 Found 979 hits of ic50 for UniProtKB: P17706
Found 979 hits of ic50 for UniProtKB: P17706 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50579997

(CHEMBL5085615)Show SMILES C[C@H](O)CCOc1ccc2cc(O)c(N3CC(=O)NS3(=O)=O)c(F)c2c1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PTPN2 (unknown origin) by Mobility shift assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00678
BindingDB Entry DOI: 10.7270/Q2SB49MH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50579998
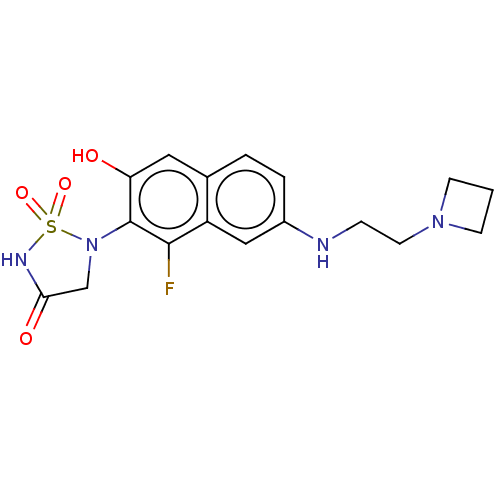
(CHEMBL5084112)Show SMILES Oc1cc2ccc(NCCN3CCC3)cc2c(F)c1N1CC(=O)NS1(=O)=O | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PTPN2 (unknown origin) by Mobility shift assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00678
BindingDB Entry DOI: 10.7270/Q2SB49MH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50579999
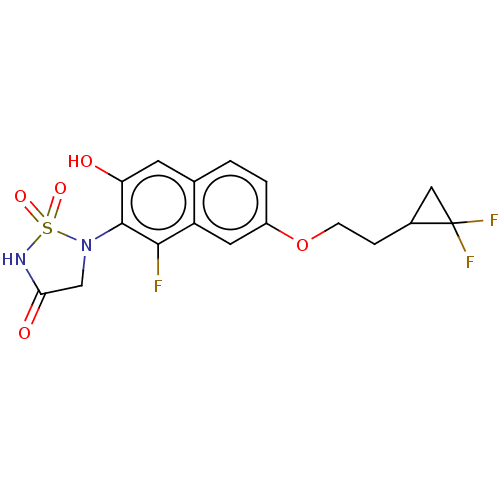
(CHEMBL5075269)Show SMILES Oc1cc2ccc(OCCC3CC3(F)F)cc2c(F)c1N1CC(=O)NS1(=O)=O | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PTPN2 (unknown origin) by Mobility shift assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00678
BindingDB Entry DOI: 10.7270/Q2SB49MH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50580000
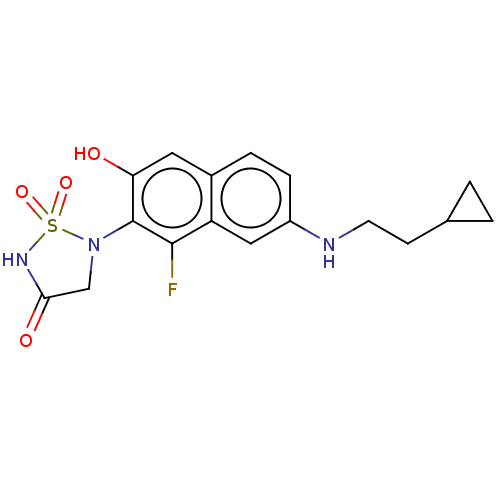
(CHEMBL5076419)Show SMILES Oc1cc2ccc(NCCC3CC3)cc2c(F)c1N1CC(=O)NS1(=O)=O | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PTPN2 (unknown origin) by Mobility shift assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00678
BindingDB Entry DOI: 10.7270/Q2SB49MH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50580001
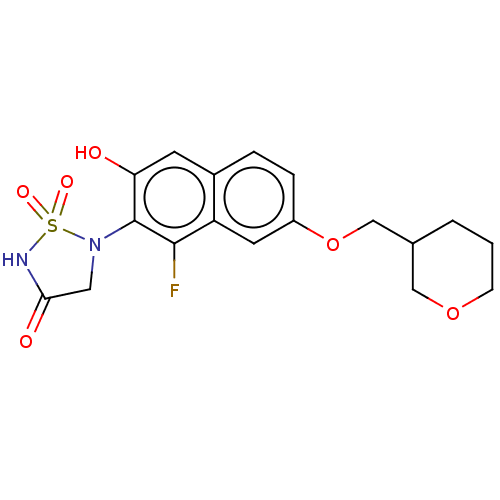
(CHEMBL5087544)Show SMILES Oc1cc2ccc(OCC3CCCOC3)cc2c(F)c1N1CC(=O)NS1(=O)=O | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PTPN2 (unknown origin) by Mobility shift assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00678
BindingDB Entry DOI: 10.7270/Q2SB49MH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50580002

(CHEMBL5084380)Show SMILES CCS(=O)(=O)N1CC=C(C1)c1ccc2cc(O)c(N3CC(=O)NS3(=O)=O)c(F)c2c1 |c:7| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PTPN2 (unknown origin) by Mobility shift assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00678
BindingDB Entry DOI: 10.7270/Q2SB49MH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50580003
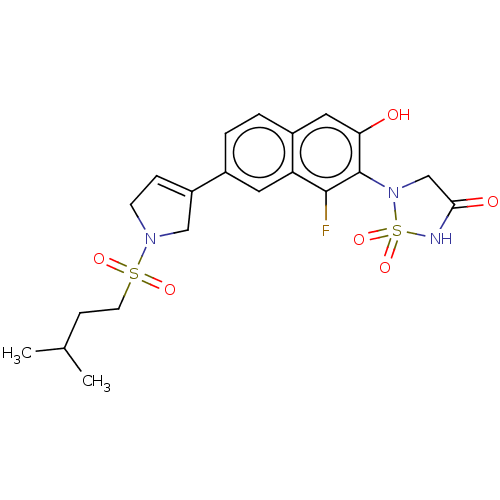
(CHEMBL5080612)Show SMILES CC(C)CCS(=O)(=O)N1CC=C(C1)c1ccc2cc(O)c(N3CC(=O)NS3(=O)=O)c(F)c2c1 |c:10| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PTPN2 (unknown origin) by Mobility shift assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00678
BindingDB Entry DOI: 10.7270/Q2SB49MH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50580005
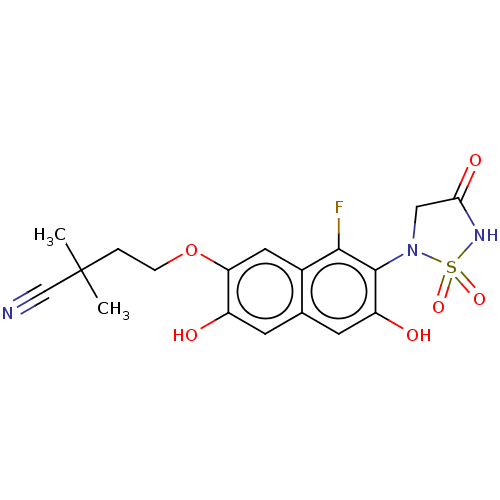
(CHEMBL5093253)Show SMILES CC(C)(CCOc1cc2c(F)c(N3CC(=O)NS3(=O)=O)c(O)cc2cc1O)C#N | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PTPN2 (unknown origin) by Mobility shift assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00678
BindingDB Entry DOI: 10.7270/Q2SB49MH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50579994
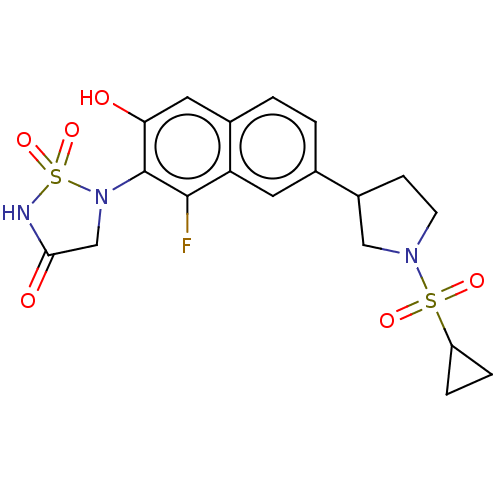
(CHEMBL5070507)Show SMILES Oc1cc2ccc(cc2c(F)c1N1CC(=O)NS1(=O)=O)C1CCN(C1)S(=O)(=O)C1CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PTPN2 (unknown origin) by Mobility shift assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00678
BindingDB Entry DOI: 10.7270/Q2SB49MH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50579995

(CHEMBL5085502)Show SMILES CC(C)(CCOc1ccc2cc(O)c(N3CC(=O)NS3(=O)=O)c(F)c2c1)C#N | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PTPN2 (unknown origin) by Mobility shift assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00678
BindingDB Entry DOI: 10.7270/Q2SB49MH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50579996

(CHEMBL5092308)Show SMILES Oc1cc2ccc(cc2c(F)c1N1CC(=O)NS1(=O)=O)-c1cnn(CC2CC2)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PTPN2 (unknown origin) by Mobility shift assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00678
BindingDB Entry DOI: 10.7270/Q2SB49MH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13599
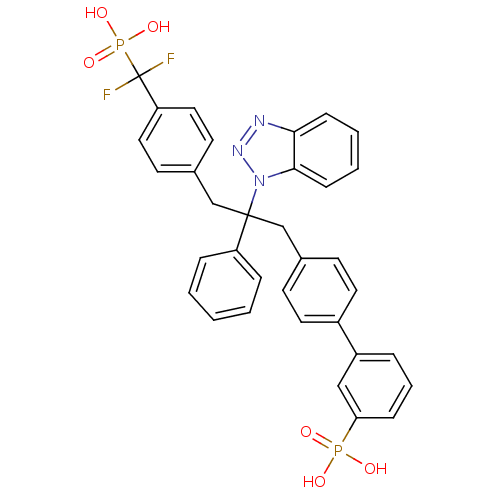
(3-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES OP(O)(=O)c1cccc(c1)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C34H29F2N3O6P2/c35-34(36,47(43,44)45)29-19-15-25(16-20-29)23-33(28-8-2-1-3-9-28,39-32-12-5-4-11-31(32)37-38-39)22-24-13-17-26(18-14-24)27-7-6-10-30(21-27)46(40,41)42/h1-21H,22-23H2,(H2,40,41,42)(H2,43,44,45) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP (unknown origin) by UV/Vis spectrophotometry |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13599

(3-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES OP(O)(=O)c1cccc(c1)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C34H29F2N3O6P2/c35-34(36,47(43,44)45)29-19-15-25(16-20-29)23-33(28-8-2-1-3-9-28,39-32-12-5-4-11-31(32)37-38-39)22-24-13-17-26(18-14-24)27-7-6-10-30(21-27)46(40,41)42/h1-21H,22-23H2,(H2,40,41,42)(H2,43,44,45) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories
| Assay Description
Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... |
Biochemistry 42: 11451-9 (2003)
Article DOI: 10.1021/bi035098j
BindingDB Entry DOI: 10.7270/Q2HX19XS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13599

(3-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES OP(O)(=O)c1cccc(c1)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C34H29F2N3O6P2/c35-34(36,47(43,44)45)29-19-15-25(16-20-29)23-33(28-8-2-1-3-9-28,39-32-12-5-4-11-31(32)37-38-39)22-24-13-17-26(18-14-24)27-7-6-10-30(21-27)46(40,41)42/h1-21H,22-23H2,(H2,40,41,42)(H2,43,44,45) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against T cell protein tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50142323
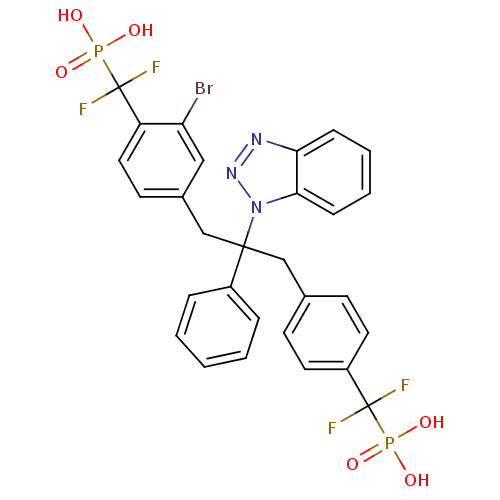
(CHEMBL267488 | [(4-{2-Benzotriazol-1-yl-3-[3-bromo...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(c(Br)c2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C29H24BrF4N3O6P2/c30-24-16-20(12-15-23(24)29(33,34)45(41,42)43)18-27(21-6-2-1-3-7-21,37-26-9-5-4-8-25(26)35-36-37)17-19-10-13-22(14-11-19)28(31,32)44(38,39)40/h1-16H,17-18H2,(H2,38,39,40)(H2,41,42,43) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against T cell protein tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13596

(({4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluoro...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccc(F)c(F)c2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C29H23F6N3O6P2/c30-23-14-13-22(15-24(23)31)27(38-26-4-2-1-3-25(26)36-37-38,16-18-5-9-20(10-6-18)28(32,33)45(39,40)41)17-19-7-11-21(12-8-19)29(34,35)46(42,43)44/h1-15H,16-17H2,(H2,39,40,41)(H2,42,43,44) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against T cell protein tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13595
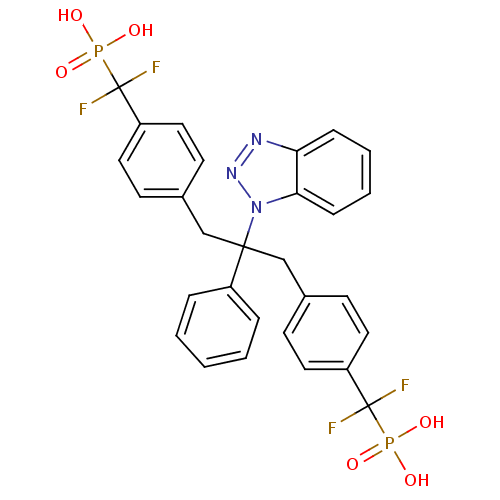
(({4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluoro...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C29H25F4N3O6P2/c30-28(31,43(37,38)39)23-14-10-20(11-15-23)18-27(22-6-2-1-3-7-22,36-26-9-5-4-8-25(26)34-35-36)19-21-12-16-24(17-13-21)29(32,33)44(40,41)42/h1-17H,18-19H2,(H2,37,38,39)(H2,40,41,42) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against T cell protein tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50580004
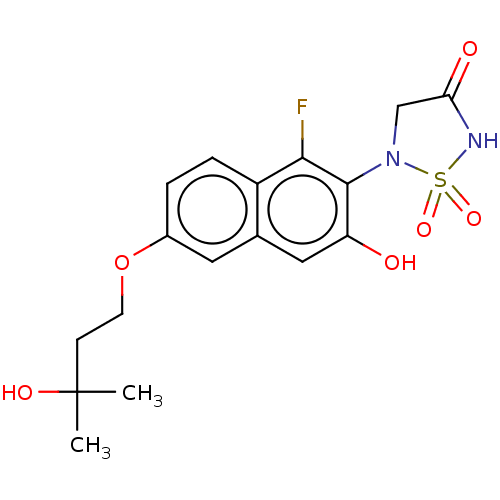
(CHEMBL5089047)Show SMILES CC(C)(O)CCOc1ccc2c(F)c(N3CC(=O)NS3(=O)=O)c(O)cc2c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PTPN2 (unknown origin) by Mobility shift assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00678
BindingDB Entry DOI: 10.7270/Q2SB49MH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50142335
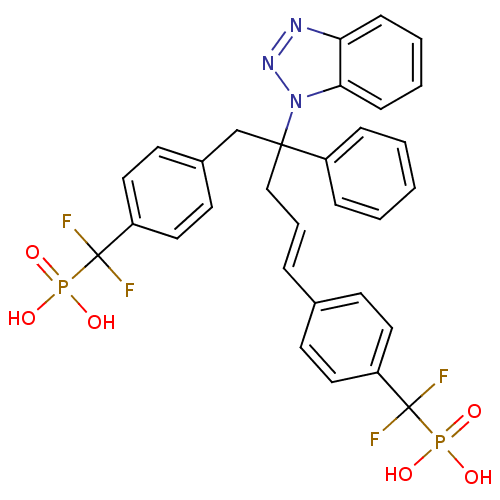
(CHEMBL274435 | [(4-{4-Benzotriazol-1-yl-5-[4-(difl...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(C\C=C\c2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C31H27F4N3O6P2/c32-30(33,45(39,40)41)25-16-12-22(13-17-25)7-6-20-29(24-8-2-1-3-9-24,38-28-11-5-4-10-27(28)36-37-38)21-23-14-18-26(19-15-23)31(34,35)46(42,43)44/h1-19H,20-21H2,(H2,39,40,41)(H2,42,43,44)/b7-6+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against T cell protein tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13595

(({4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluoro...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C29H25F4N3O6P2/c30-28(31,43(37,38)39)23-14-10-20(11-15-23)18-27(22-6-2-1-3-7-22,36-26-9-5-4-8-25(26)34-35-36)19-21-12-16-24(17-13-21)29(32,33)44(40,41)42/h1-17H,18-19H2,(H2,37,38,39)(H2,40,41,42) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories
| Assay Description
Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... |
Biochemistry 42: 11451-9 (2003)
Article DOI: 10.1021/bi035098j
BindingDB Entry DOI: 10.7270/Q2HX19XS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50115732
(CHEMBL294467 | Sodium vanadate) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human TCPTP using pNPP as substrate measured after 30 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112508
BindingDB Entry DOI: 10.7270/Q2QJ7N1M |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50391109

(CHEMBL179166 | Sodium orthovanadate (SOV) | Vanada...) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TCPTP assessed as inhibition of hydrolysis of p-nitrophenol after 10 mins by spectrophotometry |
Eur J Med Chem 57: 10-20 (2012)
Article DOI: 10.1016/j.ejmech.2012.09.015
BindingDB Entry DOI: 10.7270/Q2HX1DRS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50391109

(CHEMBL179166 | Sodium orthovanadate (SOV) | Vanada...) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Pharmacy, Shanghai Jiao Tong University, Shanghai 200240, PR China; China State Institute of Pharmaceutical Industry, Novel Technology Center of Pharmaceutical Chemistry, Shanghai Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant TCPTP (unknown origin) using pNPP as substrate measured after 30 mins |
Bioorg Med Chem Lett 27: 2166-2170 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.060
BindingDB Entry DOI: 10.7270/Q2FR0024 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50142328
(4-{1-Benzotriazol-1-yl-1-[4-(difluoro-phosphono-me...)Show SMILES COC(=O)c1ccc(cc1)C(Cc1ccc(cc1)C(F)(F)P(O)(O)=O)(Cc1ccc(cc1)C(F)(F)P(O)(O)=O)n1nnc2ccccc12 Show InChI InChI=1S/C31H27F4N3O8P2/c1-46-28(39)22-10-16-23(17-11-22)29(38-27-5-3-2-4-26(27)36-37-38,18-20-6-12-24(13-7-20)30(32,33)47(40,41)42)19-21-8-14-25(15-9-21)31(34,35)48(43,44)45/h2-17H,18-19H2,1H3,(H2,40,41,42)(H2,43,44,45) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against T cell protein tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50142324
(({4-[2-Benzotriazol-1-yl-3-[4-(difluoro-phosphono-...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2nnn[nH]2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C24H21F4N7O6P2/c25-23(26,42(36,37)38)17-9-5-15(6-10-17)13-22(21-30-32-33-31-21,35-20-4-2-1-3-19(20)29-34-35)14-16-7-11-18(12-8-16)24(27,28)43(39,40)41/h1-12H,13-14H2,(H2,36,37,38)(H2,39,40,41)(H,30,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against T cell protein tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13603
(6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES CC(C)CC(O)c1ccc2cc(cc(c2n1)P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C42H40F2N4O7P2/c1-27(2)22-38(49)36-21-18-31-23-32(24-39(40(31)45-36)56(50,51)52)30-16-12-28(13-17-30)25-41(33-8-4-3-5-9-33,48-37-11-7-6-10-35(37)46-47-48)26-29-14-19-34(20-15-29)42(43,44)57(53,54)55/h3-21,23-24,27,38,49H,22,25-26H2,1-2H3,(H2,50,51,52)(H2,53,54,55) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories
| Assay Description
Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... |
Biochemistry 42: 11451-9 (2003)
Article DOI: 10.1021/bi035098j
BindingDB Entry DOI: 10.7270/Q2HX19XS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50391109

(CHEMBL179166 | Sodium orthovanadate (SOV) | Vanada...) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University (SJTU)
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP (unknown origin) using pNPP as substrate after 30 mins |
Eur J Med Chem 164: 408-422 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.032
BindingDB Entry DOI: 10.7270/Q2KK9G90 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50142329
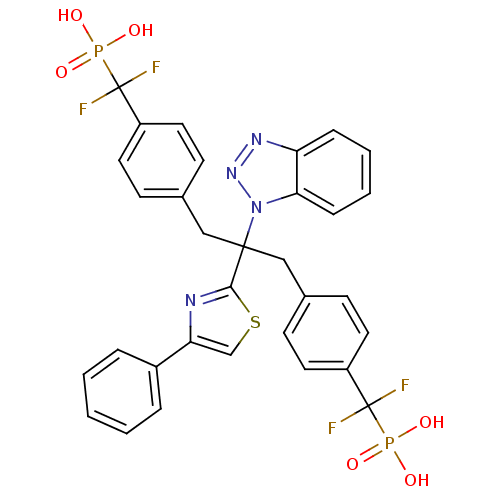
(({4-[2-Benzotriazol-1-yl-3-[4-(difluoro-phosphono-...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2nc(cs2)-c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C32H26F4N4O6P2S/c33-31(34,47(41,42)43)24-14-10-21(11-15-24)18-30(40-28-9-5-4-8-26(28)38-39-40,29-37-27(20-49-29)23-6-2-1-3-7-23)19-22-12-16-25(17-13-22)32(35,36)48(44,45)46/h1-17,20H,18-19H2,(H2,41,42,43)(H2,44,45,46) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against T cell protein tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13602

(6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES Cc1ccc2cc(cc(c2n1)P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C38H32F2N4O6P2/c1-25-11-16-29-21-30(22-35(36(29)41-25)51(45,46)47)28-17-12-26(13-18-28)23-37(31-7-3-2-4-8-31,44-34-10-6-5-9-33(34)42-43-44)24-27-14-19-32(20-15-27)38(39,40)52(48,49)50/h2-22H,23-24H2,1H3,(H2,45,46,47)(H2,48,49,50) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against T cell protein tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13601
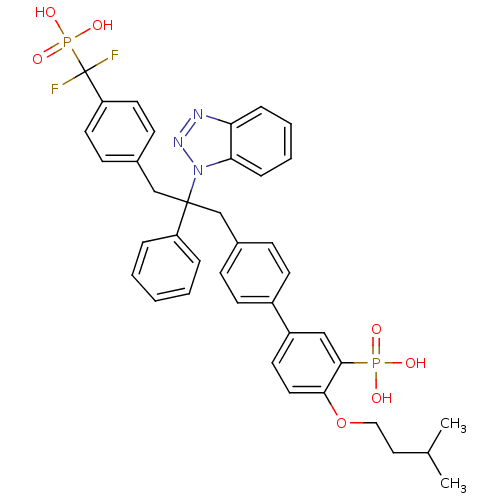
(5-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES CC(C)CCOc1ccc(cc1P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C39H39F2N3O7P2/c1-27(2)22-23-51-36-21-18-31(24-37(36)52(45,46)47)30-16-12-28(13-17-30)25-38(32-8-4-3-5-9-32,44-35-11-7-6-10-34(35)42-43-44)26-29-14-19-33(20-15-29)39(40,41)53(48,49)50/h3-21,24,27H,22-23,25-26H2,1-2H3,(H2,45,46,47)(H2,48,49,50) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories
| Assay Description
Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... |
Biochemistry 42: 11451-9 (2003)
Article DOI: 10.1021/bi035098j
BindingDB Entry DOI: 10.7270/Q2HX19XS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50142330
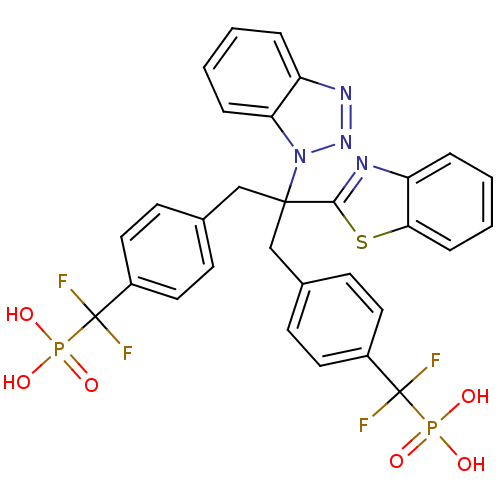
(CHEMBL8662 | [(4-{2-Benzothiazol-2-yl-2-benzotriaz...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2nc3ccccc3s2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C30H24F4N4O6P2S/c31-29(32,45(39,40)41)21-13-9-19(10-14-21)17-28(27-35-24-6-2-4-8-26(24)47-27,38-25-7-3-1-5-23(25)36-37-38)18-20-11-15-22(16-12-20)30(33,34)46(42,43)44/h1-16H,17-18H2,(H2,39,40,41)(H2,42,43,44) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against T cell protein tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13602

(6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES Cc1ccc2cc(cc(c2n1)P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C38H32F2N4O6P2/c1-25-11-16-29-21-30(22-35(36(29)41-25)51(45,46)47)28-17-12-26(13-18-28)23-37(31-7-3-2-4-8-31,44-34-10-6-5-9-33(34)42-43-44)24-27-14-19-32(20-15-27)38(39,40)52(48,49)50/h2-22H,23-24H2,1H3,(H2,45,46,47)(H2,48,49,50) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories
| Assay Description
Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... |
Biochemistry 42: 11451-9 (2003)
Article DOI: 10.1021/bi035098j
BindingDB Entry DOI: 10.7270/Q2HX19XS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13605
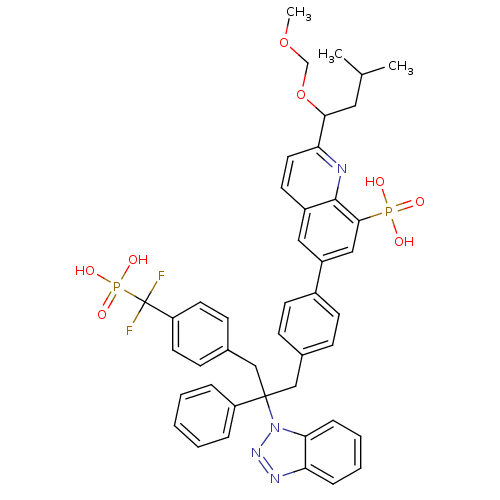
(6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES COCOC(CC(C)C)c1ccc2cc(cc(c2n1)P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C44H44F2N4O8P2/c1-29(2)23-40(58-28-57-3)38-22-19-33-24-34(25-41(42(33)47-38)59(51,52)53)32-17-13-30(14-18-32)26-43(35-9-5-4-6-10-35,50-39-12-8-7-11-37(39)48-49-50)27-31-15-20-36(21-16-31)44(45,46)60(54,55)56/h4-22,24-25,29,40H,23,26-28H2,1-3H3,(H2,51,52,53)(H2,54,55,56) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories
| Assay Description
Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... |
Biochemistry 42: 11451-9 (2003)
Article DOI: 10.1021/bi035098j
BindingDB Entry DOI: 10.7270/Q2HX19XS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13598
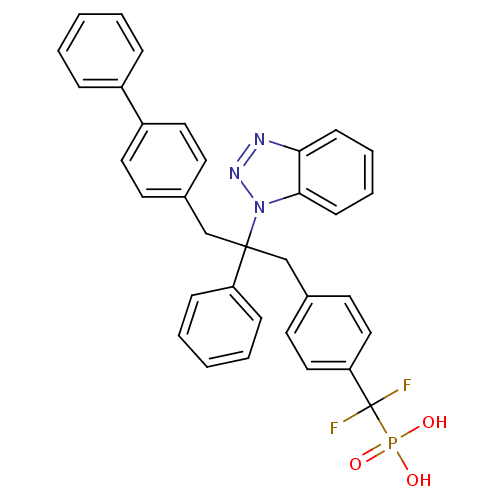
(({4-[2-(1H-1,2,3-benzotriazol-1-yl)-2-phenyl-3-(4-...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)-c2ccccc2)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C34H28F2N3O3P/c35-34(36,43(40,41)42)30-21-17-26(18-22-30)24-33(29-11-5-2-6-12-29,39-32-14-8-7-13-31(32)37-38-39)23-25-15-19-28(20-16-25)27-9-3-1-4-10-27/h1-22H,23-24H2,(H2,40,41,42) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against T cell protein tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50228024
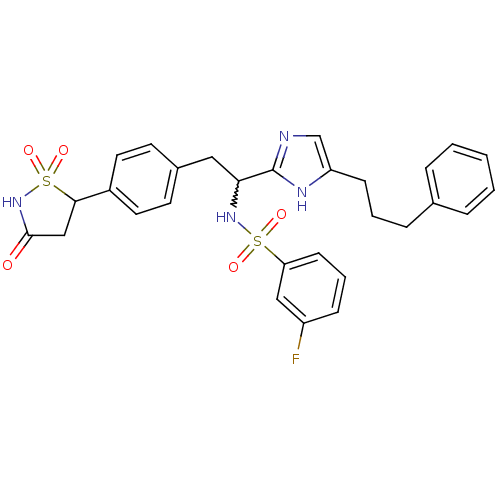
(3-fluoro-N-{1-[5-(3-phenyl-propyl)-1H-imidazol-2-y...)Show SMILES Fc1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)c1ncc(CCCc2ccccc2)[nH]1 |w:11.11| Show InChI InChI=1S/C29H29FN4O5S2/c30-23-9-5-11-25(17-23)40(36,37)33-26(29-31-19-24(32-29)10-4-8-20-6-2-1-3-7-20)16-21-12-14-22(15-13-21)27-18-28(35)34-41(27,38)39/h1-3,5-7,9,11-15,17,19,26-27,33H,4,8,10,16,18H2,(H,31,32)(H,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13598

(({4-[2-(1H-1,2,3-benzotriazol-1-yl)-2-phenyl-3-(4-...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)-c2ccccc2)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C34H28F2N3O3P/c35-34(36,43(40,41)42)30-21-17-26(18-22-30)24-33(29-11-5-2-6-12-29,39-32-14-8-7-13-31(32)37-38-39)23-25-15-19-28(20-16-25)27-9-3-1-4-10-27/h1-22H,23-24H2,(H2,40,41,42) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories
| Assay Description
Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... |
Biochemistry 42: 11451-9 (2003)
Article DOI: 10.1021/bi035098j
BindingDB Entry DOI: 10.7270/Q2HX19XS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2 [V121L]
(Homo sapiens (Human)) | BDBM13814

(({4-[(4E)-2-(1H-1,2,3-benzotriazol-1-yl)-2-[4-(met...)Show SMILES COC(=O)c1ccc(cc1)C(C\C=C\c1ccccc1)(Cc1ccc(cc1)C(F)(F)P(O)(O)=O)n1nnc2ccccc12 Show InChI InChI=1S/C32H28F2N3O5P/c1-42-30(38)25-15-19-26(20-16-25)31(21-7-10-23-8-3-2-4-9-23,37-29-12-6-5-11-28(29)35-36-37)22-24-13-17-27(18-14-24)32(33,34)43(39,40)41/h2-20H,21-22H2,1H3,(H2,39,40,41)/b10-7+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Frosst Center for Therapeutic Research
| Assay Description
Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... |
J Biol Chem 281: 8010-5 (2006)
Article DOI: 10.1074/jbc.M511827200
BindingDB Entry DOI: 10.7270/Q2C53J3V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50308844
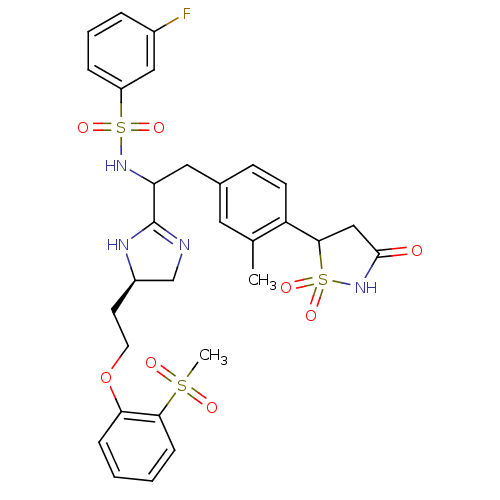
(3-Fluoro-N-{1-{(R)-4-[2-(2-methanesulfonyl-phenoxy...)Show SMILES Cc1cc(CC(NS(=O)(=O)c2cccc(F)c2)C2=NC[C@@H](CCOc3ccccc3S(C)(=O)=O)N2)ccc1C1CC(=O)NS1(=O)=O |r,t:18| Show InChI InChI=1S/C30H33FN4O8S3/c1-19-14-20(10-11-24(19)28-17-29(36)35-46(28,41)42)15-25(34-45(39,40)23-7-5-6-21(31)16-23)30-32-18-22(33-30)12-13-43-26-8-3-4-9-27(26)44(2,37)38/h3-11,14,16,22,25,28,34H,12-13,15,17-18H2,1-2H3,(H,32,33)(H,35,36)/t22-,25?,28?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM14269
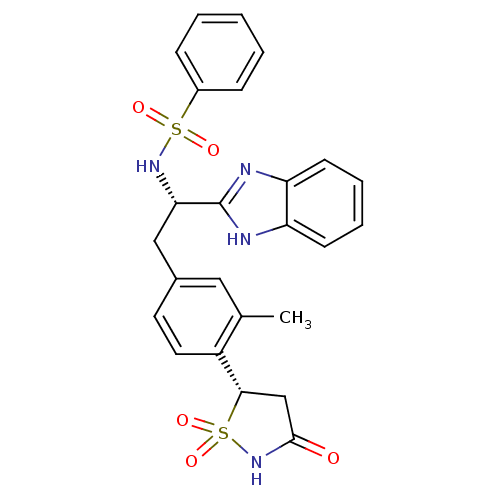
((S)-isothiazolidinone | IZD deriv. 2 | N-[(1S)-1-(...)Show SMILES Cc1cc(C[C@H](NS(=O)(=O)c2ccccc2)c2nc3ccccc3[nH]2)ccc1[C@@H]1CC(=O)NS1(=O)=O |r| Show InChI InChI=1S/C25H24N4O5S2/c1-16-13-17(11-12-19(16)23-15-24(30)29-36(23,33)34)14-22(25-26-20-9-5-6-10-21(20)27-25)28-35(31,32)18-7-3-2-4-8-18/h2-13,22-23,28H,14-15H2,1H3,(H,26,27)(H,29,30)/t22-,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50228013
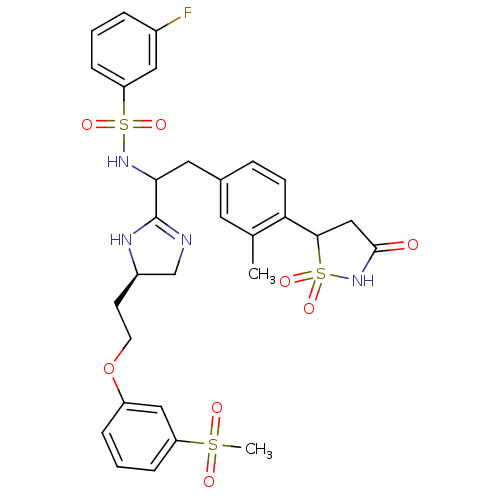
(3-fluoro-N-{1-{(R)-5-[2-(3-methanesulfonyl-phenoxy...)Show SMILES Cc1cc(CC(NS(=O)(=O)c2cccc(F)c2)C2=NC[C@@H](CCOc3cccc(c3)S(C)(=O)=O)N2)ccc1C1CC(=O)NS1(=O)=O |t:18| Show InChI InChI=1S/C30H33FN4O8S3/c1-19-13-20(9-10-26(19)28-17-29(36)35-46(28,41)42)14-27(34-45(39,40)25-8-3-5-21(31)15-25)30-32-18-22(33-30)11-12-43-23-6-4-7-24(16-23)44(2,37)38/h3-10,13,15-16,22,27-28,34H,11-12,14,17-18H2,1-2H3,(H,32,33)(H,35,36)/t22-,27?,28?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM199179
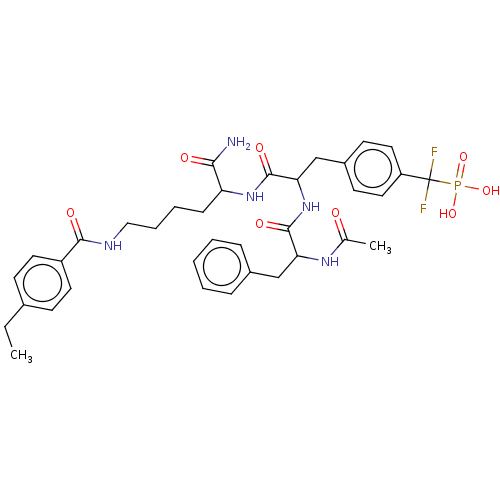
(US9217012, 8)Show SMILES CCc1ccc(cc1)C(=O)NCCCCC(NC(=O)C(Cc1ccc(cc1)C(F)(F)P(O)(O)=O)NC(=O)C(Cc1ccccc1)NC(C)=O)C(N)=O Show InChI InChI=1S/C36H44F2N5O8P/c1-3-24-12-16-27(17-13-24)33(46)40-20-8-7-11-29(32(39)45)42-35(48)31(22-26-14-18-28(19-15-26)36(37,38)52(49,50)51)43-34(47)30(41-23(2)44)21-25-9-5-4-6-10-25/h4-6,9-10,12-19,29-31H,3,7-8,11,20-22H2,1-2H3,(H2,39,45)(H,40,46)(H,41,44)(H,42,48)(H,43,47)(H2,49,50,51) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Indiana University Research and Technology Corporation
US Patent
| Assay Description
PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ... |
US Patent US9217012 (2015)
BindingDB Entry DOI: 10.7270/Q2FX788H |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50171117

(2-(4-(N-(3-bromo-4-(difluoro(phosphono)methyl)benz...)Show SMILES CN(C)S(=O)(=O)c1ccc(CN(Cc2ccc(c(Br)c2)C(F)(F)P(O)(O)=O)S(=O)(=O)c2ccc(OCC(O)=O)cc2)cc1 Show InChI InChI=1S/C25H26BrF2N2O10PS2/c1-29(2)42(36,37)20-8-3-17(4-9-20)14-30(43(38,39)21-10-6-19(7-11-21)40-16-24(31)32)15-18-5-12-22(23(26)13-18)25(27,28)41(33,34)35/h3-13H,14-16H2,1-2H3,(H,31,32)(H2,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50142325
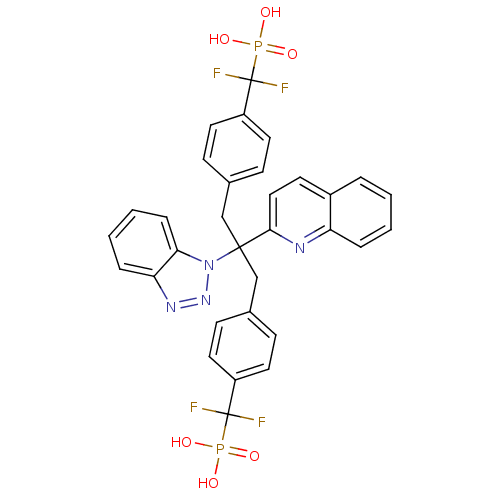
(CHEMBL273474 | [(4-{2-Benzotriazol-1-yl-3-[4-(difl...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccc3ccccc3n2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C32H26F4N4O6P2/c33-31(34,47(41,42)43)24-14-9-21(10-15-24)19-30(40-28-8-4-3-7-27(28)38-39-40,29-18-13-23-5-1-2-6-26(23)37-29)20-22-11-16-25(17-12-22)32(35,36)48(44,45)46/h1-18H,19-20H2,(H2,41,42,43)(H2,44,45,46) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against T cell protein tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13600
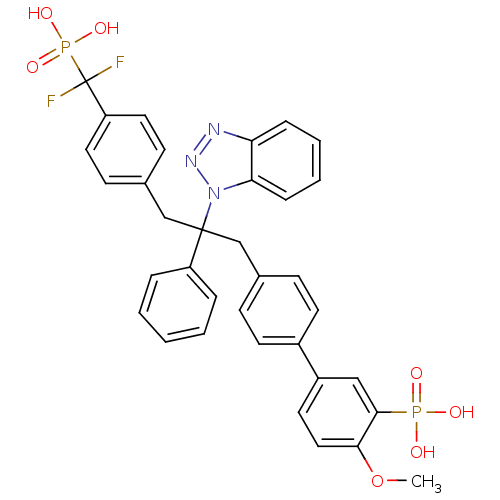
(5-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES COc1ccc(cc1P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C35H31F2N3O7P2/c1-47-32-20-17-27(21-33(32)48(41,42)43)26-15-11-24(12-16-26)22-34(28-7-3-2-4-8-28,40-31-10-6-5-9-30(31)38-39-40)23-25-13-18-29(19-14-25)35(36,37)49(44,45)46/h2-21H,22-23H2,1H3,(H2,41,42,43)(H2,44,45,46) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories
| Assay Description
Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... |
Biochemistry 42: 11451-9 (2003)
Article DOI: 10.1021/bi035098j
BindingDB Entry DOI: 10.7270/Q2HX19XS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50308854

(CHEMBL590235 | [7-(4-{1-Benzotriazol-1-yl-2-[4-(di...)Show SMILES COC(CC(C)C)c1ccc2ccc(-c3ccc(cc3)C(Cc3ccc(cc3)C(F)(F)P(O)(O)=O)(c3ccccc3)n3nnc4ccccc34)c(c2n1)P(O)(O)=O Show InChI InChI=1S/C42H40F2N4O7P2/c1-27(2)25-38(55-3)36-24-18-30-17-23-34(40(39(30)45-36)56(49,50)51)29-15-21-32(22-16-29)41(31-9-5-4-6-10-31,48-37-12-8-7-11-35(37)46-47-48)26-28-13-19-33(20-14-28)42(43,44)57(52,53)54/h4-24,27,38H,25-26H2,1-3H3,(H2,49,50,51)(H2,52,53,54) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13604

(6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES COC(CC(C)C)c1ccc2cc(cc(c2n1)P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C43H42F2N4O7P2/c1-28(2)23-39(56-3)37-22-19-32-24-33(25-40(41(32)46-37)57(50,51)52)31-17-13-29(14-18-31)26-42(34-9-5-4-6-10-34,49-38-12-8-7-11-36(38)47-48-49)27-30-15-20-35(21-16-30)43(44,45)58(53,54)55/h4-22,24-25,28,39H,23,26-27H2,1-3H3,(H2,50,51,52)(H2,53,54,55) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against T cell protein tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50535627
(CHEMBL4542052)Show SMILES COC(CC(C)C)c1ccc2ccc(-c3ccc(CC(Cc4ccc(cc4)C(F)(F)P(O)(O)=O)(c4ccccc4)n4nnc5ccccc45)cc3)c(c2n1)P(O)(O)=O Show InChI InChI=1S/C43H42F2N4O7P2/c1-28(2)25-39(56-3)37-24-20-32-19-23-35(41(40(32)46-37)57(50,51)52)31-17-13-29(14-18-31)26-42(33-9-5-4-6-10-33,49-38-12-8-7-11-36(38)47-48-49)27-30-15-21-34(22-16-30)43(44,45)58(53,54)55/h4-24,28,39H,25-27H2,1-3H3,(H2,50,51,52)(H2,53,54,55) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Sci-Tech University
Curated by ChEMBL
| Assay Description
Inhibition of human FLAG-tagged TCPTP (1 to 296) expressed in Escherichia coli using fluorescein diphosphate as substrate by fluorescence based metho... |
Bioorg Med Chem Lett 29: 2358-2363 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.011
BindingDB Entry DOI: 10.7270/Q2280C4B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13604

(6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES COC(CC(C)C)c1ccc2cc(cc(c2n1)P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C43H42F2N4O7P2/c1-28(2)23-39(56-3)37-22-19-32-24-33(25-40(41(32)46-37)57(50,51)52)31-17-13-29(14-18-31)26-42(34-9-5-4-6-10-34,49-38-12-8-7-11-36(38)47-48-49)27-30-15-20-35(21-16-30)43(44,45)58(53,54)55/h4-22,24-25,28,39H,23,26-27H2,1-3H3,(H2,50,51,52)(H2,53,54,55) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories
| Assay Description
Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... |
Biochemistry 42: 11451-9 (2003)
Article DOI: 10.1021/bi035098j
BindingDB Entry DOI: 10.7270/Q2HX19XS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13604

(6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES COC(CC(C)C)c1ccc2cc(cc(c2n1)P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C43H42F2N4O7P2/c1-28(2)23-39(56-3)37-22-19-32-24-33(25-40(41(32)46-37)57(50,51)52)31-17-13-29(14-18-31)26-42(34-9-5-4-6-10-34,49-38-12-8-7-11-36(38)47-48-49)27-30-15-20-35(21-16-30)43(44,45)58(53,54)55/h4-22,24-25,28,39H,23,26-27H2,1-3H3,(H2,50,51,52)(H2,53,54,55) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP (unknown origin) by UV/Vis spectrophotometry |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50228038

(3-fluoro-N-{1-[(R)-5-(2-fluoro-benzyl)-4,5-dihydro...)Show SMILES Fc1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)C1=NC[C@@H](Cc2ccccc2F)N1 |w:11.11,t:30| Show InChI InChI=1S/C27H26F2N4O5S2/c28-20-5-3-6-22(14-20)39(35,36)32-24(27-30-16-21(31-27)13-19-4-1-2-7-23(19)29)12-17-8-10-18(11-9-17)25-15-26(34)33-40(25,37)38/h1-11,14,21,24-25,32H,12-13,15-16H2,(H,30,31)(H,33,34)/t21-,24?,25?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data