

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM18128 ((2S)-2-[(2S)-2-amino-3-(4-hydroxyphenyl)propanamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against Bacterial Tyrosyl tRNA Synthetase | Bioorg Med Chem Lett 11: 2499-502 (2001) BindingDB Entry DOI: 10.7270/Q2Z89BPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50097749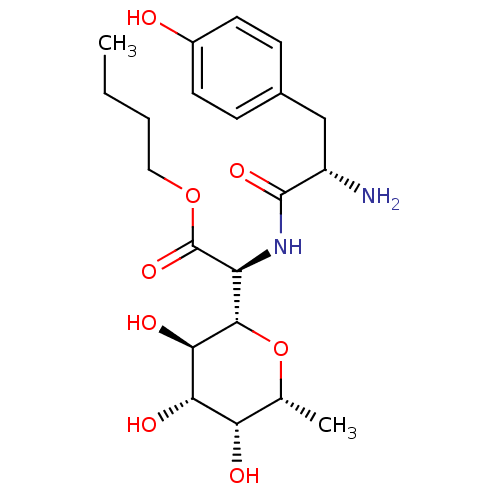 (CHEMBL163375 | [2-Amino-3-((S)-4-hydroxy-phenyl)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory activity against Staphylococcus aureus tyrosyl tRNA synthetase (YRS) using aminoacylation assay | Bioorg Med Chem Lett 11: 715-8 (2001) BindingDB Entry DOI: 10.7270/Q20C4V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50091497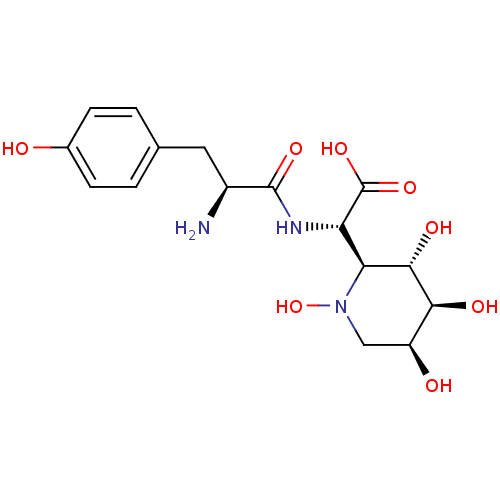 ((S)-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity of compound against tyrosyl tRNA synthetase from Staphylococcus aureus was determined | Bioorg Med Chem Lett 10: 1811-4 (2000) BindingDB Entry DOI: 10.7270/Q23F4Q5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50091497 ((S)-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory activity against Staphylococcus aureus tyrosyl tRNA synthetase (YRS) using aminoacylation assay | Bioorg Med Chem Lett 11: 711-4 (2001) BindingDB Entry DOI: 10.7270/Q2445KR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM18128 ((2S)-2-[(2S)-2-amino-3-(4-hydroxyphenyl)propanamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity of compound against tyrosyl tRNA synthetase from Staphylococcus aureus was determined | Bioorg Med Chem Lett 10: 1811-4 (2000) BindingDB Entry DOI: 10.7270/Q23F4Q5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50097746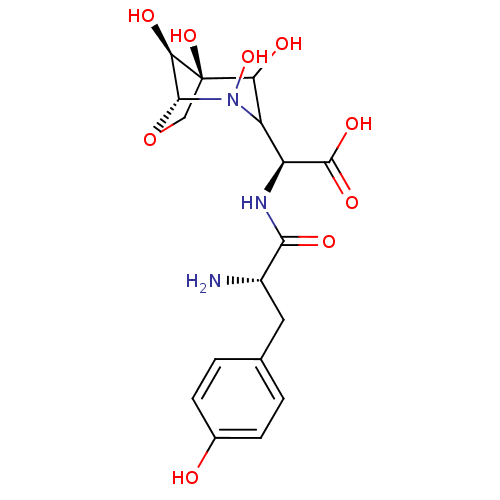 ((S)-[2-Amino-3-((S)-4-hydroxy-phenyl)-propionylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory activity against Staphylococcus aureus tyrosyl tRNA synthetase (YRS) using aminoacylation assay | Bioorg Med Chem Lett 11: 711-4 (2001) BindingDB Entry DOI: 10.7270/Q2445KR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50104311 ((S)-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against Bacterial Tyrosyl tRNA Synthetase | Bioorg Med Chem Lett 11: 2499-502 (2001) BindingDB Entry DOI: 10.7270/Q2Z89BPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50097748 (CHEMBL351127 | [2-Amino-3-((S)-4-hydroxy-phenyl)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory activity against Staphylococcus aureus tyrosyl tRNA synthetase (YRS) using aminoacylation assay | Bioorg Med Chem Lett 11: 715-8 (2001) BindingDB Entry DOI: 10.7270/Q20C4V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM18132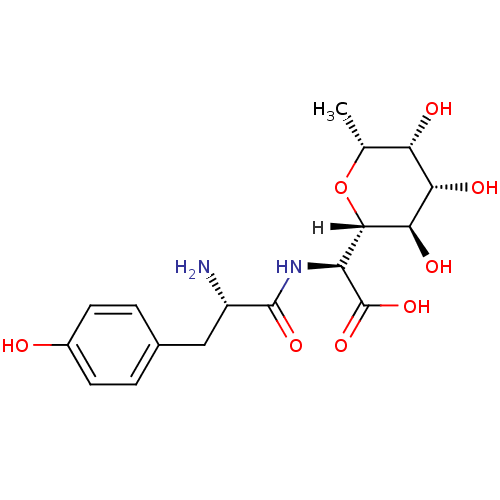 ((2S)-2-[(2S)-2-amino-3-(4-hydroxyphenyl)propanamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory activity against Staphylococcus aureus tyrosyl tRNA synthetase (YRS) using aminoacylation assay | Bioorg Med Chem Lett 11: 715-8 (2001) BindingDB Entry DOI: 10.7270/Q20C4V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50091496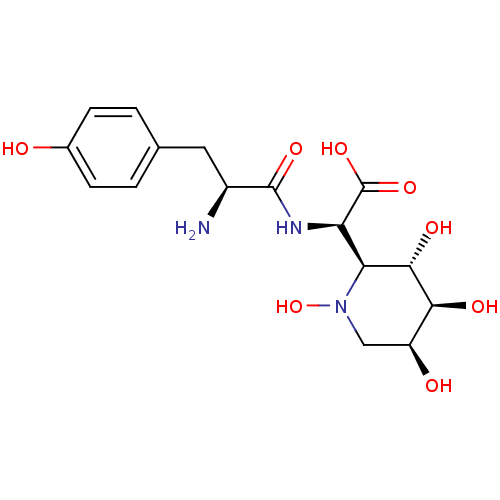 ((R)-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity of compound against tyrosyl tRNA synthetase from Staphylococcus aureus was determined | Bioorg Med Chem Lett 10: 1811-4 (2000) BindingDB Entry DOI: 10.7270/Q23F4Q5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50097744 ((S)-[2-Amino-3-((S)-4-hydroxy-phenyl)-propionylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory activity against Staphylococcus aureus tyrosyl tRNA synthetase (YRS) using aminoacylation assay | Bioorg Med Chem Lett 11: 711-4 (2001) BindingDB Entry DOI: 10.7270/Q2445KR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50097744 ((S)-[2-Amino-3-((S)-4-hydroxy-phenyl)-propionylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory activity against Staphylococcus aureus tyrosyl tRNA synthetase (YRS) using aminoacylation assay | Bioorg Med Chem Lett 11: 715-8 (2001) BindingDB Entry DOI: 10.7270/Q20C4V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50104310 ((R)-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 124 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against Bacterial Tyrosyl tRNA Synthetase | Bioorg Med Chem Lett 11: 2499-502 (2001) BindingDB Entry DOI: 10.7270/Q2Z89BPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50097747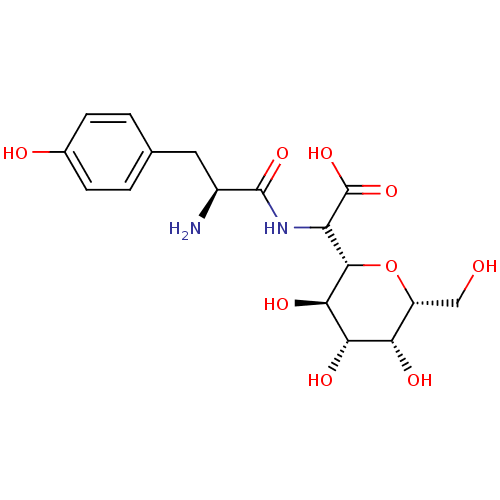 (CHEMBL162552 | [2-Amino-3-((S)-4-hydroxy-phenyl)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory activity against Staphylococcus aureus tyrosyl tRNA synthetase (YRS) using aminoacylation assay | Bioorg Med Chem Lett 11: 715-8 (2001) BindingDB Entry DOI: 10.7270/Q20C4V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50104313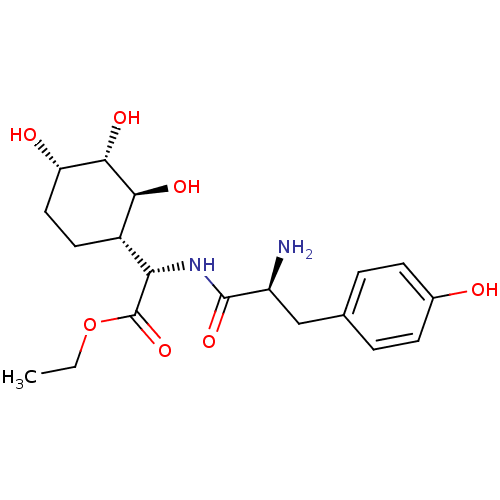 ((S)-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 245 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against Bacterial Tyrosyl tRNA Synthetase | Bioorg Med Chem Lett 11: 2499-502 (2001) BindingDB Entry DOI: 10.7270/Q2Z89BPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50097745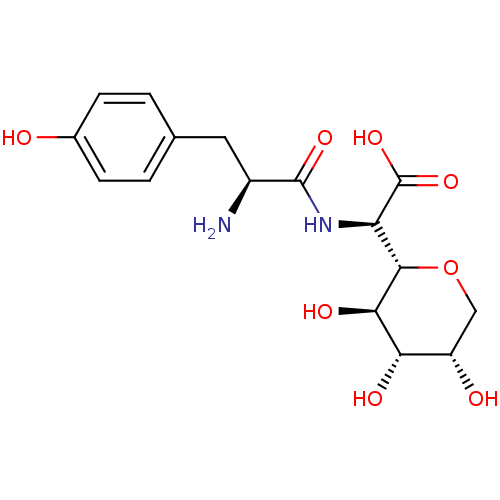 (CHEMBL159436 | [2-Amino-3-((S)-4-hydroxy-phenyl)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory activity against Staphylococcus aureus tyrosyl tRNA synthetase (YRS) using aminoacylation assay | Bioorg Med Chem Lett 11: 711-4 (2001) BindingDB Entry DOI: 10.7270/Q2445KR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50081838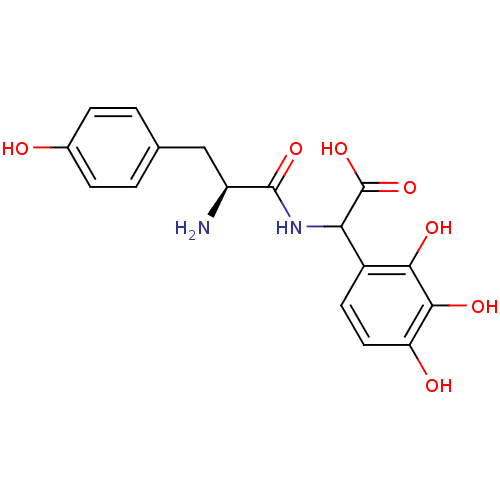 (CHEMBL318019 | [(S)-2-Amino-3-(4-hydroxy-phenyl)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Compound was tested for inhibition of Staphylococcus aureus tyrosyl tRNA Synthetase | Bioorg Med Chem Lett 9: 2859-62 (1999) BindingDB Entry DOI: 10.7270/Q2F18XX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50081839 (CHEMBL98455 | [(S)-2-Amino-3-(4-hydroxy-phenyl)-pr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Compound was tested for inhibition of Staphylococcus aureus tyrosyl tRNA Synthetase | Bioorg Med Chem Lett 9: 2859-62 (1999) BindingDB Entry DOI: 10.7270/Q2F18XX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50081834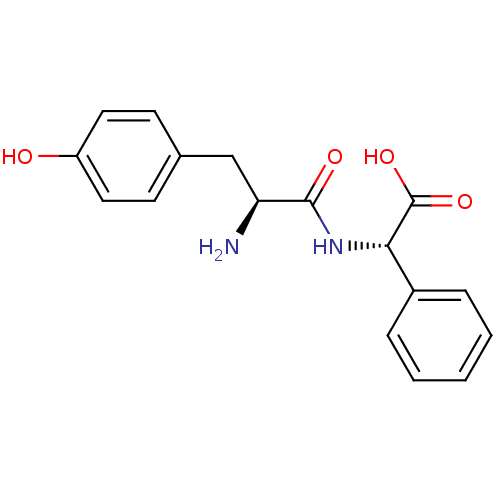 ((S)-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Compound was tested for inhibition of Staphylococcus aureus tyrosyl tRNA Synthetase | Bioorg Med Chem Lett 9: 2859-62 (1999) BindingDB Entry DOI: 10.7270/Q2F18XX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50081837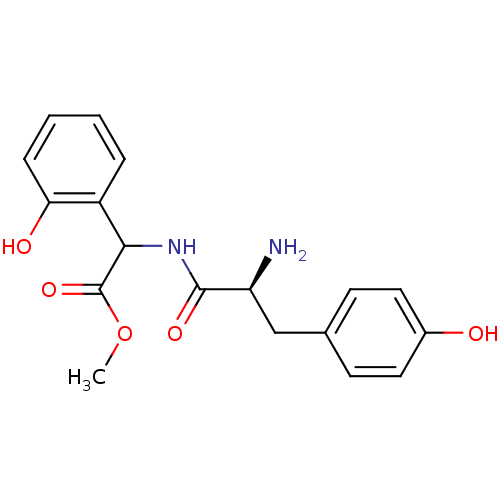 (CHEMBL99347 | [(S)-2-Amino-3-(4-hydroxy-phenyl)-pr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Compound was tested for inhibition of Staphylococcus aureus tyrosyl tRNA Synthetase | Bioorg Med Chem Lett 9: 2859-62 (1999) BindingDB Entry DOI: 10.7270/Q2F18XX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50104314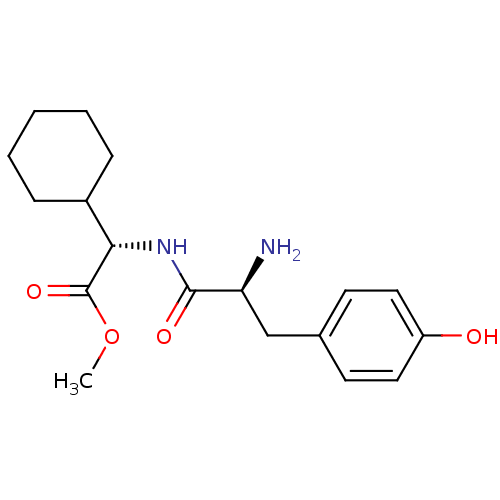 ((S)-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against Bacterial Tyrosyl tRNA Synthetase | Bioorg Med Chem Lett 11: 2499-502 (2001) BindingDB Entry DOI: 10.7270/Q2Z89BPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50104312 ((R)-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against Bacterial Tyrosyl tRNA Synthetase | Bioorg Med Chem Lett 11: 2499-502 (2001) BindingDB Entry DOI: 10.7270/Q2Z89BPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50081841 (CHEMBL95581 | [(S)-2-Amino-3-(4-hydroxy-phenyl)-pr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Compound was tested for inhibition of Staphylococcus aureus tyrosyl tRNA Synthetase | Bioorg Med Chem Lett 9: 2859-62 (1999) BindingDB Entry DOI: 10.7270/Q2F18XX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50081840 ((S)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Compound was tested for inhibition of Staphylococcus aureus tyrosyl tRNA Synthetase | Bioorg Med Chem Lett 9: 2859-62 (1999) BindingDB Entry DOI: 10.7270/Q2F18XX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50081836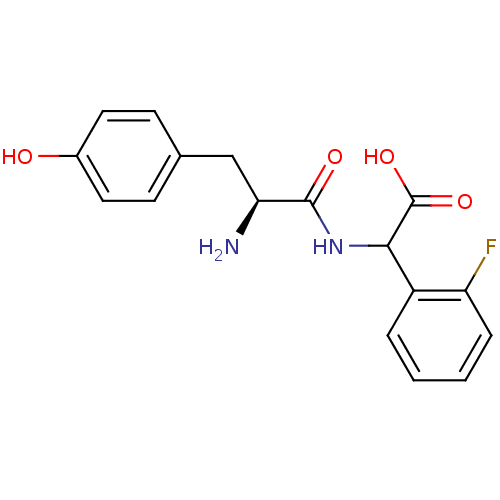 (CHEMBL95616 | [(S)-2-Amino-3-(4-hydroxy-phenyl)-pr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Compound was tested for inhibition of Staphylococcus aureus tyrosyl tRNA Synthetase | Bioorg Med Chem Lett 9: 2859-62 (1999) BindingDB Entry DOI: 10.7270/Q2F18XX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50081835 ((S)-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Compound was tested for inhibition of Staphylococcus aureus tyrosyl tRNA Synthetase | Bioorg Med Chem Lett 9: 2859-62 (1999) BindingDB Entry DOI: 10.7270/Q2F18XX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM18128 ((2S)-2-[(2S)-2-amino-3-(4-hydroxyphenyl)propanamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity of compound against tyrosyl tRNA synthetase from mammalian cells was determined | Bioorg Med Chem Lett 10: 1811-4 (2000) BindingDB Entry DOI: 10.7270/Q23F4Q5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||