

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2C18 (Homo sapiens (Human)) | BDBM50105809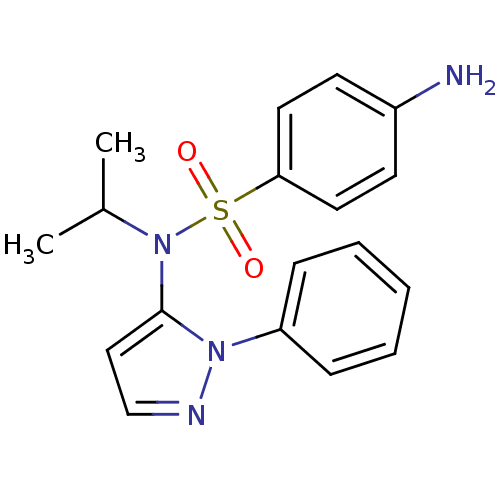 (4-Amino-N-isopropyl-N-(2-phenyl-2H-pyrazol-3-yl)-b...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris V Curated by ChEMBL | Assay Description Inhibitory effect on human recombinant liver cytochrome P450 2C18 expressed in yeast strain | J Med Chem 44: 3622-31 (2001) BindingDB Entry DOI: 10.7270/Q20Z72KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C18 (Homo sapiens (Human)) | BDBM50105810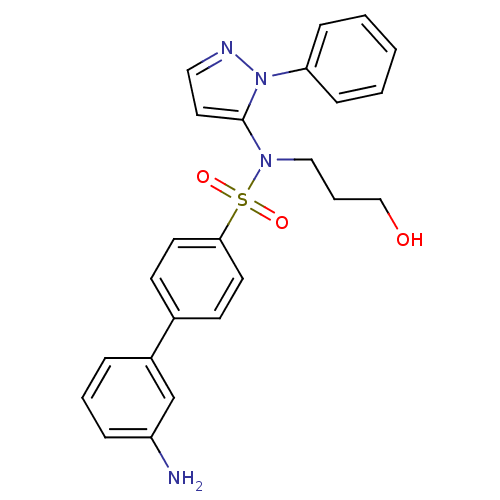 (3'-Amino-biphenyl-4-sulfonic acid (3-hydroxy-propy...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris V Curated by ChEMBL | Assay Description Inhibitory effect on human recombinant liver cytochrome P450 2C18 expressed in yeast strain | J Med Chem 44: 3622-31 (2001) BindingDB Entry DOI: 10.7270/Q20Z72KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C18 (Homo sapiens (Human)) | BDBM50105811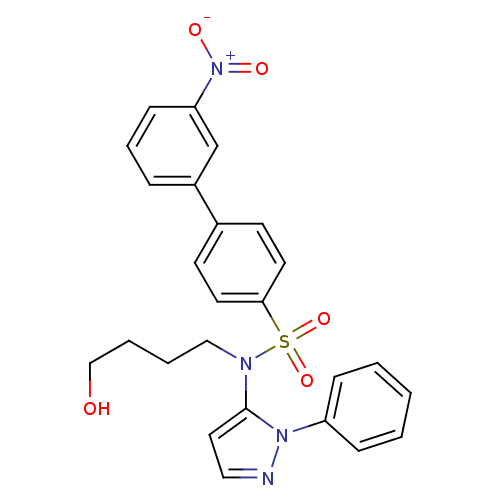 (3'-Nitro-biphenyl-4-sulfonic acid (4-hydroxy-butyl...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris V Curated by ChEMBL | Assay Description Inhibitory effect on human recombinant liver cytochrome P450 2C18 expressed in yeast strain | J Med Chem 44: 3622-31 (2001) BindingDB Entry DOI: 10.7270/Q20Z72KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C18 (Homo sapiens (Human)) | BDBM50105812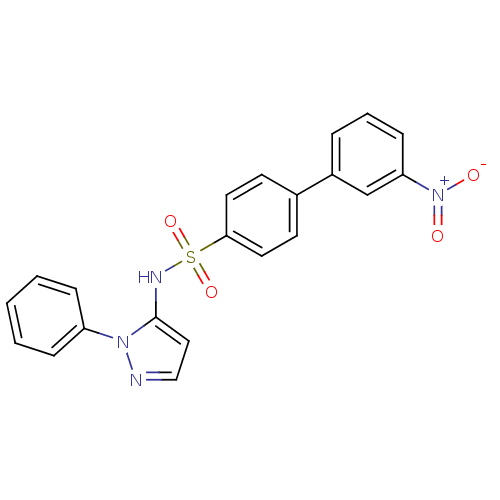 (3'-Nitro-biphenyl-4-sulfonic acid (2-phenyl-2H-pyr...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris V Curated by ChEMBL | Assay Description Inhibitory effect on human recombinant liver cytochrome P450 2C18 expressed in yeast strain | J Med Chem 44: 3622-31 (2001) BindingDB Entry DOI: 10.7270/Q20Z72KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2/2C18/2C19 (Homo sapiens (Human)) | BDBM461264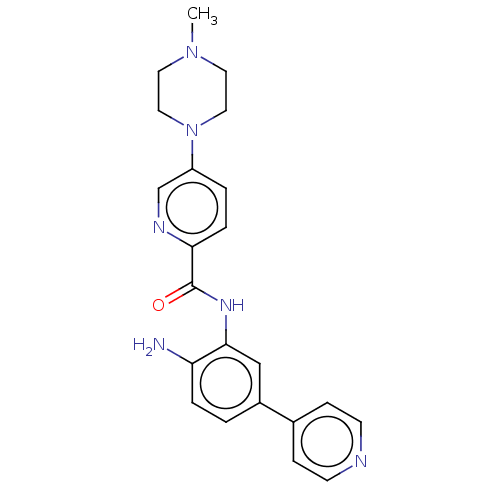 (US10774056, Compound 028 | US11542242, Compound 02...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
REGENACY PHARMACEUTICALS, INC. US Patent | Assay Description For Cyp inhibition, human liver microsomes from BD Gentest were incubated with Compound 028 or Compound 032 (10, 3.33, 1.11, 0.37, 0.12, 0.04, 0.01 &... | US Patent US10774056 (2020) BindingDB Entry DOI: 10.7270/Q25D8VXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C18 (Homo sapiens (Human)) | BDBM50105825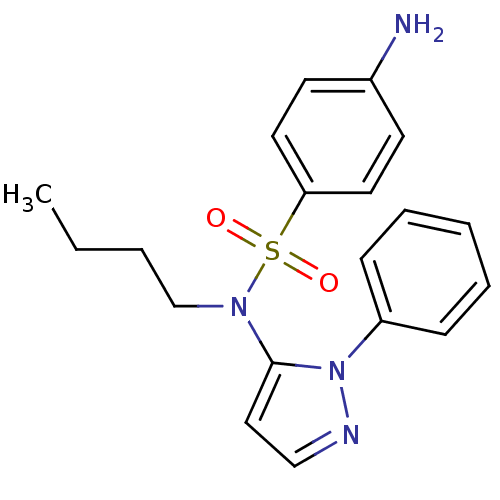 (4-Amino-N-butyl-N-(2-phenyl-2H-pyrazol-3-yl)-benze...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris V Curated by ChEMBL | Assay Description Inhibitory effect on human recombinant liver cytochrome P450 2C18 expressed in yeast strain | J Med Chem 44: 3622-31 (2001) BindingDB Entry DOI: 10.7270/Q20Z72KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C18 (Homo sapiens (Human)) | BDBM50105820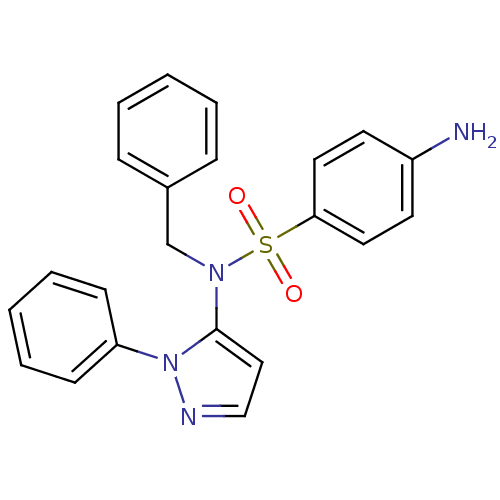 (4-Amino-N-benzyl-N-(2-phenyl-2H-pyrazol-3-yl)-benz...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris V Curated by ChEMBL | Assay Description Inhibitory effect on human recombinant liver cytochrome P450 2C18 expressed in yeast strain | J Med Chem 44: 3622-31 (2001) BindingDB Entry DOI: 10.7270/Q20Z72KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C18 (Homo sapiens (Human)) | BDBM50105821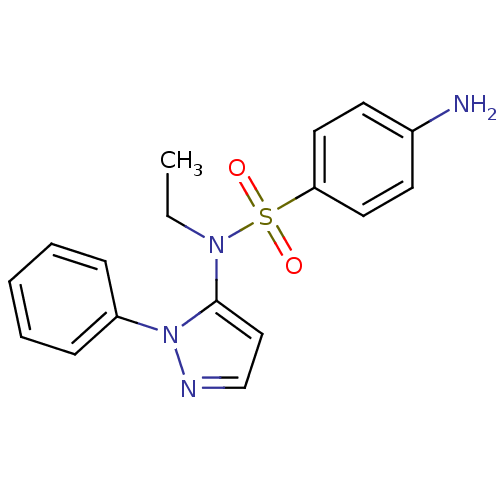 (4-Amino-N-ethyl-N-(2-phenyl-2H-pyrazol-3-yl)-benze...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris V Curated by ChEMBL | Assay Description Inhibitory effect on human recombinant liver cytochrome P450 2C18 expressed in yeast strain | J Med Chem 44: 3622-31 (2001) BindingDB Entry DOI: 10.7270/Q20Z72KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C18 (Homo sapiens (Human)) | BDBM50105816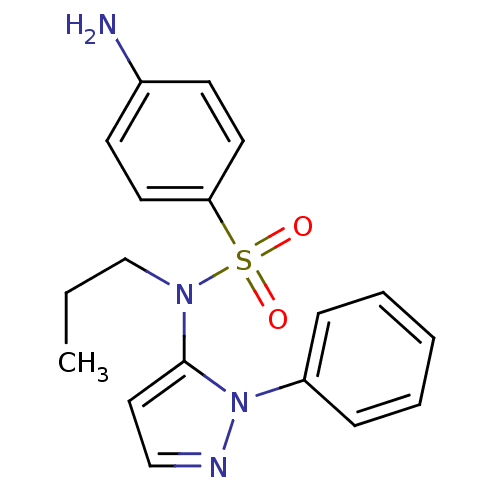 (4-Amino-N-(2-phenyl-2H-pyrazol-3-yl)-N-propyl-benz...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris V Curated by ChEMBL | Assay Description Inhibitory effect on human recombinant liver cytochrome P450 2C18 expressed in yeast strain | J Med Chem 44: 3622-31 (2001) BindingDB Entry DOI: 10.7270/Q20Z72KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C18 (Homo sapiens (Human)) | BDBM50105808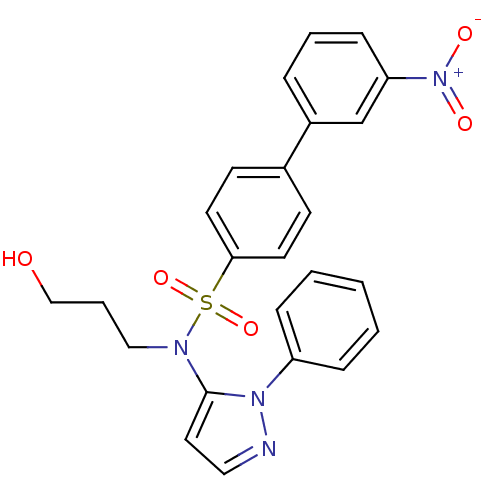 (3'-Nitro-biphenyl-4-sulfonic acid (3-hydroxy-propy...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris V Curated by ChEMBL | Assay Description Inhibitory effect on human recombinant liver cytochrome P450 2C18 expressed in yeast strain | J Med Chem 44: 3622-31 (2001) BindingDB Entry DOI: 10.7270/Q20Z72KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C18 (Homo sapiens (Human)) | BDBM50105814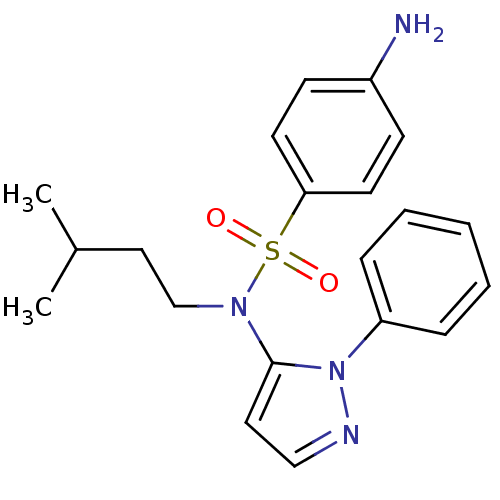 (4-Amino-N-(3-methyl-butyl)-N-(2-phenyl-2H-pyrazol-...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris V Curated by ChEMBL | Assay Description Inhibitory effect on human recombinant liver cytochrome P450 2C18 expressed in yeast strain | J Med Chem 44: 3622-31 (2001) BindingDB Entry DOI: 10.7270/Q20Z72KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C18 (Homo sapiens (Human)) | BDBM50105817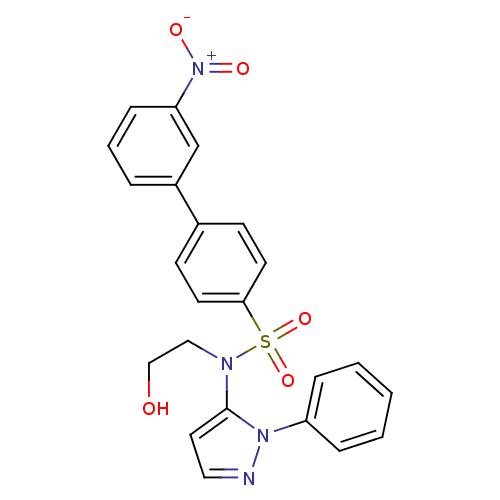 (3'-Nitro-biphenyl-4-sulfonic acid (2-hydroxy-ethyl...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris V Curated by ChEMBL | Assay Description Inhibitory effect on human recombinant liver cytochrome P450 2C18 expressed in yeast strain | J Med Chem 44: 3622-31 (2001) BindingDB Entry DOI: 10.7270/Q20Z72KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2/2C18/2C19 (Homo sapiens (Human)) | BDBM461268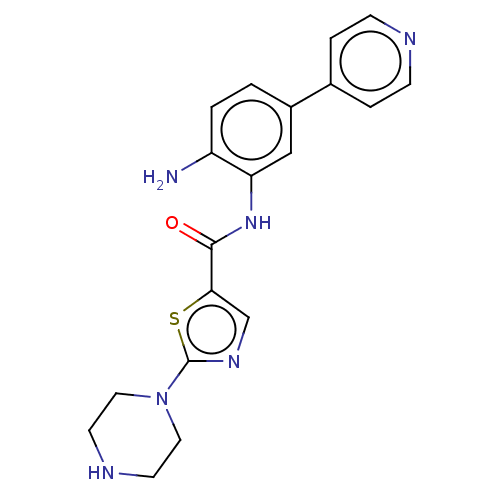 (US10774056, Compound 032 | US11542242, Compound 03...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
REGENACY PHARMACEUTICALS, INC. US Patent | Assay Description For Cyp inhibition, human liver microsomes from BD Gentest were incubated with Compound 028 or Compound 032 (10, 3.33, 1.11, 0.37, 0.12, 0.04, 0.01 &... | US Patent US10774056 (2020) BindingDB Entry DOI: 10.7270/Q25D8VXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2/2C18/2C19 (Homo sapiens (Human)) | BDBM461261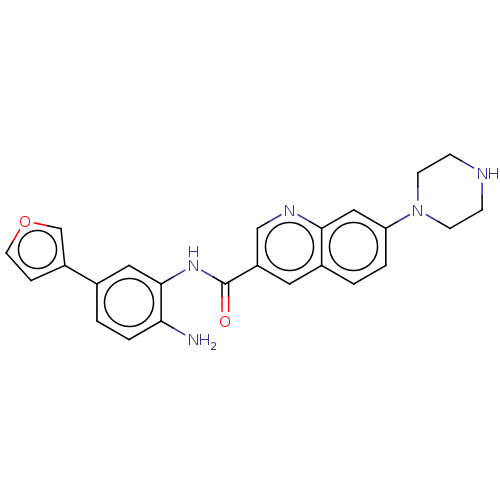 (US10774056, Compound 025 | US11542242, Compound 02...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
REGENACY PHARMACEUTICALS, INC. US Patent | Assay Description For Cyp inhibition, human liver microsomes from BD Gentest were incubated with Compound 028 or Compound 032 (10, 3.33, 1.11, 0.37, 0.12, 0.04, 0.01 &... | US Patent US10774056 (2020) BindingDB Entry DOI: 10.7270/Q25D8VXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C18 (Homo sapiens (Human)) | BDBM50105828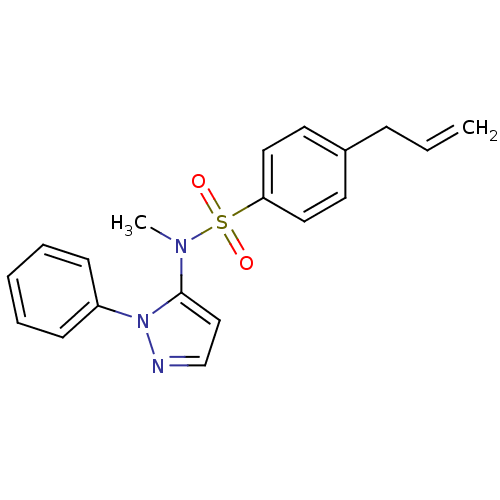 (4-Allyl-N-methyl-N-(2-phenyl-2H-pyrazol-3-yl)-benz...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris V Curated by ChEMBL | Assay Description Inhibitory effect on human recombinant liver cytochrome P450 2C18 expressed in yeast strain | J Med Chem 44: 3622-31 (2001) BindingDB Entry DOI: 10.7270/Q20Z72KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2/2C18/2C19 (Homo sapiens (Human)) | BDBM461263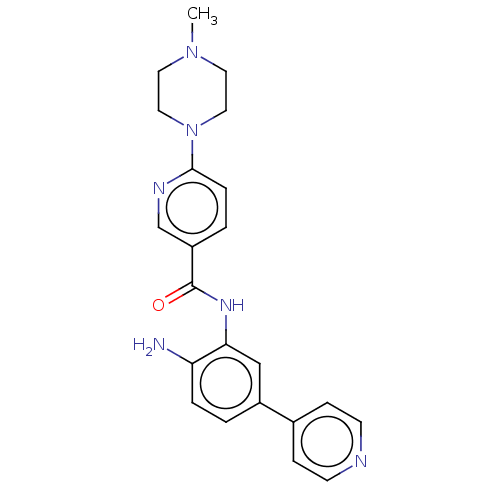 (US10774056, Compound 027 | US11542242, Compound 02...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
REGENACY PHARMACEUTICALS, INC. US Patent | Assay Description For Cyp inhibition, human liver microsomes from BD Gentest were incubated with Compound 028 or Compound 032 (10, 3.33, 1.11, 0.37, 0.12, 0.04, 0.01 &... | US Patent US10774056 (2020) BindingDB Entry DOI: 10.7270/Q25D8VXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2/2C18/2C19 (Homo sapiens (Human)) | BDBM461266 (US10774056, Compound 030 | US11542242, Compound 03...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
REGENACY PHARMACEUTICALS, INC. US Patent | Assay Description For Cyp inhibition, human liver microsomes from BD Gentest were incubated with Compound 028 or Compound 032 (10, 3.33, 1.11, 0.37, 0.12, 0.04, 0.01 &... | US Patent US10774056 (2020) BindingDB Entry DOI: 10.7270/Q25D8VXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2/2C18/2C19 (Homo sapiens (Human)) | BDBM461256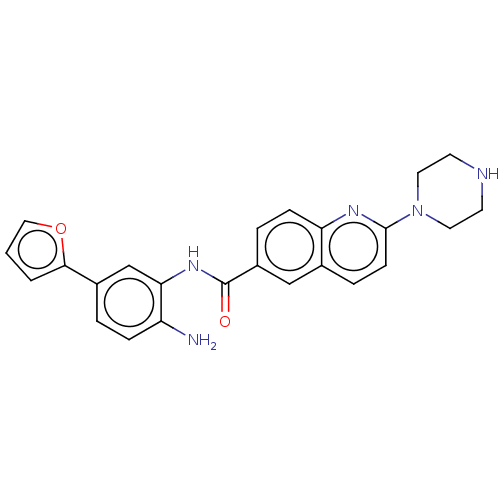 (US10774056, Compound 003 | US11542242, Compound 00...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
REGENACY PHARMACEUTICALS, INC. US Patent | Assay Description For Cyp inhibition, human liver microsomes from BD Gentest were incubated with Compound 028 or Compound 032 (10, 3.33, 1.11, 0.37, 0.12, 0.04, 0.01 &... | US Patent US10774056 (2020) BindingDB Entry DOI: 10.7270/Q25D8VXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2/2C18/2C19 (Homo sapiens (Human)) | BDBM461258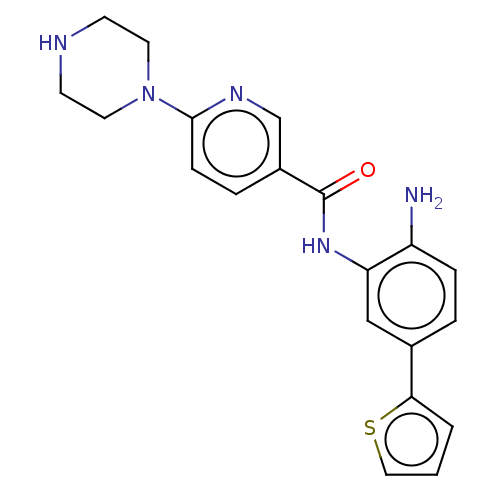 (US10774056, Compound 007 | US11542242, Compound 00...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
REGENACY PHARMACEUTICALS, INC. US Patent | Assay Description For Cyp inhibition, human liver microsomes from BD Gentest were incubated with Compound 028 or Compound 032 (10, 3.33, 1.11, 0.37, 0.12, 0.04, 0.01 &... | US Patent US10774056 (2020) BindingDB Entry DOI: 10.7270/Q25D8VXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2/2C18/2C19 (Homo sapiens (Human)) | BDBM461257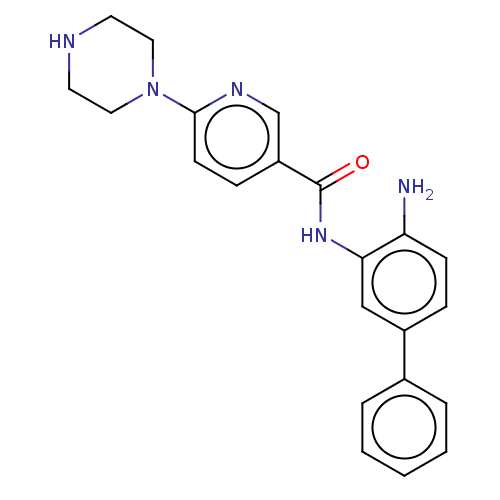 (US10774056, Compound 006 | US11542242, Compound 00...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
REGENACY PHARMACEUTICALS, INC. US Patent | Assay Description For Cyp inhibition, human liver microsomes from BD Gentest were incubated with Compound 028 or Compound 032 (10, 3.33, 1.11, 0.37, 0.12, 0.04, 0.01 &... | US Patent US10774056 (2020) BindingDB Entry DOI: 10.7270/Q25D8VXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 (Homo sapiens (Human)) | BDBM50508661 (CHEMBL4524830) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human CYP450 | Bioorg Med Chem Lett 29: 659-663 (2019) Article DOI: 10.1016/j.bmcl.2018.12.013 BindingDB Entry DOI: 10.7270/Q2K64NC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2/2C18/2C19 (Homo sapiens (Human)) | BDBM461260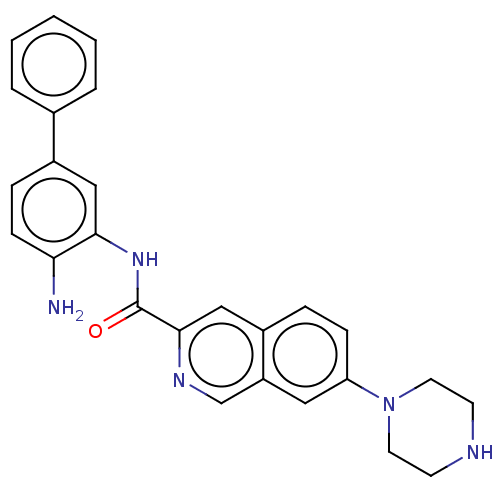 (US10774056, Compound 022 | US11542242, Compound 02...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
REGENACY PHARMACEUTICALS, INC. US Patent | Assay Description For Cyp inhibition, human liver microsomes from BD Gentest were incubated with Compound 028 or Compound 032 (10, 3.33, 1.11, 0.37, 0.12, 0.04, 0.01 &... | US Patent US10774056 (2020) BindingDB Entry DOI: 10.7270/Q25D8VXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2/2C18/2C19 (Homo sapiens (Human)) | BDBM461267 (US10774056, Compound 031 | US11542242, Compound 03...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
REGENACY PHARMACEUTICALS, INC. US Patent | Assay Description For Cyp inhibition, human liver microsomes from BD Gentest were incubated with Compound 028 or Compound 032 (10, 3.33, 1.11, 0.37, 0.12, 0.04, 0.01 &... | US Patent US10774056 (2020) BindingDB Entry DOI: 10.7270/Q25D8VXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C18 (Homo sapiens (Human)) | BDBM50105824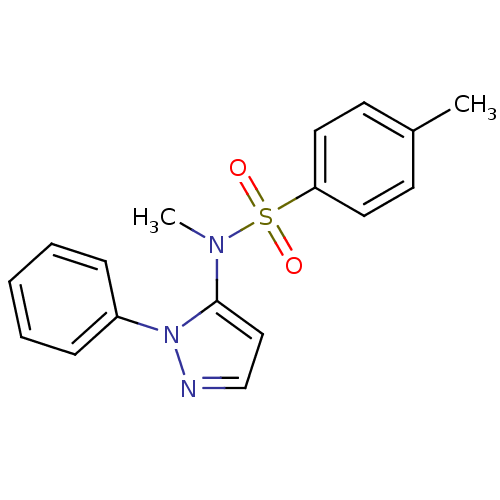 (4,N-Dimethyl-N-(2-phenyl-2H-pyrazol-3-yl)-benzenes...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris V Curated by ChEMBL | Assay Description Inhibitory effect on human recombinant liver cytochrome P450 2C18 expressed in yeast strain | J Med Chem 44: 3622-31 (2001) BindingDB Entry DOI: 10.7270/Q20Z72KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C18 (Homo sapiens (Human)) | BDBM50105818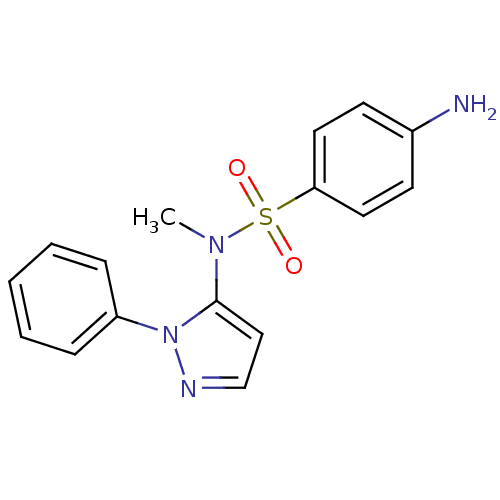 (4-Amino-N-methyl-N-(2-phenyl-2H-pyrazol-3-yl)-benz...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris V Curated by ChEMBL | Assay Description Inhibitory effect on human recombinant liver cytochrome P450 2C18 expressed in yeast strain | J Med Chem 44: 3622-31 (2001) BindingDB Entry DOI: 10.7270/Q20Z72KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C18 (Homo sapiens (Human)) | BDBM50105815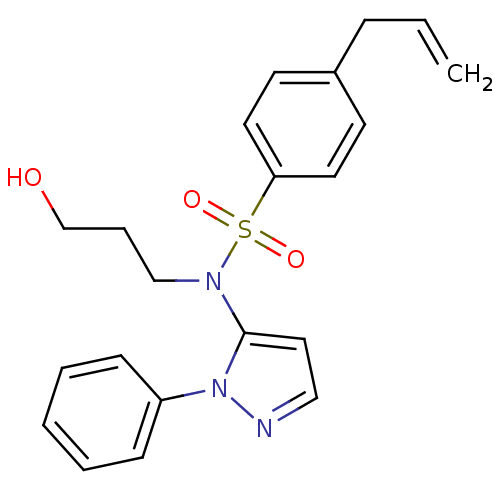 (4-Allyl-N-(3-hydroxy-propyl)-N-(2-phenyl-2H-pyrazo...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris V Curated by ChEMBL | Assay Description Inhibitory effect on human recombinant liver cytochrome P450 2C18 expressed in yeast strain | J Med Chem 44: 3622-31 (2001) BindingDB Entry DOI: 10.7270/Q20Z72KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C18 (Homo sapiens (Human)) | BDBM50105822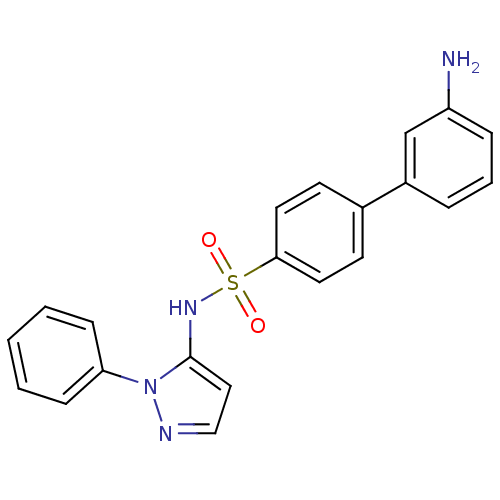 (3'-Amino-biphenyl-4-sulfonic acid (2-phenyl-2H-pyr...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris V Curated by ChEMBL | Assay Description Inhibitory effect on human recombinant liver cytochrome P450 2C18 expressed in yeast strain | J Med Chem 44: 3622-31 (2001) BindingDB Entry DOI: 10.7270/Q20Z72KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C18 (Homo sapiens (Human)) | BDBM50105823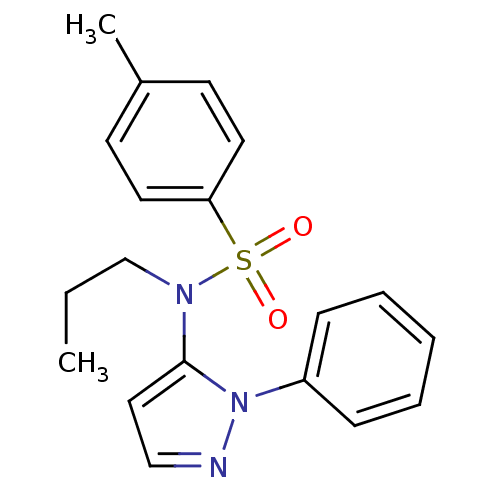 (4-Methyl-N-(2-phenyl-2H-pyrazol-3-yl)-N-propyl-ben...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris V Curated by ChEMBL | Assay Description Inhibitory effect on human recombinant liver cytochrome P450 2C18 expressed in yeast strain | J Med Chem 44: 3622-31 (2001) BindingDB Entry DOI: 10.7270/Q20Z72KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C18 (Homo sapiens (Human)) | BDBM50548260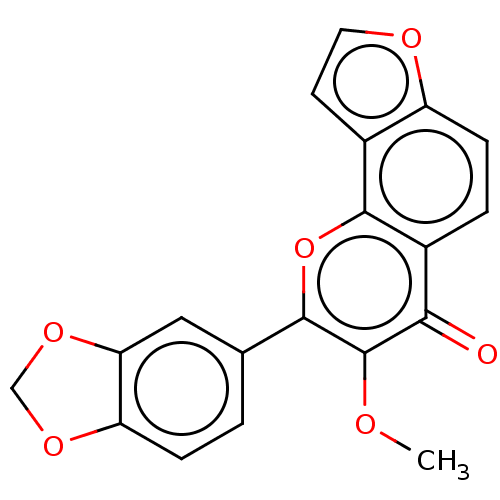 (Pongapin) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human CYP2C18 expressed in Sacchrosomes by fluorescence based assay | Citation and Details Article DOI: 10.1016/j.bmc.2018.11.013 BindingDB Entry DOI: 10.7270/Q20V8HDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C18 (Homo sapiens (Human)) | BDBM50548258 (LANCEOLATIN B) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human CYP2C18 expressed in Sacchrosomes by fluorescence based assay | Citation and Details Article DOI: 10.1016/j.bmc.2018.11.013 BindingDB Entry DOI: 10.7270/Q20V8HDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C18 (Homo sapiens (Human)) | BDBM50105819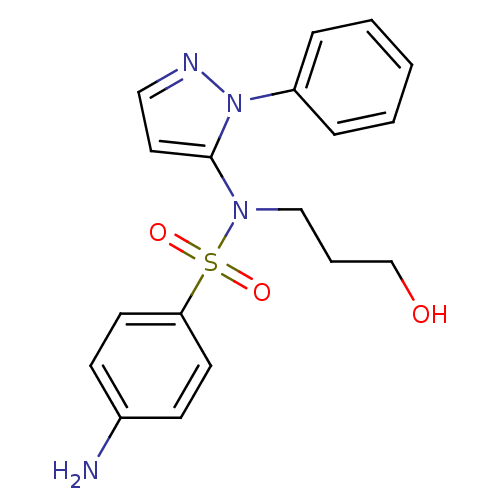 (4-Amino-N-(3-hydroxy-propyl)-N-(2-phenyl-2H-pyrazo...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris V Curated by ChEMBL | Assay Description Inhibitory effect on human recombinant liver cytochrome P450 2C18 expressed in yeast strain | J Med Chem 44: 3622-31 (2001) BindingDB Entry DOI: 10.7270/Q20Z72KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C18 (Homo sapiens (Human)) | BDBM50105826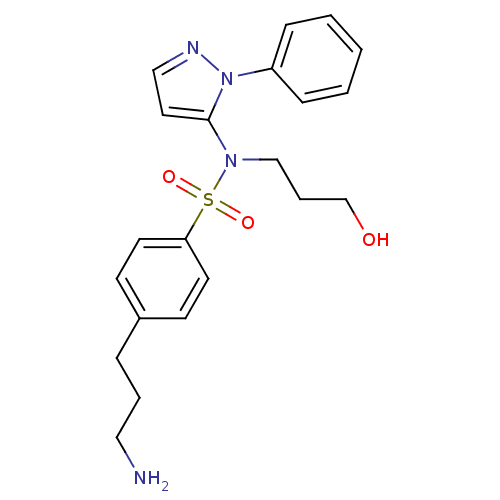 (4-(3-Amino-propyl)-N-(3-hydroxy-propyl)-N-(2-pheny...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris V Curated by ChEMBL | Assay Description Inhibitory effect on human recombinant liver cytochrome P450 2C18 expressed in yeast strain | J Med Chem 44: 3622-31 (2001) BindingDB Entry DOI: 10.7270/Q20Z72KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C18 (Homo sapiens (Human)) | BDBM50090677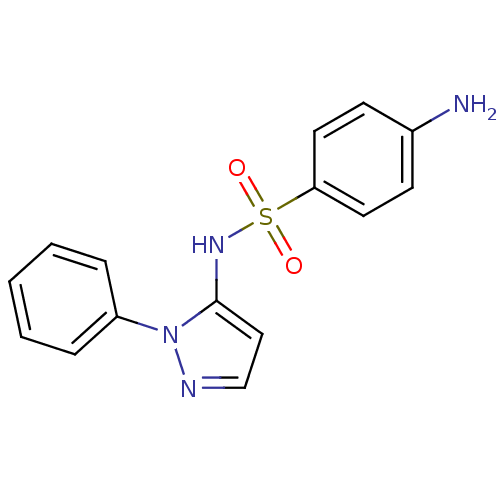 (4-Amino-N-(2-phenyl-2H-pyrazol-3-yl)-benzenesulfon...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris V Curated by ChEMBL | Assay Description Inhibitory effect on human recombinant liver cytochrome P450 2C18 expressed in yeast strain | J Med Chem 44: 3622-31 (2001) BindingDB Entry DOI: 10.7270/Q20Z72KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C18 (Homo sapiens (Human)) | BDBM50105807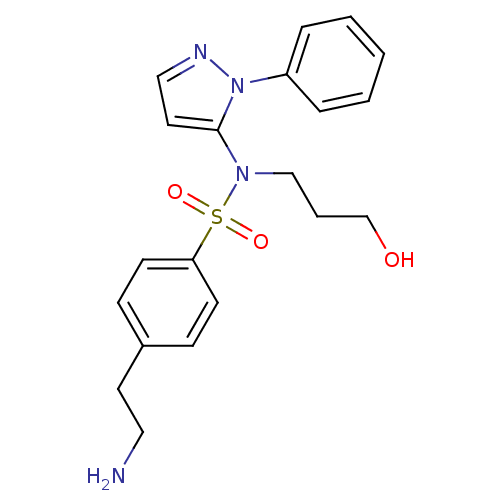 (4-(2-Amino-ethyl)-N-(3-hydroxy-propyl)-N-(2-phenyl...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris V Curated by ChEMBL | Assay Description Inhibitory effect on human recombinant liver cytochrome P450 2C18 expressed in yeast strain | J Med Chem 44: 3622-31 (2001) BindingDB Entry DOI: 10.7270/Q20Z72KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C18 (Homo sapiens (Human)) | BDBM50105813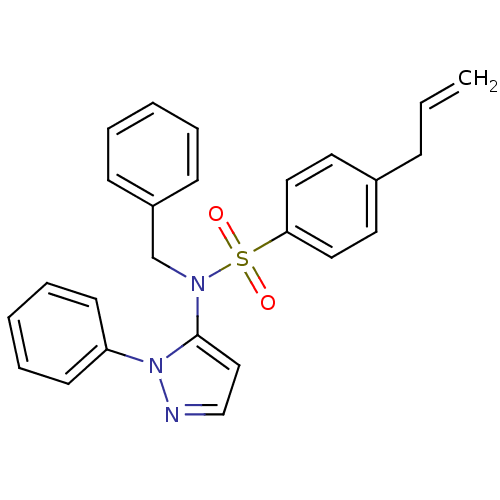 (4-Allyl-N-benzyl-N-(2-phenyl-2H-pyrazol-3-yl)-benz...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris V Curated by ChEMBL | Assay Description Inhibitory effect on human recombinant liver cytochrome P450 2C18 expressed in yeast strain | J Med Chem 44: 3622-31 (2001) BindingDB Entry DOI: 10.7270/Q20Z72KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C18 (Homo sapiens (Human)) | BDBM50105827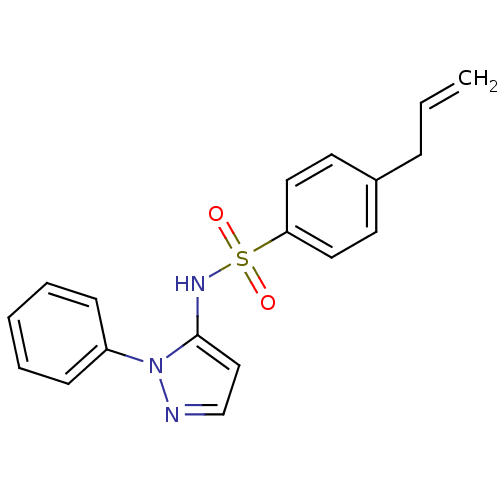 (4-Allyl-N-(2-phenyl-2H-pyrazol-3-yl)-benzenesulfon...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris V Curated by ChEMBL | Assay Description Inhibitory effect on human recombinant liver cytochrome P450 2C18 expressed in yeast strain | J Med Chem 44: 3622-31 (2001) BindingDB Entry DOI: 10.7270/Q20Z72KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C18 (Homo sapiens (Human)) | BDBM50090669 (4-Methyl-N-(2-phenyl-2H-pyrazol-3-yl)-benzenesulfo...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris V Curated by ChEMBL | Assay Description Inhibitory effect on human recombinant liver cytochrome P450 2C18 expressed in yeast strain | J Med Chem 44: 3622-31 (2001) BindingDB Entry DOI: 10.7270/Q20Z72KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||