57,68)(H,58,67)(H,59,72)(H,60,71)(H,61,70)(H,62,73)(H4,53,54,55)/t31-,32-,33-,34-,35-,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institut f£r Biophysik
Curated by ChEMBL
| Assay Description
Inhibition of radioligand [3H]OT binding to oxytocin receptor (OT) in guinea pig myometrium membrane |
J Med Chem 37: 255-9 (1994)
BindingDB Entry DOI: 10.7270/Q2H995TM |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50407342

(CHEMBL415160)Show SMILES CN1[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](CC(N)=O)C(=O)N[C@@H](CSSC2(CCCCC2)CC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C52H74N14O12S2/c1-65-39(25-31-14-16-32(67)17-15-31)49(77)63-35(24-30-10-4-2-5-11-30)46(74)60-34(18-19-40(53)68)45(73)62-36(26-41(54)69)47(75)64-37(29-79-80-52(27-43(65)71)20-6-3-7-21-52)50(78)66-23-9-13-38(66)48(76)61-33(12-8-22-58-51(56)57)44(72)59-28-42(55)70/h2,4-5,10-11,14-17,33-39,67H,3,6-9,12-13,18-29H2,1H3,(H2,53,68)(H2,54,69)(H2,55,70)(H,59,72)(H,60,74)(H,61,76)(H,62,73)(H,63,77)(H,64,75)(H4,56,57,58)/t33-,34-,35+,36+,37-,38-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
In vitro activity for the anti-oxytocic activity with out Mg2+ |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50030079
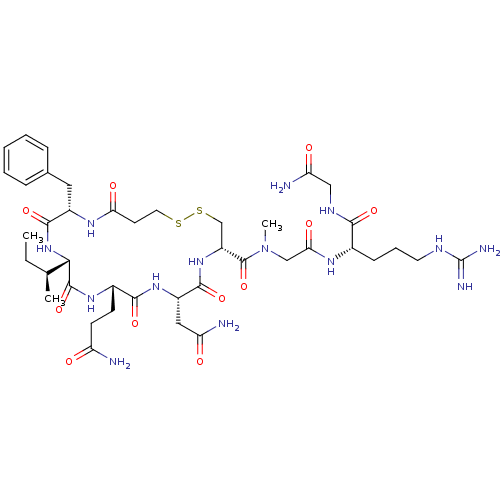
(CHEMBL87636 | [Mpa1,D-Phe2,Sar7]AVT)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)CCSSC[C@@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N(C)CC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C41H64N14O11S2/c1-4-22(2)34-39(65)51-25(12-13-29(42)56)36(62)52-27(18-30(43)57)37(63)53-28(21-68-67-16-14-32(59)50-26(38(64)54-34)17-23-9-6-5-7-10-23)40(66)55(3)20-33(60)49-24(11-8-15-47-41(45)46)35(61)48-19-31(44)58/h5-7,9-10,22,24-28,34H,4,8,11-21H2,1-3H3,(H2,42,56)(H2,43,57)(H2,44,58)(H,48,61)(H,49,60)(H,50,59)(H,51,65)(H,52,62)(H,53,63)(H,54,64)(H4,45,46,47)/t22-,24-,25-,26-,27-,28+,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institut f£r Biophysik
Curated by ChEMBL
| Assay Description
Inhibition of radioligand [3H]OT binding to oxytocin receptor (OT) in guinea pig myometrium membrane |
J Med Chem 37: 255-9 (1994)
BindingDB Entry DOI: 10.7270/Q2H995TM |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50075818
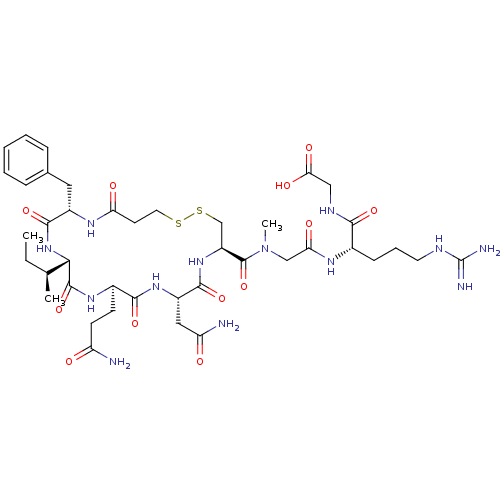
(CHEMBL157988 | Mpa1,D-Phe2,Sar7AVT)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](CCC(N)=O)NC1=O)C(=O)N(C)CC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(O)=O Show InChI InChI=1S/C41H63N13O12S2/c1-4-22(2)34-39(65)50-25(12-13-29(42)55)36(62)51-27(18-30(43)56)37(63)52-28(21-68-67-16-14-31(57)49-26(38(64)53-34)17-23-9-6-5-7-10-23)40(66)54(3)20-32(58)48-24(11-8-15-46-41(44)45)35(61)47-19-33(59)60/h5-7,9-10,22,24-28,34H,4,8,11-21H2,1-3H3,(H2,42,55)(H2,43,56)(H,47,61)(H,48,58)(H,49,57)(H,50,65)(H,51,62)(H,52,63)(H,53,64)(H,59,60)(H4,44,45,46)/t22-,24-,25+,26-,27-,28-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a |
Albert Szent-Gy£rgyi Medical University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its dissociation constant (Kd) to guinea pig myometrial Oxytocin receptor |
Bioorg Med Chem Lett 9: 667-72 (1999)
BindingDB Entry DOI: 10.7270/Q2QC040K |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50030083

(CHEMBL414582 | [Mca1,D-Phe2,Sar7]AVT)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)CC2(CCCCC2)SSC[C@@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N(C)CC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C46H72N14O11S2/c1-4-26(2)38-43(70)56-29(15-16-33(47)61)40(67)57-31(21-34(48)62)41(68)58-32(44(71)60(3)24-37(65)54-28(14-11-19-52-45(50)51)39(66)53-23-35(49)63)25-72-73-46(17-9-6-10-18-46)22-36(64)55-30(42(69)59-38)20-27-12-7-5-8-13-27/h5,7-8,12-13,26,28-32,38H,4,6,9-11,14-25H2,1-3H3,(H2,47,61)(H2,48,62)(H2,49,63)(H,53,66)(H,54,65)(H,55,64)(H,56,70)(H,57,67)(H,58,68)(H,59,69)(H4,50,51,52)/t26-,28-,29-,30-,31-,32+,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institut f£r Biophysik
Curated by ChEMBL
| Assay Description
Inhibition of radioligand [3H]OT binding to oxytocin receptor (OT) in guinea pig myometrium membrane |
J Med Chem 37: 255-9 (1994)
BindingDB Entry DOI: 10.7270/Q2H995TM |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50407348
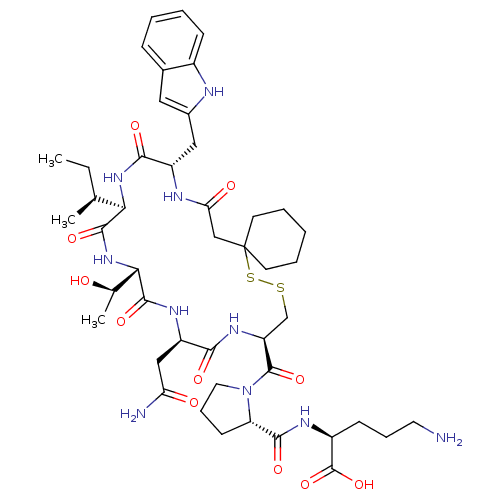
(CHEMBL1790312)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H](Cc2cc3ccccc3[nH]2)NC(=O)CC2(CCCCC2)SSC[C@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC1=O)[C@@H](C)O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN)C(O)=O Show InChI InChI=1S/C46H68N10O11S2/c1-4-25(2)37-42(63)55-38(26(3)57)43(64)52-32(22-35(48)58)39(60)53-33(44(65)56-19-11-15-34(56)41(62)51-30(45(66)67)14-10-18-47)24-68-69-46(16-8-5-9-17-46)23-36(59)50-31(40(61)54-37)21-28-20-27-12-6-7-13-29(27)49-28/h6-7,12-13,20,25-26,30-34,37-38,49,57H,4-5,8-11,14-19,21-24,47H2,1-3H3,(H2,48,58)(H,50,59)(H,51,62)(H,52,64)(H,53,60)(H,54,61)(H,55,63)(H,66,67)/t25-,26+,30-,31-,32+,33-,34-,37+,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
In vitro anti-oxytocic activity with out Mg2+. |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50407338
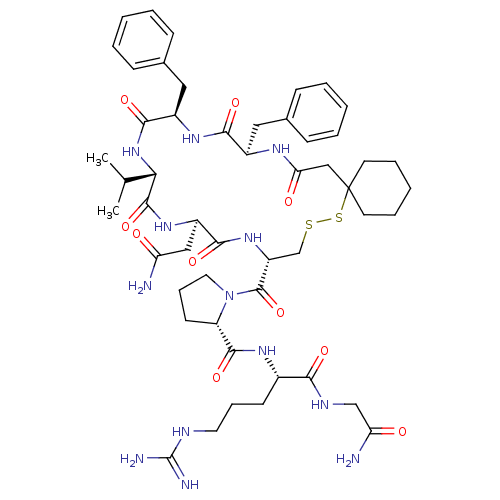
(CHEMBL385062)Show SMILES CC(C)[C@@H]1NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccccc2)NC(=O)CC2(CCCCC2)SSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C51H73N13O10S2/c1-30(2)42-48(73)61-36(26-39(52)65)45(70)62-37(49(74)64-23-13-19-38(64)47(72)59-33(18-12-22-56-50(54)55)43(68)57-28-40(53)66)29-75-76-51(20-10-5-11-21-51)27-41(67)58-34(24-31-14-6-3-7-15-31)44(69)60-35(46(71)63-42)25-32-16-8-4-9-17-32/h3-4,6-9,14-17,30,33-38,42H,5,10-13,18-29H2,1-2H3,(H2,52,65)(H2,53,66)(H,57,68)(H,58,67)(H,59,72)(H,60,69)(H,61,73)(H,62,70)(H,63,71)(H4,54,55,56)/t33-,34+,35+,36+,37+,38-,42-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 10.7 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
In vitro activity was determinedfor the anti-oxytocic activity with 0.5 mM Mg2+. |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50407340
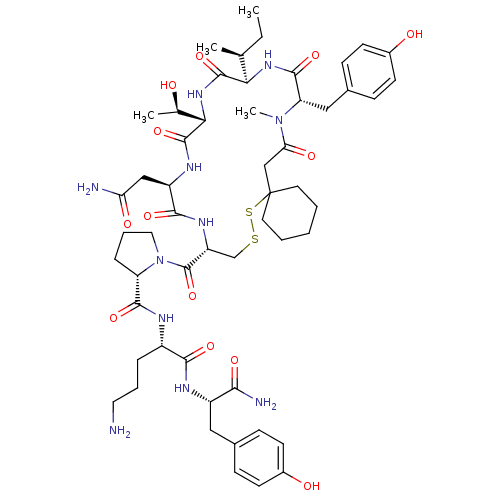
(CHEMBL1790310)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H](Cc2ccc(O)cc2)N(C)C(=O)CC2(CCCCC2)SSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC1=O)[C@@H](C)O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C54H79N11O13S2/c1-5-30(2)44-51(76)63-45(31(3)66)52(77)60-38(27-42(56)69)48(73)61-39(29-79-80-54(21-7-6-8-22-54)28-43(70)64(4)41(50(75)62-44)26-33-15-19-35(68)20-16-33)53(78)65-24-10-12-40(65)49(74)58-36(11-9-23-55)47(72)59-37(46(57)71)25-32-13-17-34(67)18-14-32/h13-20,30-31,36-41,44-45,66-68H,5-12,21-29,55H2,1-4H3,(H2,56,69)(H2,57,71)(H,58,74)(H,59,72)(H,60,77)(H,61,73)(H,62,75)(H,63,76)/t30-,31+,36-,37-,38+,39+,40-,41-,44+,45-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
In vitro antioxycic activity with out Mg2+. |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50407332

(CHEMBL405005)Show SMILES CCN1[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](CC(N)=O)C(=O)N[C@@H](CSSC2(CCCCC2)CC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C53H77N13O11S2/c1-4-65-40(26-33-17-19-34(67)20-18-33)49(75)61-36(25-32-13-7-5-8-14-32)47(73)64-44(31(2)3)50(76)62-37(27-41(54)68)46(72)63-38(30-78-79-53(28-43(65)70)21-9-6-10-22-53)51(77)66-24-12-16-39(66)48(74)60-35(15-11-23-58-52(56)57)45(71)59-29-42(55)69/h5,7-8,13-14,17-20,31,35-40,44,67H,4,6,9-12,15-16,21-30H2,1-3H3,(H2,54,68)(H2,55,69)(H,59,71)(H,60,74)(H,61,75)(H,62,76)(H,63,72)(H,64,73)(H4,56,57,58)/t35-,36+,37+,38-,39-,40-,44-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
In vitro activity for the anti-oxytocic activity with out Mg2+. |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50407337

(CHEMBL1790314)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H](Cc2ccc(O)cc2)N(C)C(=O)CC2(CCCCC2)SSC[C@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC1=O)[C@@H](C)O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN)C(O)=O Show InChI InChI=1S/C45H69N9O12S2/c1-5-25(2)36-41(62)52-37(26(3)55)42(63)49-30(22-34(47)57)38(59)50-31(43(64)54-20-10-12-32(54)39(60)48-29(44(65)66)11-9-19-46)24-67-68-45(17-7-6-8-18-45)23-35(58)53(4)33(40(61)51-36)21-27-13-15-28(56)16-14-27/h13-16,25-26,29-33,36-37,55-56H,5-12,17-24,46H2,1-4H3,(H2,47,57)(H,48,60)(H,49,63)(H,50,59)(H,51,61)(H,52,62)(H,65,66)/t25-,26+,29-,30+,31-,32-,33-,36+,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
In vitro anti-oxytocic activity with out Mg2+. |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50407350

(CHEMBL263521)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H](Cc2ccc(O)cc2)N(C)C(=O)CC2(CCCCC2)SSC[C@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN)C(=O)NCC(N)=O Show InChI InChI=1S/C48H74N12O12S2/c1-4-27(2)40-46(71)55-31(16-17-36(50)62)42(67)56-32(23-37(51)63)43(68)57-33(47(72)60-21-9-11-34(60)44(69)54-30(10-8-20-49)41(66)53-25-38(52)64)26-73-74-48(18-6-5-7-19-48)24-39(65)59(3)35(45(70)58-40)22-28-12-14-29(61)15-13-28/h12-15,27,30-35,40,61H,4-11,16-26,49H2,1-3H3,(H2,50,62)(H2,51,63)(H2,52,64)(H,53,66)(H,54,69)(H,55,71)(H,56,67)(H,57,68)(H,58,70)/t27-,30-,31-,32+,33-,34-,35-,40+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 13.2 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
In vitro anti-oxytocic activity with 0.5 mM Mg2+. |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50407336

(CHEMBL412616)Show SMILES CN1[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](CC(N)=O)C(=O)N[C@H](CSSC2(CCCCC2)CC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C52H74N14O12S2/c1-65-39(25-31-14-16-32(67)17-15-31)49(77)63-35(24-30-10-4-2-5-11-30)46(74)60-34(18-19-40(53)68)45(73)62-36(26-41(54)69)47(75)64-37(29-79-80-52(27-43(65)71)20-6-3-7-21-52)50(78)66-23-9-13-38(66)48(76)61-33(12-8-22-58-51(56)57)44(72)59-28-42(55)70/h2,4-5,10-11,14-17,33-39,67H,3,6-9,12-13,18-29H2,1H3,(H2,53,68)(H2,54,69)(H2,55,70)(H,59,72)(H,60,74)(H,61,76)(H,62,73)(H,63,77)(H,64,75)(H4,56,57,58)/t33-,34-,35+,36+,37+,38-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 13.5 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
In vitro activity for the anti-oxytocic activity with out Mg2+. |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50407339
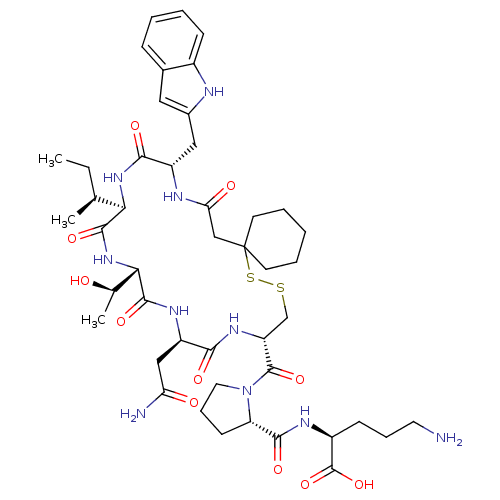
(CHEMBL1790307)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H](Cc2cc3ccccc3[nH]2)NC(=O)CC2(CCCCC2)SSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC1=O)[C@@H](C)O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN)C(O)=O Show InChI InChI=1S/C46H68N10O11S2/c1-4-25(2)37-42(63)55-38(26(3)57)43(64)52-32(22-35(48)58)39(60)53-33(44(65)56-19-11-15-34(56)41(62)51-30(45(66)67)14-10-18-47)24-68-69-46(16-8-5-9-17-46)23-36(59)50-31(40(61)54-37)21-28-20-27-12-6-7-13-29(27)49-28/h6-7,12-13,20,25-26,30-34,37-38,49,57H,4-5,8-11,14-19,21-24,47H2,1-3H3,(H2,48,58)(H,50,59)(H,51,62)(H,52,64)(H,53,60)(H,54,61)(H,55,63)(H,66,67)/t25-,26+,30-,31-,32+,33+,34-,37+,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
In vitro anti-oxytocic activity with out Mg2+. |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50030080
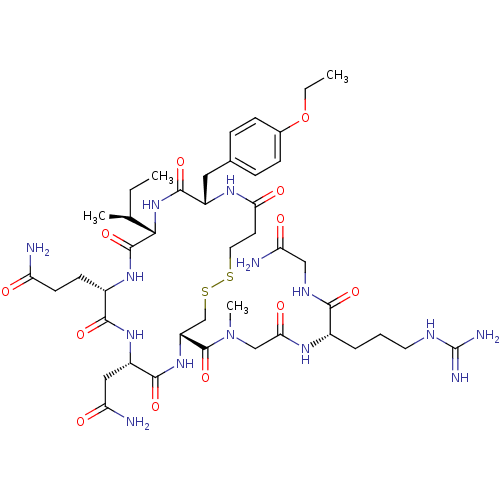
(CHEMBL411420 | [Mca1,D-Tyr(OEt)2,Sar7]AVT)Show SMILES CCOc1ccc(C[C@@H]2NC(=O)CCSSC[C@@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC2=O)[C@@H](C)CC)C(=O)N(C)CC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O)cc1 Show InChI InChI=1S/C43H68N14O12S2/c1-5-23(3)36-41(67)53-27(13-14-31(44)58)38(64)54-29(19-32(45)59)39(65)55-30(42(68)57(4)21-35(62)51-26(8-7-16-49-43(47)48)37(63)50-20-33(46)60)22-71-70-17-15-34(61)52-28(40(66)56-36)18-24-9-11-25(12-10-24)69-6-2/h9-12,23,26-30,36H,5-8,13-22H2,1-4H3,(H2,44,58)(H2,45,59)(H2,46,60)(H,50,63)(H,51,62)(H,52,61)(H,53,67)(H,54,64)(H,55,65)(H,56,66)(H4,47,48,49)/t23-,26-,27-,28-,29-,30+,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institut f£r Biophysik
Curated by ChEMBL
| Assay Description
Inhibition of radioligand [3H]OT binding to oxytocin receptor (OT) in guinea pig myometrium membrane |
J Med Chem 37: 255-9 (1994)
BindingDB Entry DOI: 10.7270/Q2H995TM |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50407335

(CHEMBL428187)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H](Cc2cc3ccccc3[nH]2)NC(=O)CC2(CCCCC2)SSC[C@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C50H75N15O11S2/c1-3-27(2)41-47(75)61-32(15-16-37(51)66)43(71)62-34(23-38(52)67)44(72)63-35(48(76)65-20-10-14-36(65)46(74)60-31(13-9-19-56-49(54)55)42(70)57-25-39(53)68)26-77-78-50(17-7-4-8-18-50)24-40(69)59-33(45(73)64-41)22-29-21-28-11-5-6-12-30(28)58-29/h5-6,11-12,21,27,31-36,41,58H,3-4,7-10,13-20,22-26H2,1-2H3,(H2,51,66)(H2,52,67)(H2,53,68)(H,57,70)(H,59,69)(H,60,74)(H,61,75)(H,62,71)(H,63,72)(H,64,73)(H4,54,55,56)/t27-,31-,32-,33-,34+,35-,36-,41+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
In vitro anti-oxytocic activity with out Mg2+. |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50030077

(CHEMBL330022 | [Mca1,D-Tyr(OMe)2,Sar7]AVT)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(OC)cc2)NC(=O)CCSSC[C@@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N(C)CC(=O)NCC(N)=O Show InChI InChI=1S/C41H61N11O12S2/c1-5-22(2)35-39(61)47-25(12-13-30(42)53)36(58)48-27(18-31(43)54)37(59)49-28(40(62)52-15-6-7-29(52)41(63)51(3)20-34(57)45-19-32(44)55)21-66-65-16-14-33(56)46-26(38(60)50-35)17-23-8-10-24(64-4)11-9-23/h8-11,22,25-29,35H,5-7,12-21H2,1-4H3,(H2,42,53)(H2,43,54)(H2,44,55)(H,45,57)(H,46,56)(H,47,61)(H,48,58)(H,49,59)(H,50,60)/t22-,25-,26-,27-,28+,29-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institut f£r Biophysik
Curated by ChEMBL
| Assay Description
Inhibition of radioligand [3H]OT binding to oxytocin receptor (OT) in guinea pig myometrium membrane |
J Med Chem 37: 255-9 (1994)
BindingDB Entry DOI: 10.7270/Q2H995TM |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50407334

(CHEMBL1790313)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H](Cc2ccc(O)cc2)N(C)C(=O)CC2(CCCCC2)SSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC1=O)[C@@H](C)O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN)C(O)=O Show InChI InChI=1S/C45H69N9O12S2/c1-5-25(2)36-41(62)52-37(26(3)55)42(63)49-30(22-34(47)57)38(59)50-31(43(64)54-20-10-12-32(54)39(60)48-29(44(65)66)11-9-19-46)24-67-68-45(17-7-6-8-18-45)23-35(58)53(4)33(40(61)51-36)21-27-13-15-28(56)16-14-27/h13-16,25-26,29-33,36-37,55-56H,5-12,17-24,46H2,1-4H3,(H2,47,57)(H,48,60)(H,49,63)(H,50,59)(H,51,61)(H,52,62)(H,65,66)/t25-,26+,29-,30+,31+,32-,33-,36+,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
In vitro anti-oxytocic activity with out Mg2+. |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50030088

(CHEMBL438387 | [Mca1,D-Tyr(OEt)2,Sar7]AVP)Show SMILES CCOc1ccc(C[C@@H]2NC(=O)CC3(CCCCC3)SSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc3ccccc3)NC2=O)C(=O)N(C)CC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O)cc1 Show InChI InChI=1S/C51H74N14O12S2/c1-3-77-32-16-14-31(15-17-32)24-35-46(73)62-36(23-30-11-6-4-7-12-30)47(74)61-34(18-19-39(52)66)45(72)63-37(25-40(53)67)48(75)64-38(29-78-79-51(26-42(69)60-35)20-8-5-9-21-51)49(76)65(2)28-43(70)59-33(13-10-22-57-50(55)56)44(71)58-27-41(54)68/h4,6-7,11-12,14-17,33-38H,3,5,8-10,13,18-29H2,1-2H3,(H2,52,66)(H2,53,67)(H2,54,68)(H,58,71)(H,59,70)(H,60,69)(H,61,74)(H,62,73)(H,63,72)(H,64,75)(H4,55,56,57)/t33-,34-,35-,36-,37-,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institut f£r Biophysik
Curated by ChEMBL
| Assay Description
Inhibition of radioligand [3H]OT binding to oxytocin receptor (OT) in guinea pig myometrium membrane |
J Med Chem 37: 255-9 (1994)
BindingDB Entry DOI: 10.7270/Q2H995TM |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50407344
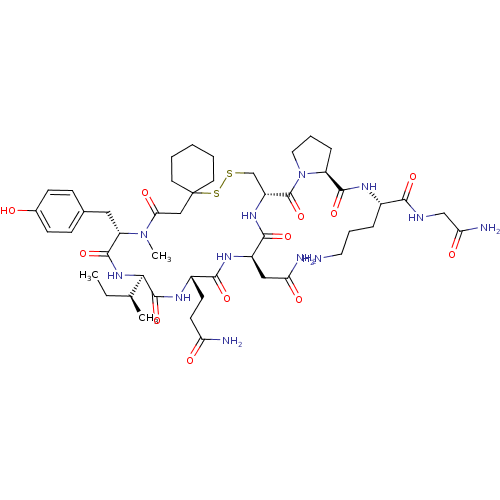
(CHEMBL261914)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H](Cc2ccc(O)cc2)N(C)C(=O)CC2(CCCCC2)SSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN)C(=O)NCC(N)=O Show InChI InChI=1S/C48H74N12O12S2/c1-4-27(2)40-46(71)55-31(16-17-36(50)62)42(67)56-32(23-37(51)63)43(68)57-33(47(72)60-21-9-11-34(60)44(69)54-30(10-8-20-49)41(66)53-25-38(52)64)26-73-74-48(18-6-5-7-19-48)24-39(65)59(3)35(45(70)58-40)22-28-12-14-29(61)15-13-28/h12-15,27,30-35,40,61H,4-11,16-26,49H2,1-3H3,(H2,50,62)(H2,51,63)(H2,52,64)(H,53,66)(H,54,69)(H,55,71)(H,56,67)(H,57,68)(H,58,70)/t27-,30-,31-,32+,33+,34-,35-,40+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
In vitro anti-oxytocic activity with out Mg2+. |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50407339

(CHEMBL1790307)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H](Cc2cc3ccccc3[nH]2)NC(=O)CC2(CCCCC2)SSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC1=O)[C@@H](C)O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN)C(O)=O Show InChI InChI=1S/C46H68N10O11S2/c1-4-25(2)37-42(63)55-38(26(3)57)43(64)52-32(22-35(48)58)39(60)53-33(44(65)56-19-11-15-34(56)41(62)51-30(45(66)67)14-10-18-47)24-68-69-46(16-8-5-9-17-46)23-36(59)50-31(40(61)54-37)21-28-20-27-12-6-7-13-29(27)49-28/h6-7,12-13,20,25-26,30-34,37-38,49,57H,4-5,8-11,14-19,21-24,47H2,1-3H3,(H2,48,58)(H,50,59)(H,51,62)(H,52,64)(H,53,60)(H,54,61)(H,55,63)(H,66,67)/t25-,26+,30-,31-,32+,33+,34-,37+,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 22.4 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
In vitro anti-oxytocic activity with 0.5 mM Mg2+. |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50407348

(CHEMBL1790312)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H](Cc2cc3ccccc3[nH]2)NC(=O)CC2(CCCCC2)SSC[C@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC1=O)[C@@H](C)O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN)C(O)=O Show InChI InChI=1S/C46H68N10O11S2/c1-4-25(2)37-42(63)55-38(26(3)57)43(64)52-32(22-35(48)58)39(60)53-33(44(65)56-19-11-15-34(56)41(62)51-30(45(66)67)14-10-18-47)24-68-69-46(16-8-5-9-17-46)23-36(59)50-31(40(61)54-37)21-28-20-27-12-6-7-13-29(27)49-28/h6-7,12-13,20,25-26,30-34,37-38,49,57H,4-5,8-11,14-19,21-24,47H2,1-3H3,(H2,48,58)(H,50,59)(H,51,62)(H,52,64)(H,53,60)(H,54,61)(H,55,63)(H,66,67)/t25-,26+,30-,31-,32+,33-,34-,37+,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 22.9 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
In vitro anti-oxytocic activity with 0.5 mM Mg2+. |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50407345

(CHEMBL1790315)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H](Cc2ccc(O)cc2)N(C)C(=O)CC2(CCCCC2)SSC[C@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC1=O)[C@@H](C)O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C54H79N11O13S2/c1-5-30(2)44-51(76)63-45(31(3)66)52(77)60-38(27-42(56)69)48(73)61-39(29-79-80-54(21-7-6-8-22-54)28-43(70)64(4)41(50(75)62-44)26-33-15-19-35(68)20-16-33)53(78)65-24-10-12-40(65)49(74)58-36(11-9-23-55)47(72)59-37(46(57)71)25-32-13-17-34(67)18-14-32/h13-20,30-31,36-41,44-45,66-68H,5-12,21-29,55H2,1-4H3,(H2,56,69)(H2,57,71)(H,58,74)(H,59,72)(H,60,77)(H,61,73)(H,62,75)(H,63,76)/t30-,31+,36-,37-,38+,39-,40-,41-,44+,45-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
In vitro antioxycic activity with out Mg2+. |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50407336

(CHEMBL412616)Show SMILES CN1[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](CC(N)=O)C(=O)N[C@H](CSSC2(CCCCC2)CC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C52H74N14O12S2/c1-65-39(25-31-14-16-32(67)17-15-31)49(77)63-35(24-30-10-4-2-5-11-30)46(74)60-34(18-19-40(53)68)45(73)62-36(26-41(54)69)47(75)64-37(29-79-80-52(27-43(65)71)20-6-3-7-21-52)50(78)66-23-9-13-38(66)48(76)61-33(12-8-22-58-51(56)57)44(72)59-28-42(55)70/h2,4-5,10-11,14-17,33-39,67H,3,6-9,12-13,18-29H2,1H3,(H2,53,68)(H2,54,69)(H2,55,70)(H,59,72)(H,60,74)(H,61,76)(H,62,73)(H,63,77)(H,64,75)(H4,56,57,58)/t33-,34-,35+,36+,37+,38-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
In vitro activity was determinedfor the anti-oxytocic activity with 0.5 mM Mg2+. |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50407334

(CHEMBL1790313)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H](Cc2ccc(O)cc2)N(C)C(=O)CC2(CCCCC2)SSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC1=O)[C@@H](C)O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN)C(O)=O Show InChI InChI=1S/C45H69N9O12S2/c1-5-25(2)36-41(62)52-37(26(3)55)42(63)49-30(22-34(47)57)38(59)50-31(43(64)54-20-10-12-32(54)39(60)48-29(44(65)66)11-9-19-46)24-67-68-45(17-7-6-8-18-45)23-35(58)53(4)33(40(61)51-36)21-27-13-15-28(56)16-14-27/h13-16,25-26,29-33,36-37,55-56H,5-12,17-24,46H2,1-4H3,(H2,47,57)(H,48,60)(H,49,63)(H,50,59)(H,51,61)(H,52,62)(H,65,66)/t25-,26+,29-,30+,31+,32-,33-,36+,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 25.1 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
In vitro anti-oxytocic activity with 0.5 mM Mg2+. |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50407341

(CHEMBL264449)Show SMILES CCN1[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](CC(N)=O)C(=O)N[C@H](CSSC2(CCCCC2)CC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C53H77N13O11S2/c1-4-65-40(26-33-17-19-34(67)20-18-33)49(75)61-36(25-32-13-7-5-8-14-32)47(73)64-44(31(2)3)50(76)62-37(27-41(54)68)46(72)63-38(30-78-79-53(28-43(65)70)21-9-6-10-22-53)51(77)66-24-12-16-39(66)48(74)60-35(15-11-23-58-52(56)57)45(71)59-29-42(55)69/h5,7-8,13-14,17-20,31,35-40,44,67H,4,6,9-12,15-16,21-30H2,1-3H3,(H2,54,68)(H2,55,69)(H,59,71)(H,60,74)(H,61,75)(H,62,76)(H,63,72)(H,64,73)(H4,56,57,58)/t35-,36+,37+,38+,39-,40-,44-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
In vitro activity for the anti-oxytocic activity with out Mg2+. |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50030085
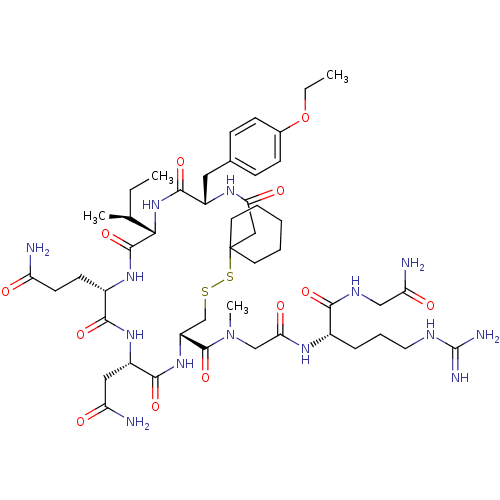
(CHEMBL415230 | [Mpa1,D-Tyr(OEt)2,Sar7]AVT)Show SMILES CCOc1ccc(C[C@@H]2NC(=O)CC3(CCCCC3)SSC[C@@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC2=O)[C@@H](C)CC)C(=O)N(C)CC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O)cc1 Show InChI InChI=1S/C48H76N14O12S2/c1-5-27(3)40-45(72)58-31(16-17-35(49)63)42(69)59-33(22-36(50)64)43(70)60-34(46(73)62(4)25-39(67)56-30(11-10-20-54-47(52)53)41(68)55-24-37(51)65)26-75-76-48(18-8-7-9-19-48)23-38(66)57-32(44(71)61-40)21-28-12-14-29(15-13-28)74-6-2/h12-15,27,30-34,40H,5-11,16-26H2,1-4H3,(H2,49,63)(H2,50,64)(H2,51,65)(H,55,68)(H,56,67)(H,57,66)(H,58,72)(H,59,69)(H,60,70)(H,61,71)(H4,52,53,54)/t27-,30-,31-,32-,33-,34+,40-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institut f£r Biophysik
Curated by ChEMBL
| Assay Description
Inhibition of radioligand [3H]OT binding to oxytocin receptor (OT) in guinea pig myometrium membrane |
J Med Chem 37: 255-9 (1994)
BindingDB Entry DOI: 10.7270/Q2H995TM |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50407335

(CHEMBL428187)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H](Cc2cc3ccccc3[nH]2)NC(=O)CC2(CCCCC2)SSC[C@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C50H75N15O11S2/c1-3-27(2)41-47(75)61-32(15-16-37(51)66)43(71)62-34(23-38(52)67)44(72)63-35(48(76)65-20-10-14-36(65)46(74)60-31(13-9-19-56-49(54)55)42(70)57-25-39(53)68)26-77-78-50(17-7-4-8-18-50)24-40(69)59-33(45(73)64-41)22-29-21-28-11-5-6-12-30(28)58-29/h5-6,11-12,21,27,31-36,41,58H,3-4,7-10,13-20,22-26H2,1-2H3,(H2,51,66)(H2,52,67)(H2,53,68)(H,57,70)(H,59,69)(H,60,74)(H,61,75)(H,62,71)(H,63,72)(H,64,73)(H4,54,55,56)/t27-,31-,32-,33-,34+,35-,36-,41+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 28.8 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
In vitro anti-oxytocic activity with 0.5 mM Mg2+. |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50407332

(CHEMBL405005)Show SMILES CCN1[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](CC(N)=O)C(=O)N[C@@H](CSSC2(CCCCC2)CC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C53H77N13O11S2/c1-4-65-40(26-33-17-19-34(67)20-18-33)49(75)61-36(25-32-13-7-5-8-14-32)47(73)64-44(31(2)3)50(76)62-37(27-41(54)68)46(72)63-38(30-78-79-53(28-43(65)70)21-9-6-10-22-53)51(77)66-24-12-16-39(66)48(74)60-35(15-11-23-58-52(56)57)45(71)59-29-42(55)69/h5,7-8,13-14,17-20,31,35-40,44,67H,4,6,9-12,15-16,21-30H2,1-3H3,(H2,54,68)(H2,55,69)(H,59,71)(H,60,74)(H,61,75)(H,62,76)(H,63,72)(H,64,73)(H4,56,57,58)/t35-,36+,37+,38-,39-,40-,44-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 29.5 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
In vitro activity was determinedfor the anti-oxytocic activity with 0.5 mM Mg2+. |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50407343

(CHEMBL412806)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H](Cc2cc3ccccc3[nH]2)NC(=O)CC2(CCCCC2)SSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C50H75N15O11S2/c1-3-27(2)41-47(75)61-32(15-16-37(51)66)43(71)62-34(23-38(52)67)44(72)63-35(48(76)65-20-10-14-36(65)46(74)60-31(13-9-19-56-49(54)55)42(70)57-25-39(53)68)26-77-78-50(17-7-4-8-18-50)24-40(69)59-33(45(73)64-41)22-29-21-28-11-5-6-12-30(28)58-29/h5-6,11-12,21,27,31-36,41,58H,3-4,7-10,13-20,22-26H2,1-2H3,(H2,51,66)(H2,52,67)(H2,53,68)(H,57,70)(H,59,69)(H,60,74)(H,61,75)(H,62,71)(H,63,72)(H,64,73)(H4,54,55,56)/t27-,31-,32-,33-,34+,35+,36-,41+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 31.6 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
In vitro anti-oxytocic activity with 0.5 mM Mg2+. |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50407344

(CHEMBL261914)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H](Cc2ccc(O)cc2)N(C)C(=O)CC2(CCCCC2)SSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN)C(=O)NCC(N)=O Show InChI InChI=1S/C48H74N12O12S2/c1-4-27(2)40-46(71)55-31(16-17-36(50)62)42(67)56-32(23-37(51)63)43(68)57-33(47(72)60-21-9-11-34(60)44(69)54-30(10-8-20-49)41(66)53-25-38(52)64)26-73-74-48(18-6-5-7-19-48)24-39(65)59(3)35(45(70)58-40)22-28-12-14-29(61)15-13-28/h12-15,27,30-35,40,61H,4-11,16-26,49H2,1-3H3,(H2,50,62)(H2,51,63)(H2,52,64)(H,53,66)(H,54,69)(H,55,71)(H,56,67)(H,57,68)(H,58,70)/t27-,30-,31-,32+,33+,34-,35-,40+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 36.3 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
In vitro anti-oxytocic activity with 0.5 mM Mg2+. |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50407349

(CHEMBL265357)Show SMILES CCN1[C@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](CC(N)=O)C(=O)N[C@H](CSSC2(CCCCC2)CC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C53H77N13O11S2/c1-4-65-40(26-33-17-19-34(67)20-18-33)49(75)61-36(25-32-13-7-5-8-14-32)47(73)64-44(31(2)3)50(76)62-37(27-41(54)68)46(72)63-38(30-78-79-53(28-43(65)70)21-9-6-10-22-53)51(77)66-24-12-16-39(66)48(74)60-35(15-11-23-58-52(56)57)45(71)59-29-42(55)69/h5,7-8,13-14,17-20,31,35-40,44,67H,4,6,9-12,15-16,21-30H2,1-3H3,(H2,54,68)(H2,55,69)(H,59,71)(H,60,74)(H,61,75)(H,62,76)(H,63,72)(H,64,73)(H4,56,57,58)/t35-,36+,37+,38+,39-,40+,44-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 37.1 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
In vitro activity for the anti-oxytocic activity with out Mg2+. |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data