

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lethal factor (Bacillus anthracis) | BDBM8509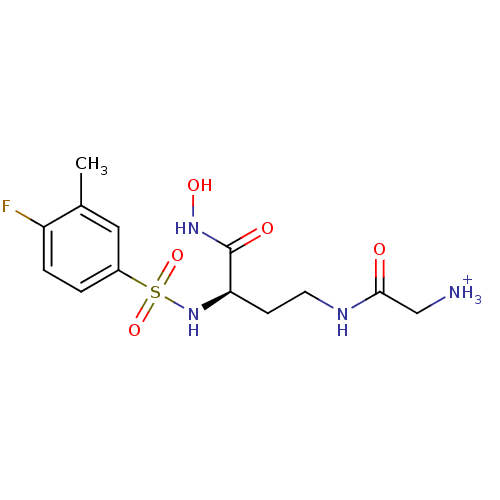 ((2R)-2-[(4-fluoro-3-methylbenzene)sulfonamido]-4-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories | Assay Description The assay was performed in a 96-well plate, each well contained substrate peptide, LF, and the test compound. The C-terminally fluorophore of substra... | Bioorg Med Chem Lett 16: 964-8 (2006) Article DOI: 10.1016/j.bmcl.2005.10.088 BindingDB Entry DOI: 10.7270/Q2K35RV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8513 ((2R)-5-[(aminiumylmethanimidoyl)amino]-2-[(4-fluor...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories | Assay Description The assay was performed in a 96-well plate, each well contained substrate peptide, LF, and the test compound. The C-terminally fluorophore of substra... | Bioorg Med Chem Lett 16: 964-8 (2006) Article DOI: 10.1016/j.bmcl.2005.10.088 BindingDB Entry DOI: 10.7270/Q2K35RV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8501 ((2R)-2-cyclohexyl-2-[(4-fluoro-3-methylbenzene)sul...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories | Assay Description The assay was performed in a 96-well plate, each well contained substrate peptide, LF, and the test compound. The C-terminally fluorophore of substra... | Bioorg Med Chem Lett 16: 964-8 (2006) Article DOI: 10.1016/j.bmcl.2005.10.088 BindingDB Entry DOI: 10.7270/Q2K35RV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50236202 (CHEMBL4074171) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis lethal factor protease preincubated for 30 mins prior to addition of substrate containing fluorescent proteins CyPet... | J Med Chem 60: 1916-1927 (2017) Article DOI: 10.1021/acs.jmedchem.6b01689 BindingDB Entry DOI: 10.7270/Q2377C05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8512 ((2R)-3-(2-aminiumylethoxy)-2-[(4-fluoro-3-methylbe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories | Assay Description The assay was performed in a 96-well plate, each well contained substrate peptide, LF, and the test compound. The C-terminally fluorophore of substra... | Bioorg Med Chem Lett 16: 964-8 (2006) Article DOI: 10.1016/j.bmcl.2005.10.088 BindingDB Entry DOI: 10.7270/Q2K35RV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8502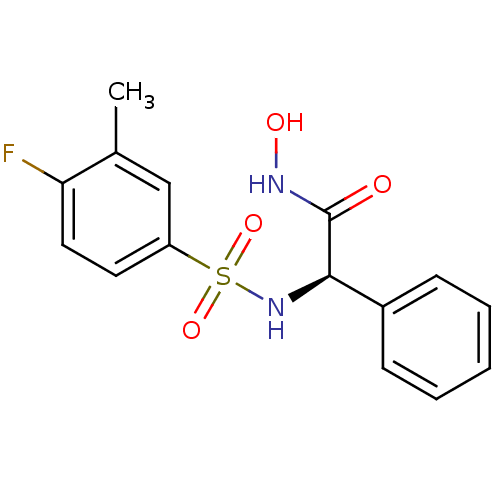 ((2R)-2-[(4-fluoro-3-methylbenzene)sulfonamido]-N-h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories | Assay Description The assay was performed in a 96-well plate, each well contained substrate peptide, LF, and the test compound. The C-terminally fluorophore of substra... | Bioorg Med Chem Lett 16: 964-8 (2006) Article DOI: 10.1016/j.bmcl.2005.10.088 BindingDB Entry DOI: 10.7270/Q2K35RV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8507 ((2R)-3-cyclopropyl-2-[(4-fluoro-3-methylbenzene)su...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories | Assay Description The assay was performed in a 96-well plate, each well contained substrate peptide, LF, and the test compound. The C-terminally fluorophore of substra... | Bioorg Med Chem Lett 16: 964-8 (2006) Article DOI: 10.1016/j.bmcl.2005.10.088 BindingDB Entry DOI: 10.7270/Q2K35RV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8503 ((2R)-2-[(4-fluoro-3-methylbenzene)sulfonamido]-N-h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories | Assay Description The assay was performed in a 96-well plate, each well contained substrate peptide, LF, and the test compound. The C-terminally fluorophore of substra... | Bioorg Med Chem Lett 16: 964-8 (2006) Article DOI: 10.1016/j.bmcl.2005.10.088 BindingDB Entry DOI: 10.7270/Q2K35RV4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8511 ((2R)-2-[(4-fluoro-3-methylbenzene)sulfonamido]-4-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories | Assay Description The assay was performed in a 96-well plate, each well contained substrate peptide, LF, and the test compound. The C-terminally fluorophore of substra... | Bioorg Med Chem Lett 16: 964-8 (2006) Article DOI: 10.1016/j.bmcl.2005.10.088 BindingDB Entry DOI: 10.7270/Q2K35RV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8510 ((2R)-2-[(4-fluoro-3-methylbenzene)sulfonamido]-4-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories | Assay Description The assay was performed in a 96-well plate, each well contained substrate peptide, LF, and the test compound. The C-terminally fluorophore of substra... | Bioorg Med Chem Lett 16: 964-8 (2006) Article DOI: 10.1016/j.bmcl.2005.10.088 BindingDB Entry DOI: 10.7270/Q2K35RV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50236194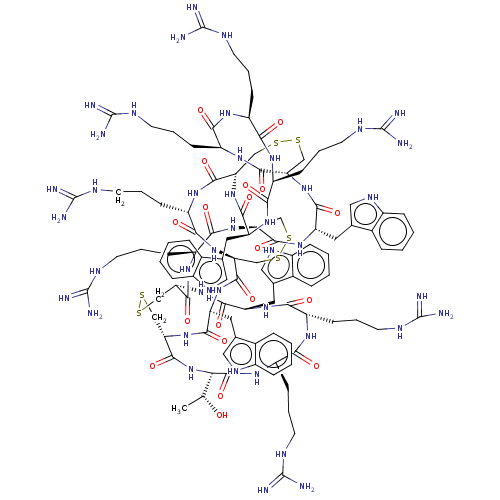 (CHEMBL4094675) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis lethal factor protease preincubated for 30 mins prior to addition of substrate containing fluorescent proteins CyPet... | J Med Chem 60: 1916-1927 (2017) Article DOI: 10.1021/acs.jmedchem.6b01689 BindingDB Entry DOI: 10.7270/Q2377C05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8500 ((2R)-2-cyclopentyl-2-[(4-fluoro-3-methylbenzene)su...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories | Assay Description The assay was performed in a 96-well plate, each well contained substrate peptide, LF, and the test compound. The C-terminally fluorophore of substra... | Bioorg Med Chem Lett 16: 964-8 (2006) Article DOI: 10.1016/j.bmcl.2005.10.088 BindingDB Entry DOI: 10.7270/Q2K35RV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8508 ((2R,3S)-3-cyclopropyl-2-[(4-fluoro-3-methylbenzene...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories | Assay Description The assay was performed in a 96-well plate, each well contained substrate peptide, LF, and the test compound. The C-terminally fluorophore of substra... | Bioorg Med Chem Lett 16: 964-8 (2006) Article DOI: 10.1016/j.bmcl.2005.10.088 BindingDB Entry DOI: 10.7270/Q2K35RV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8506 ((2R)-2-[(4-fluoro-3-methylbenzene)sulfonamido]-N-h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories | Assay Description The assay was performed in a 96-well plate, each well contained substrate peptide, LF, and the test compound. The C-terminally fluorophore of substra... | Bioorg Med Chem Lett 16: 964-8 (2006) Article DOI: 10.1016/j.bmcl.2005.10.088 BindingDB Entry DOI: 10.7270/Q2K35RV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8505 ((2R,3S)-2-[(4-fluoro-3-methylbenzene)sulfonamido]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories | Assay Description The assay was performed in a 96-well plate, each well contained substrate peptide, LF, and the test compound. The C-terminally fluorophore of substra... | Bioorg Med Chem Lett 16: 964-8 (2006) Article DOI: 10.1016/j.bmcl.2005.10.088 BindingDB Entry DOI: 10.7270/Q2K35RV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8496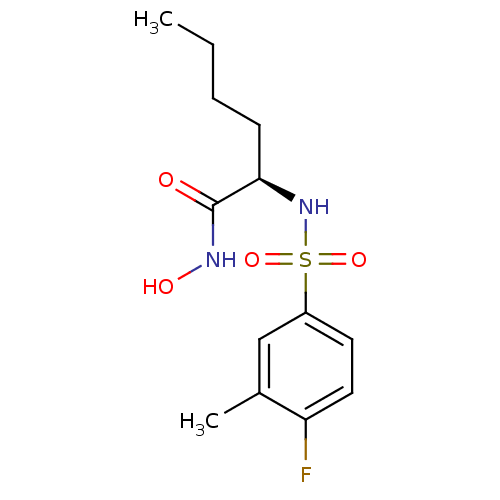 ((2R)-2-[(4-fluoro-3-methylbenzene)sulfonamido]-N-h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories | Assay Description The assay was performed in a 96-well plate, each well contained substrate peptide, LF, and the test compound. The C-terminally fluorophore of substra... | Bioorg Med Chem Lett 16: 964-8 (2006) Article DOI: 10.1016/j.bmcl.2005.10.088 BindingDB Entry DOI: 10.7270/Q2K35RV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8514 (4-[(3R)-3-[(4-fluoro-3-methylbenzene)sulfonamido]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories | Assay Description The assay was performed in a 96-well plate, each well contained substrate peptide, LF, and the test compound. The C-terminally fluorophore of substra... | Bioorg Med Chem Lett 16: 964-8 (2006) Article DOI: 10.1016/j.bmcl.2005.10.088 BindingDB Entry DOI: 10.7270/Q2K35RV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50236206 (CHEMBL4070379) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Inhibitor constant was measured against Muscle phosphorylase a in rabbit | J Med Chem 60: 1916-1927 (2017) Article DOI: 10.1021/acs.jmedchem.6b01689 BindingDB Entry DOI: 10.7270/Q2377C05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50236208 (CHEMBL4081011) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis lethal factor protease preincubated for 30 mins prior to addition of substrate containing fluorescent proteins CyPet... | J Med Chem 60: 1916-1927 (2017) Article DOI: 10.1021/acs.jmedchem.6b01689 BindingDB Entry DOI: 10.7270/Q2377C05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50070942 ((-)-Epigallocatechin gallate | (-)-Epigallocatechi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113877 BindingDB Entry DOI: 10.7270/Q2PK0M78 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50236204 (CHEMBL4091315) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis lethal factor protease preincubated for 30 mins prior to addition of substrate containing fluorescent proteins CyPet... | J Med Chem 60: 1916-1927 (2017) Article DOI: 10.1021/acs.jmedchem.6b01689 BindingDB Entry DOI: 10.7270/Q2377C05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8495 ((2R)-2-[(4-fluoro-3-methylbenzene)sulfonamido]-N-h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories | Assay Description The assay was performed in a 96-well plate, each well contained substrate peptide, LF, and the test compound. The C-terminally fluorophore of substra... | Bioorg Med Chem Lett 16: 964-8 (2006) Article DOI: 10.1016/j.bmcl.2005.10.088 BindingDB Entry DOI: 10.7270/Q2K35RV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8498 ((2R)-2-cyclopropyl-2-[(4-fluoro-3-methylbenzene)su...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories | Assay Description The assay was performed in a 96-well plate, each well contained substrate peptide, LF, and the test compound. The C-terminally fluorophore of substra... | Bioorg Med Chem Lett 16: 964-8 (2006) Article DOI: 10.1016/j.bmcl.2005.10.088 BindingDB Entry DOI: 10.7270/Q2K35RV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8504 (2-[(4-fluoro-3-methylbenzene)sulfonamido]-N-hydrox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories | Assay Description The assay was performed in a 96-well plate, each well contained substrate peptide, LF, and the test compound. The C-terminally fluorophore of substra... | Bioorg Med Chem Lett 16: 964-8 (2006) Article DOI: 10.1016/j.bmcl.2005.10.088 BindingDB Entry DOI: 10.7270/Q2K35RV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50236197 (CHEMBL4099044) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis lethal factor protease preincubated for 30 mins prior to addition of substrate containing fluorescent proteins CyPet... | J Med Chem 60: 1916-1927 (2017) Article DOI: 10.1021/acs.jmedchem.6b01689 BindingDB Entry DOI: 10.7270/Q2377C05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8494 ((2R)-2-[(4-fluoro-3-methylbenzene)sulfonamido]-N-h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories | Assay Description The assay was performed in a 96-well plate, each well contained substrate peptide, LF, and the test compound. The C-terminally fluorophore of substra... | Bioorg Med Chem Lett 16: 964-8 (2006) Article DOI: 10.1016/j.bmcl.2005.10.088 BindingDB Entry DOI: 10.7270/Q2K35RV4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8479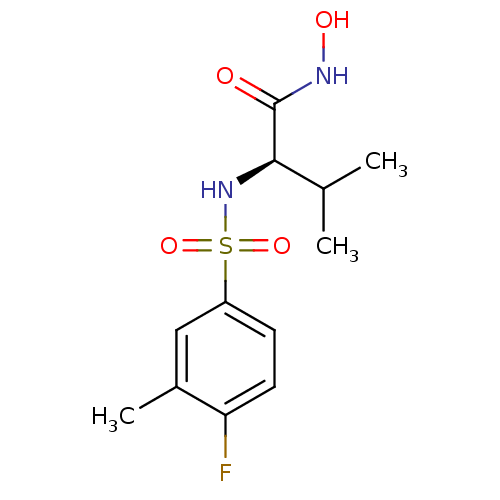 ((2R)-2-[(4-fluoro-3-methylbenzene)sulfonamido]-N-h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Merck Research Laboratories | Assay Description The assay was performed in a 96-well plate, each well contained substrate peptide, LF, and the test compound. The C-terminally fluorophore of substra... | Bioorg Med Chem Lett 16: 964-8 (2006) Article DOI: 10.1016/j.bmcl.2005.10.088 BindingDB Entry DOI: 10.7270/Q2K35RV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8499 ((2R)-2-cyclobutyl-2-[(4-fluoro-3-methylbenzene)sul...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories | Assay Description The assay was performed in a 96-well plate, each well contained substrate peptide, LF, and the test compound. The C-terminally fluorophore of substra... | Bioorg Med Chem Lett 16: 964-8 (2006) Article DOI: 10.1016/j.bmcl.2005.10.088 BindingDB Entry DOI: 10.7270/Q2K35RV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8443 (2-[(5Z)-5-({5-[2-chloro-5-(trifluoromethyl)phenyl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute for Medical Research | Assay Description The fluorescence peptide cleavage assay was performed in a 96-well plate in which each reaction mixture contained MAPKKide, LF (List Biological Labor... | Chem Biol Drug Des 71: 131-9 (2008) Article DOI: 10.1111/j.1747-0285.2007.00617.x BindingDB Entry DOI: 10.7270/Q2KW5DCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50236205 (CHEMBL4064688) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 197 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis lethal factor protease preincubated for 30 mins prior to addition of substrate containing fluorescent proteins CyPet... | J Med Chem 60: 1916-1927 (2017) Article DOI: 10.1021/acs.jmedchem.6b01689 BindingDB Entry DOI: 10.7270/Q2377C05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50236199 (CHEMBL4065687) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 205 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis lethal factor protease preincubated for 30 mins prior to addition of substrate containing fluorescent proteins CyPet... | J Med Chem 60: 1916-1927 (2017) Article DOI: 10.1021/acs.jmedchem.6b01689 BindingDB Entry DOI: 10.7270/Q2377C05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50236193 (CHEMBL4104995) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 221 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Inhibitor constant was measured against Muscle phosphorylase b in rabbit | J Med Chem 60: 1916-1927 (2017) Article DOI: 10.1021/acs.jmedchem.6b01689 BindingDB Entry DOI: 10.7270/Q2377C05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50236202 (CHEMBL4074171) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis lethal factor protease preincubated for 30 mins prior to addition of substrate containing fluorescent proteins CyPet... | J Med Chem 60: 1916-1927 (2017) Article DOI: 10.1021/acs.jmedchem.6b01689 BindingDB Entry DOI: 10.7270/Q2377C05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50236204 (CHEMBL4091315) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis lethal factor protease preincubated for 30 mins prior to addition of substrate containing fluorescent proteins CyPet... | J Med Chem 60: 1916-1927 (2017) Article DOI: 10.1021/acs.jmedchem.6b01689 BindingDB Entry DOI: 10.7270/Q2377C05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8442 (2-[(5Z)-5-{[5-(3,4-dichlorophenyl)furan-2-yl]methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute for Medical Research | Assay Description The fluorescence peptide cleavage assay was performed in a 96-well plate in which each reaction mixture contained MAPKKide, LF (List Biological Labor... | Chem Biol Drug Des 71: 131-9 (2008) Article DOI: 10.1111/j.1747-0285.2007.00617.x BindingDB Entry DOI: 10.7270/Q2KW5DCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50236209 (CHEMBL4067395) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 262 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis lethal factor protease preincubated for 30 mins prior to addition of substrate containing fluorescent proteins CyPet... | J Med Chem 60: 1916-1927 (2017) Article DOI: 10.1021/acs.jmedchem.6b01689 BindingDB Entry DOI: 10.7270/Q2377C05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8442 (2-[(5Z)-5-{[5-(3,4-dichlorophenyl)furan-2-yl]methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 265 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute for Medical Research | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained MAPKKide, LF, and the small-molecule inhibitor. The C-t... | Proc Natl Acad Sci U S A 102: 9499-504 (2005) Article DOI: 10.1073/pnas.0502733102 BindingDB Entry DOI: 10.7270/Q2TM78BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8476 ((2R)-2-[(4-fluorobenzene)sulfonamido]-N-hydroxy-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Merck Research Laboratories | Assay Description The assay was performed in a 96-well plate, each well contained substrate peptide, LF, and the test compound. The C-terminally fluorophore of substra... | Bioorg Med Chem Lett 16: 964-8 (2006) Article DOI: 10.1016/j.bmcl.2005.10.088 BindingDB Entry DOI: 10.7270/Q2K35RV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8441 (2-[(5Z)-5-{[5-(3-chloro-4-methoxyphenyl)furan-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 298 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute for Medical Research | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained MAPKKide, LF, and the small-molecule inhibitor. The C-t... | Proc Natl Acad Sci U S A 102: 9499-504 (2005) Article DOI: 10.1073/pnas.0502733102 BindingDB Entry DOI: 10.7270/Q2TM78BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50328675 (CHEMBL1255963) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis lethal factor | Antimicrob Agents Chemother 52: 2239-41 (2008) Article DOI: 10.1128/AAC.00009-08 BindingDB Entry DOI: 10.7270/Q2K35TWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8497 ((2R)-2-[(4-fluoro-3-methylbenzene)sulfonamido]-N-h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories | Assay Description The assay was performed in a 96-well plate, each well contained substrate peptide, LF, and the test compound. The C-terminally fluorophore of substra... | Bioorg Med Chem Lett 16: 964-8 (2006) Article DOI: 10.1016/j.bmcl.2005.10.088 BindingDB Entry DOI: 10.7270/Q2K35RV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8447 (2-[(5Z)-5-[(5-{[(5Z)-3-(carboxymethyl)-4-oxo-2-sul...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute for Medical Research | Assay Description The fluorescence peptide cleavage assay was performed in a 96-well plate in which each reaction mixture contained MAPKKide, LF (List Biological Labor... | Chem Biol Drug Des 71: 131-9 (2008) Article DOI: 10.1111/j.1747-0285.2007.00617.x BindingDB Entry DOI: 10.7270/Q2KW5DCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50236198 (CHEMBL4073105) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis lethal factor protease preincubated for 30 mins prior to addition of substrate containing fluorescent proteins CyPet... | J Med Chem 60: 1916-1927 (2017) Article DOI: 10.1021/acs.jmedchem.6b01689 BindingDB Entry DOI: 10.7270/Q2377C05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8478 ((2R)-N-hydroxy-3-methyl-2-[(3-methylbenzene)sulfon...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Merck Research Laboratories | Assay Description The assay was performed in a 96-well plate, each well contained substrate peptide, LF, and the test compound. The C-terminally fluorophore of substra... | Bioorg Med Chem Lett 16: 964-8 (2006) Article DOI: 10.1016/j.bmcl.2005.10.088 BindingDB Entry DOI: 10.7270/Q2K35RV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50453861 (CHEMBL4207547) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 406 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis lethal factor protease using substrate containing CyPet and YPet linked by RRKKVYPYPMEGTIA sequence preincubated for... | Bioorg Med Chem 26: 1212-1219 (2018) Article DOI: 10.1016/j.bmc.2017.09.002 BindingDB Entry DOI: 10.7270/Q2HD7Z7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8488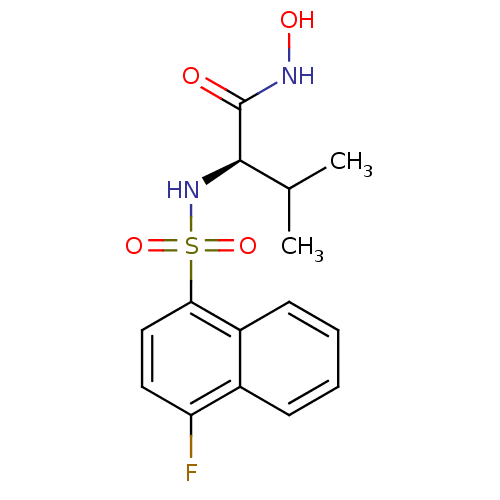 ((2R)-2-[(4-fluoronaphthalene-1-)sulfonamido]-N-hyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories | Assay Description The assay was performed in a 96-well plate, each well contained substrate peptide, LF, and the test compound. The C-terminally fluorophore of substra... | Bioorg Med Chem Lett 16: 964-8 (2006) Article DOI: 10.1016/j.bmcl.2005.10.088 BindingDB Entry DOI: 10.7270/Q2K35RV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8494 ((2R)-2-[(4-fluoro-3-methylbenzene)sulfonamido]-N-h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis Lethal factor after 10 mins by mobility shift protease assay using 8 uM FITC as substrate | J Med Chem 58: 8723-33 (2015) Article DOI: 10.1021/acs.jmedchem.5b01446 BindingDB Entry DOI: 10.7270/Q2TQ63CR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8439 (2-[(5Z)-5-{[5-(4-chloro-2-nitrophenyl)furan-2-yl]m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute for Medical Research | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained MAPKKide, LF, and the small-molecule inhibitor. The C-t... | Proc Natl Acad Sci U S A 102: 9499-504 (2005) Article DOI: 10.1073/pnas.0502733102 BindingDB Entry DOI: 10.7270/Q2TM78BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50328676 (CHEMBL1255964) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis lethal factor | Antimicrob Agents Chemother 52: 2239-41 (2008) Article DOI: 10.1128/AAC.00009-08 BindingDB Entry DOI: 10.7270/Q2K35TWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50236203 (CHEMBL4091910) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 558 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis lethal factor protease preincubated for 30 mins prior to addition of substrate containing fluorescent proteins CyPet... | J Med Chem 60: 1916-1927 (2017) Article DOI: 10.1021/acs.jmedchem.6b01689 BindingDB Entry DOI: 10.7270/Q2377C05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 577 total ) | Next | Last >> |