 Found 17 hits of ki for UniProtKB: P29350
Found 17 hits of ki for UniProtKB: P29350 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 6
(Homo sapiens (Human)) | BDBM50299153

((Z)-5-{2-[3,5-bis(trifluoromethyl)benzyloxy]-5-bro...)Show SMILES OC1=NC(=O)C(S1)=Cc1cc(Br)ccc1OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |w:7.8,t:1| Show InChI InChI=1S/C19H10BrF6NO3S/c20-13-1-2-14(10(5-13)6-15-16(28)27-17(29)31-15)30-8-9-3-11(18(21,22)23)7-12(4-9)19(24,25)26/h1-7H,8H2,(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University
Curated by ChEMBL
| Assay Description
Inhibition of SHP1 catalytic domain expressed in Escherichia coli |
Bioorg Med Chem Lett 19: 6161-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.020
BindingDB Entry DOI: 10.7270/Q20P102S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 6
(Homo sapiens (Human)) | BDBM50219479

(3,3'-dicarboxy-2,2'-dihydroxy-5,5'-diphenyldipheny...)Show SMILES OC(=O)c1cc(cc(Cc2cc(cc(C(O)=O)c2O)-c2ccccc2)c1O)-c1ccccc1 Show InChI InChI=1S/C27H20O6/c28-24-20(11-18(14-22(24)26(30)31)16-7-3-1-4-8-16)13-21-12-19(17-9-5-2-6-10-17)15-23(25(21)29)27(32)33/h1-12,14-15,28-29H,13H2,(H,30,31)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University
Curated by ChEMBL
| Assay Description
Inhibition of SHP-1 catalytic domain |
Bioorg Med Chem 15: 6535-48 (2007)
Article DOI: 10.1016/j.bmc.2007.07.010
BindingDB Entry DOI: 10.7270/Q2GM871J |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 6
(Homo sapiens (Human)) | BDBM50208362
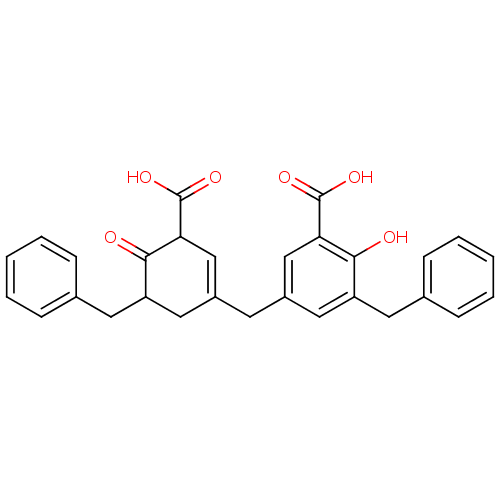
(3-benzyl-5-[(5-benzyl-3-carboxy-4-hydroxycyclohexa...)Show SMILES OC(=O)C1C=C(Cc2cc(Cc3ccccc3)c(O)c(c2)C(O)=O)CC(Cc2ccccc2)C1=O |t:4| Show InChI InChI=1S/C29H26O6/c30-26-22(12-18-7-3-1-4-8-18)14-20(16-24(26)28(32)33)11-21-15-23(13-19-9-5-2-6-10-19)27(31)25(17-21)29(34)35/h1-10,14,16-17,23,25,30H,11-13,15H2,(H,32,33)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University
Curated by ChEMBL
| Assay Description
Inhibition of SHP-1 catalytic domain |
Bioorg Med Chem Lett 17: 2760-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.069
BindingDB Entry DOI: 10.7270/Q2445N91 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 6
(Homo sapiens (Human)) | BDBM50118796

(6-(OXALYL-AMINO)-1H-INDOLE-5-CARBOXYLIC ACID | 6-(...)Show InChI InChI=1S/C11H8N2O5/c14-9(11(17)18)13-8-4-7-5(1-2-12-7)3-6(8)10(15)16/h1-4,12H,(H,13,14)(H,15,16)(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN6 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 6
(Homo sapiens (Human)) | BDBM50208359
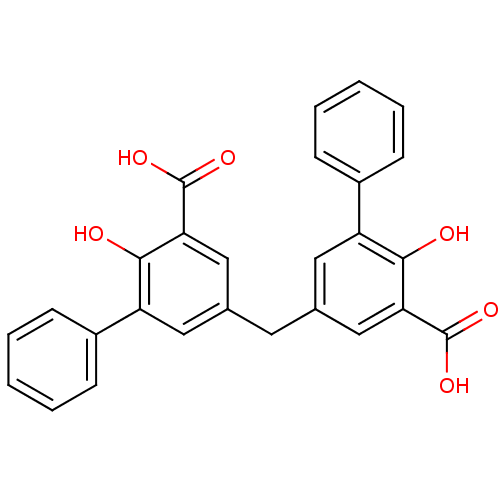
(5,5'-methylenebis(2-hydroxybiphenyl-3-carboxylic a...)Show SMILES OC(=O)c1cc(Cc2cc(C(O)=O)c(O)c(c2)-c2ccccc2)cc(c1O)-c1ccccc1 Show InChI InChI=1S/C27H20O6/c28-24-20(18-7-3-1-4-8-18)12-16(14-22(24)26(30)31)11-17-13-21(19-9-5-2-6-10-19)25(29)23(15-17)27(32)33/h1-10,12-15,28-29H,11H2,(H,30,31)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University
Curated by ChEMBL
| Assay Description
Inhibition of SHP-1 catalytic domain |
Bioorg Med Chem Lett 17: 2760-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.069
BindingDB Entry DOI: 10.7270/Q2445N91 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 6
(Homo sapiens (Human)) | BDBM50118751
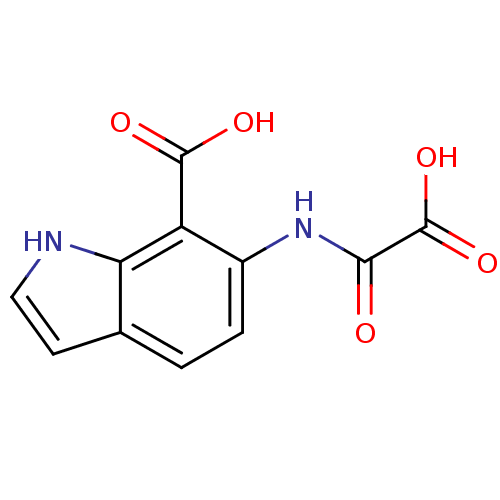
(6-(Oxalyl-amino)-1H-indole-7-carboxylic acid | 6-(...)Show InChI InChI=1S/C11H8N2O5/c14-9(11(17)18)13-6-2-1-5-3-4-12-8(5)7(6)10(15)16/h1-4,12H,(H,13,14)(H,15,16)(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN6 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 6
(Homo sapiens (Human)) | BDBM50119679

(2-[(S)-2-(2-{2-[4-(2-Bromo-acetyl)-phenoxy]-acetyl...)Show SMILES OC(=O)CCC(NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)COc1ccc(cc1)C(=O)CBr)C(O)=O Show InChI InChI=1S/C22H26BrN3O11/c23-9-16(27)12-1-3-13(4-2-12)37-11-18(29)24-10-17(28)25-14(5-7-19(30)31)21(34)26-15(22(35)36)6-8-20(32)33/h1-4,14-15H,5-11H2,(H,24,29)(H,25,28)(H,26,34)(H,30,31)(H,32,33)(H,35,36)/t14-,15?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Dissociation constant of the compound towards SHP 1 receptor-inhibitor complex was determined using PNP as substrate |
Bioorg Med Chem Lett 12: 3047-50 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8KCX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 6
(Homo sapiens (Human)) | BDBM50118749
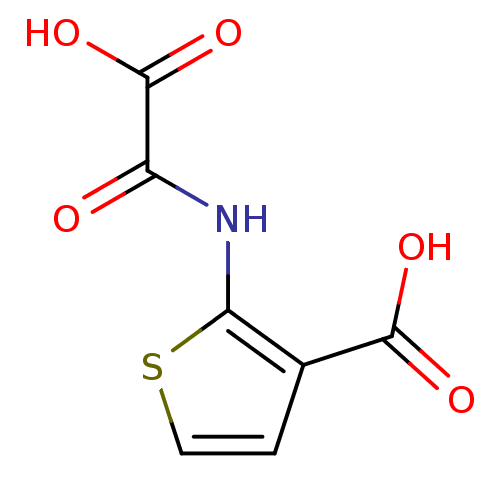
(2-(Oxalyl-amino)-thiophene-3-carboxylic acid | 2-(...)Show InChI InChI=1S/C7H5NO5S/c9-4(7(12)13)8-5-3(6(10)11)1-2-14-5/h1-2H,(H,8,9)(H,10,11)(H,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN6 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 6
(Homo sapiens (Human)) | BDBM50118778

(5-iodo-2-(oxaloamino)benzoic acid | CHEMBL336908)Show InChI InChI=1S/C9H6INO5/c10-4-1-2-6(5(3-4)8(13)14)11-7(12)9(15)16/h1-3H,(H,11,12)(H,13,14)(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN6 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 6
(Homo sapiens (Human)) | BDBM50299462

(2-(3-(dihydroxymethyl)naphthalen-2-ylamino)-2-oxoa...)Show InChI InChI=1S/C13H11NO5/c15-11(13(18)19)14-10-6-8-4-2-1-3-7(8)5-9(10)12(16)17/h1-6,12,16-17H,(H,14,15)(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 9.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN6 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 6
(Homo sapiens (Human)) | BDBM50118762
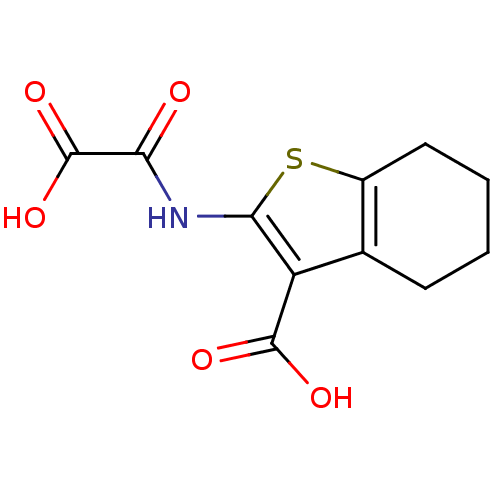
(2-(Oxalyl-amino)-4,5,6,7-tetrahydro-benzo[b]thioph...)Show InChI InChI=1S/C11H11NO5S/c13-8(11(16)17)12-9-7(10(14)15)5-3-1-2-4-6(5)18-9/h1-4H2,(H,12,13)(H,14,15)(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN6 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 6
(Homo sapiens (Human)) | BDBM50119683

(2-[(S)-2-(2-{[4'-(2-Bromo-acetyl)-biphenyl-2-carbo...)Show SMILES OC(=O)CCC(NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)c1ccccc1-c1ccc(cc1)C(=O)CBr)C(O)=O Show InChI InChI=1S/C27H28BrN3O10/c28-13-21(32)16-7-5-15(6-8-16)17-3-1-2-4-18(17)25(38)29-14-22(33)30-19(9-11-23(34)35)26(39)31-20(27(40)41)10-12-24(36)37/h1-8,19-20H,9-14H2,(H,29,38)(H,30,33)(H,31,39)(H,34,35)(H,36,37)(H,40,41)/t19-,20?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Dissociation constant of the compound towards SHP 1 receptor-inhibitor complex was determined using PNP as substrate |
Bioorg Med Chem Lett 12: 3047-50 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8KCX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 6
(Homo sapiens (Human)) | BDBM50299461
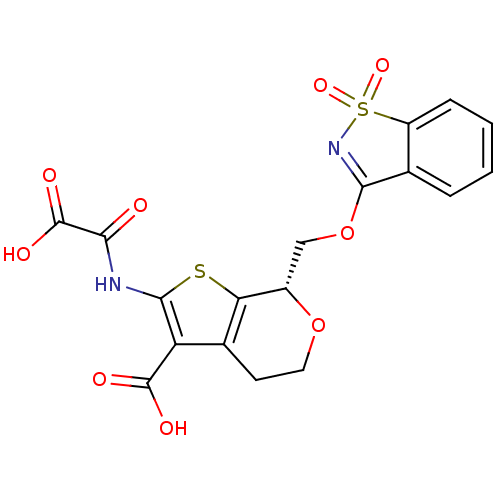
((S)-7-(1,1-Dioxo-1H-1lambda*6*-benzo[d]isothiazol-...)Show SMILES OC(=O)C(=O)Nc1sc2[C@H](COC3=NS(=O)(=O)c4ccccc34)OCCc2c1C(O)=O |r,t:12| Show InChI InChI=1S/C18H14N2O9S2/c21-14(18(24)25)19-16-12(17(22)23)9-5-6-28-10(13(9)30-16)7-29-15-8-3-1-2-4-11(8)31(26,27)20-15/h1-4,10H,5-7H2,(H,19,21)(H,22,23)(H,24,25)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 2.89E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN6 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 6
(Homo sapiens (Human)) | BDBM50118744
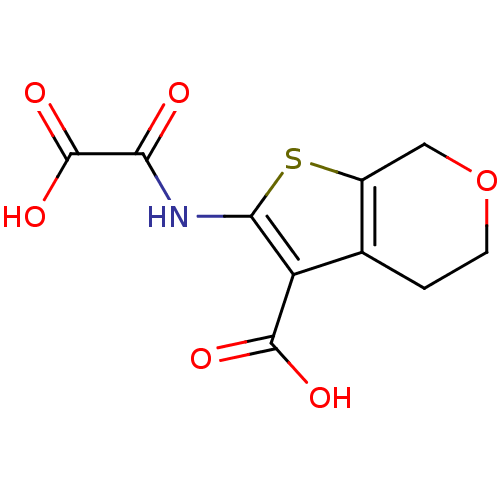
(2-(Oxalyl-amino)-4,7-dihydro-5H-thieno[2,3-c]pyran...)Show InChI InChI=1S/C10H9NO6S/c12-7(10(15)16)11-8-6(9(13)14)4-1-2-17-3-5(4)18-8/h1-3H2,(H,11,12)(H,13,14)(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 3.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN6 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 6
(Homo sapiens (Human)) | BDBM50118789
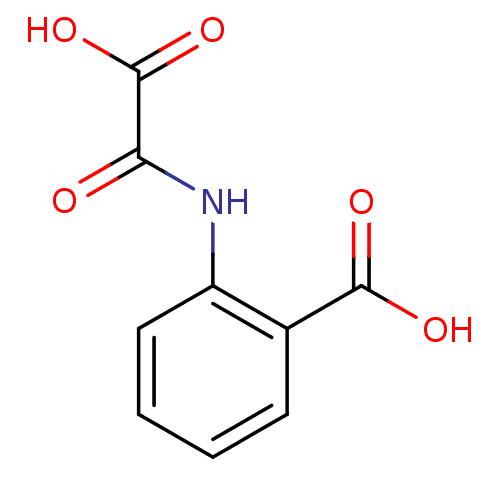
(2-(oxaloamino)benzoic acid | CHEMBL139050)Show InChI InChI=1S/C9H7NO5/c11-7(9(14)15)10-6-4-2-1-3-5(6)8(12)13/h1-4H,(H,10,11)(H,12,13)(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN6 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 6
(Homo sapiens (Human)) | BDBM50118779
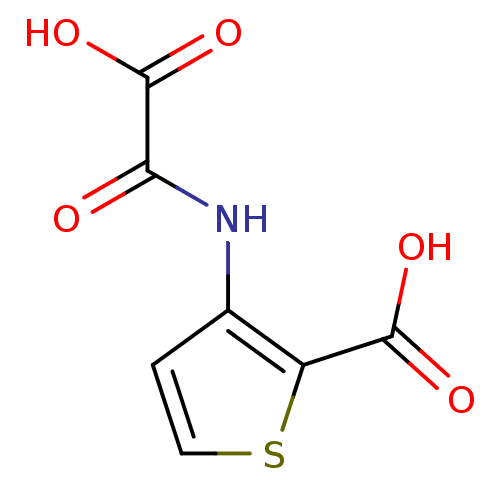
(3-(Oxalyl-amino)-thiophene-2-carboxylic acid | 3-(...)Show InChI InChI=1S/C7H5NO5S/c9-5(7(12)13)8-3-1-2-14-4(3)6(10)11/h1-2H,(H,8,9)(H,10,11)(H,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN6 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 6
(Homo sapiens (Human)) | BDBM50118792
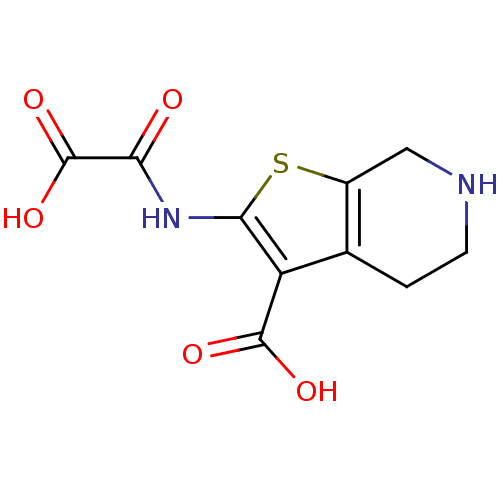
(2-(OXALYL-AMINO)-4,5,6,7-TETRAHYDRO-THIENO[2,3-C]P...)Show InChI InChI=1S/C10H10N2O5S/c13-7(10(16)17)12-8-6(9(14)15)4-1-2-11-3-5(4)18-8/h11H,1-3H2,(H,12,13)(H,14,15)(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN6 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data