 Found 252 hits of ec50 for UniProtKB: P20309
Found 252 hits of ec50 for UniProtKB: P20309 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50538220

(CHEMBL4638964)Show SMILES [Br-].[H][C@]12O[C@@]1([H])[C@]1([H])C[C@H](C[C@@]2([H])[N+]1(C)C)OC(=O)C(O)(c1cccs1)c1cccs1 |r| Show InChI InChI=1S/C19H22NO4S2.BrH/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15;/h3-8,11-13,16-17,22H,9-10H2,1-2H3;1H/q+1;/p-1/t11-,12+,13-,16+,17-; | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg
Curated by ChEMBL
| Assay Description
Antagonist activity at muscarinic M3 receptor (unknown origin) expressed in HEK293 cells coexpressing EA tagged beta-arrestin2 assessed as inhibition... |
J Med Chem 63: 4349-4369 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00297
BindingDB Entry DOI: 10.7270/Q2CR5XWB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50475448
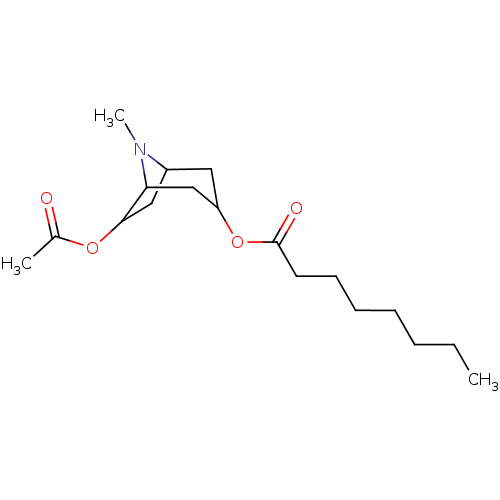
(CHEMBL195011)Show SMILES CCCCCCCC(=O)OC1CC2CC(OC(C)=O)C(C1)N2C |TLB:9:10:21:14.13,THB:15:14:21:20.11.10| Show InChI InChI=1S/C18H31NO4/c1-4-5-6-7-8-9-18(21)23-15-10-14-11-17(22-13(2)20)16(12-15)19(14)3/h14-17H,4-12H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.74 | n/a | n/a | n/a | n/a |
Shanghai Second Medical University
Curated by ChEMBL
| Assay Description
Agonistic activity against Muscarinic acetylcholine receptor of guinea pig ileal longitudinal muscle |
Bioorg Med Chem Lett 15: 4814-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.045
BindingDB Entry DOI: 10.7270/Q23T9KZT |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50538203
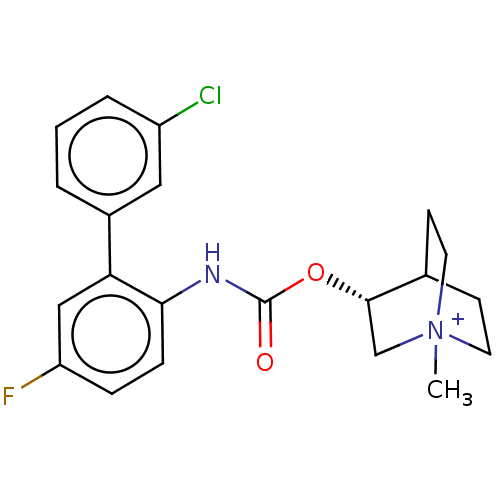
(CHEMBL4647437)Show SMILES C[N+]12CCC(CC1)[C@H](C2)OC(=O)Nc1ccc(F)cc1-c1cccc(Cl)c1 |r| Show InChI InChI=1S/C21H22ClFN2O2.CH2O2/c1-25-9-7-14(8-10-25)20(13-25)27-21(26)24-19-6-5-17(23)12-18(19)15-3-2-4-16(22)11-15;2-1-3/h2-6,11-12,14,20H,7-10,13H2,1H3;1H,(H,2,3)/t14?,20-,25?;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg
Curated by ChEMBL
| Assay Description
Antagonist activity at muscarinic M3 receptor (unknown origin) expressed in HEK293 cells coexpressing EA tagged beta-arrestin2 assessed as inhibition... |
J Med Chem 63: 4349-4369 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00297
BindingDB Entry DOI: 10.7270/Q2CR5XWB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50538202

(CHEMBL4633816)Show SMILES C[N+]12CCC(CC1)[C@H](C2)OC(=O)Nc1ccc(F)cc1-c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C21H21ClF2N2O2.CH2O2/c1-26-8-6-13(7-9-26)20(12-26)28-21(27)25-19-5-3-15(23)11-16(19)14-2-4-18(24)17(22)10-14;2-1-3/h2-5,10-11,13,20H,6-9,12H2,1H3;1H,(H,2,3)/t13?,20-,26?;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg
Curated by ChEMBL
| Assay Description
Antagonist activity at muscarinic M3 receptor (unknown origin) expressed in HEK293 cells coexpressing EA tagged beta-arrestin2 assessed as inhibition... |
J Med Chem 63: 4349-4369 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00297
BindingDB Entry DOI: 10.7270/Q2CR5XWB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM10759

(2-acetoxyethyl(trimethyl)ammonium;bromide | 2-acet...)Show InChI InChI=1S/C7H16NO2/c1-7(9)10-6-5-8(2,3)4/h5-6H2,1-4H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a |
H. Lundbeck A/S
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M3 receptor expressed in BHK-21 cells assessed as increase of acetylcholine-induced calcium flux by FLIPR assay |
J Med Chem 53: 6386-97 (2010)
Article DOI: 10.1021/jm100697g
BindingDB Entry DOI: 10.7270/Q2765G9K |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM10759

(2-acetoxyethyl(trimethyl)ammonium;bromide | 2-acet...)Show InChI InChI=1S/C7H16NO2/c1-7(9)10-6-5-8(2,3)4/h5-6H2,1-4H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a |
H. Lundbeck A/S
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M3 receptor expressed in BHK-21 cells assessed as calcium mobilization for 6 mins by Calcium4-based staining |
Bioorg Med Chem Lett 22: 5134-40 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.048
BindingDB Entry DOI: 10.7270/Q24M95MM |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50079583
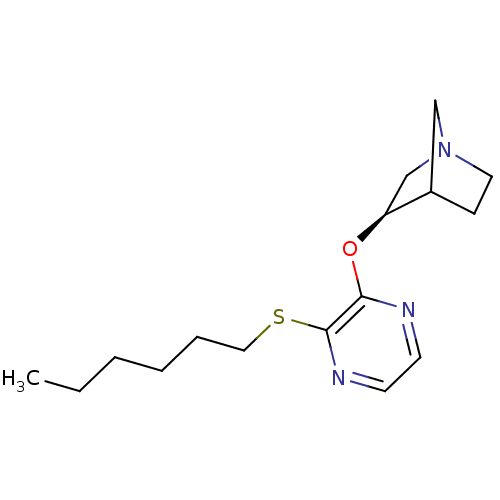
((R)-3-(3-Hexylsulfanyl-pyrazin-2-yloxy)-1-aza-bicy...)Show SMILES CCCCCCSc1nccnc1O[C@H]1CN2CCC1C2 |TLB:13:14:20:18.17| Show InChI InChI=1S/C16H25N3OS/c1-2-3-4-5-10-21-16-15(17-7-8-18-16)20-14-12-19-9-6-13(14)11-19/h7-8,13-14H,2-6,9-12H2,1H3/t13?,14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro functional agonism against M3 muscarinic receptor (PI) |
Bioorg Med Chem Lett 9: 1895-900 (1999)
BindingDB Entry DOI: 10.7270/Q2G161CX |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50538205
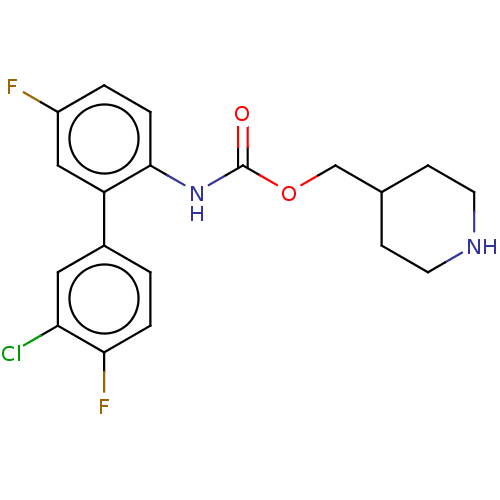
(CHEMBL4636528)Show SMILES Fc1ccc(NC(=O)OCC2CCNCC2)c(c1)-c1ccc(F)c(Cl)c1 Show InChI InChI=1S/C19H19ClF2N2O2/c20-16-9-13(1-3-17(16)22)15-10-14(21)2-4-18(15)24-19(25)26-11-12-5-7-23-8-6-12/h1-4,9-10,12,23H,5-8,11H2,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M3 receptor expressed in HEK293 cells assessed as increase in IP1 accumulation incubated for 90 min by TR-HTRF a... |
J Med Chem 63: 4349-4369 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00297
BindingDB Entry DOI: 10.7270/Q2CR5XWB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50538205

(CHEMBL4636528)Show SMILES Fc1ccc(NC(=O)OCC2CCNCC2)c(c1)-c1ccc(F)c(Cl)c1 Show InChI InChI=1S/C19H19ClF2N2O2/c20-16-9-13(1-3-17(16)22)15-10-14(21)2-4-18(15)24-19(25)26-11-12-5-7-23-8-6-12/h1-4,9-10,12,23H,5-8,11H2,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg
Curated by ChEMBL
| Assay Description
Antagonist activity at muscarinic M3 receptor (unknown origin) expressed in HEK293 cells coexpressing EA tagged beta-arrestin2 assessed as inhibition... |
J Med Chem 63: 4349-4369 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00297
BindingDB Entry DOI: 10.7270/Q2CR5XWB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50475454

(CHEMBL196218)Show SMILES CCCCCC(=O)OC1CC2CC(CC1N2C)OC(=O)CCCCC |TLB:17:12:15:8.9,THB:7:8:15:13.11.12| Show InChI InChI=1S/C20H35NO4/c1-4-6-8-10-19(22)24-16-12-15-13-18(17(14-16)21(15)3)25-20(23)11-9-7-5-2/h15-18H,4-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.79 | n/a | n/a | n/a | n/a |
Shanghai Second Medical University
Curated by ChEMBL
| Assay Description
Agonistic activity against Muscarinic acetylcholine receptor of guinea pig ileal longitudinal muscle |
Bioorg Med Chem Lett 15: 4814-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.045
BindingDB Entry DOI: 10.7270/Q23T9KZT |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50538220

(CHEMBL4638964)Show SMILES [Br-].[H][C@]12O[C@@]1([H])[C@]1([H])C[C@H](C[C@@]2([H])[N+]1(C)C)OC(=O)C(O)(c1cccs1)c1cccs1 |r| Show InChI InChI=1S/C19H22NO4S2.BrH/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15;/h3-8,11-13,16-17,22H,9-10H2,1-2H3;1H/q+1;/p-1/t11-,12+,13-,16+,17-; | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg
Curated by ChEMBL
| Assay Description
Antagonist activity at human muscarinic M3 receptor expressed in HEK293 assessed as inhibition of carbachol-induced IP1 accumulation preincubated for... |
J Med Chem 63: 4349-4369 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00297
BindingDB Entry DOI: 10.7270/Q2CR5XWB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50064609
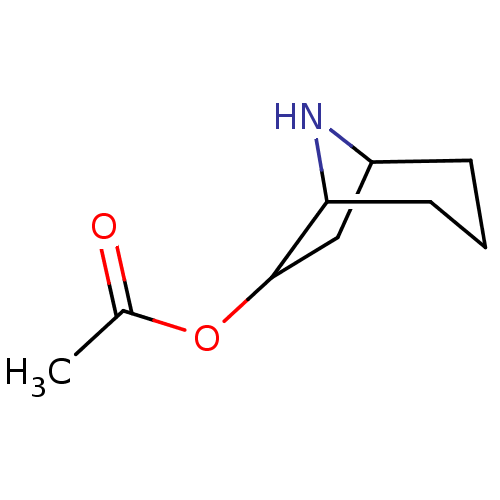
(6-acetoxy-8-azonia-bicyclo[3.2.1]octane | Acetic a...)Show InChI InChI=1S/C9H15NO2/c1-6(11)12-9-5-7-3-2-4-8(9)10-7/h7-10H,2-5H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.17 | n/a | n/a | n/a | n/a |
Shanghai Second Medical University
Curated by ChEMBL
| Assay Description
Agonistic activity against Muscarinic acetylcholine receptor of guinea pig ileal longitudinal muscle |
Bioorg Med Chem Lett 15: 4814-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.045
BindingDB Entry DOI: 10.7270/Q23T9KZT |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50538203

(CHEMBL4647437)Show SMILES C[N+]12CCC(CC1)[C@H](C2)OC(=O)Nc1ccc(F)cc1-c1cccc(Cl)c1 |r| Show InChI InChI=1S/C21H22ClFN2O2.CH2O2/c1-25-9-7-14(8-10-25)20(13-25)27-21(26)24-19-6-5-17(23)12-18(19)15-3-2-4-16(22)11-15;2-1-3/h2-6,11-12,14,20H,7-10,13H2,1H3;1H,(H,2,3)/t14?,20-,25?;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg
Curated by ChEMBL
| Assay Description
Antagonist activity at human muscarinic M3 receptor expressed in HEK293 assessed as inhibition of carbachol-induced IP1 accumulation preincubated for... |
J Med Chem 63: 4349-4369 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00297
BindingDB Entry DOI: 10.7270/Q2CR5XWB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50475459

(CHEMBL194008)Show SMILES CCCCCC(=O)OC1CC2CC(OC(C)=O)C(C1)N2C |TLB:7:8:19:12.11,THB:13:12:19:18.9.8| Show InChI InChI=1S/C16H27NO4/c1-4-5-6-7-16(19)21-13-8-12-9-15(20-11(2)18)14(10-13)17(12)3/h12-15H,4-10H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.46 | n/a | n/a | n/a | n/a |
Shanghai Second Medical University
Curated by ChEMBL
| Assay Description
Agonistic activity against Muscarinic acetylcholine receptor of guinea pig ileal longitudinal muscle |
Bioorg Med Chem Lett 15: 4814-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.045
BindingDB Entry DOI: 10.7270/Q23T9KZT |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50538202

(CHEMBL4633816)Show SMILES C[N+]12CCC(CC1)[C@H](C2)OC(=O)Nc1ccc(F)cc1-c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C21H21ClF2N2O2.CH2O2/c1-26-8-6-13(7-9-26)20(12-26)28-21(27)25-19-5-3-15(23)11-16(19)14-2-4-18(24)17(22)10-14;2-1-3/h2-5,10-11,13,20H,6-9,12H2,1H3;1H,(H,2,3)/t13?,20-,26?;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg
Curated by ChEMBL
| Assay Description
Antagonist activity at human muscarinic M3 receptor expressed in HEK293 assessed as inhibition of carbachol-induced IP1 accumulation preincubated for... |
J Med Chem 63: 4349-4369 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00297
BindingDB Entry DOI: 10.7270/Q2CR5XWB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50538206
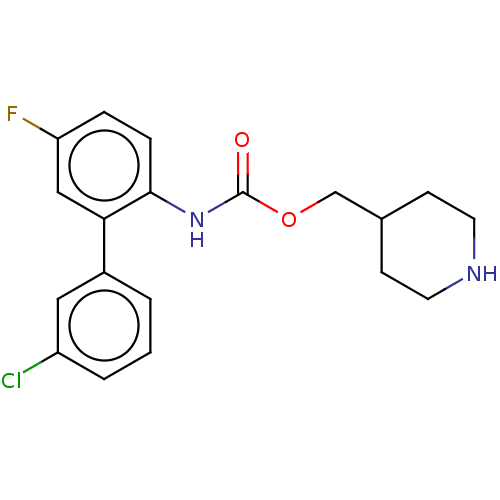
(CHEMBL4640703)Show InChI InChI=1S/C19H20ClFN2O2/c20-15-3-1-2-14(10-15)17-11-16(21)4-5-18(17)23-19(24)25-12-13-6-8-22-9-7-13/h1-5,10-11,13,22H,6-9,12H2,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M3 receptor expressed in HEK293 cells assessed as increase in IP1 accumulation incubated for 90 min by TR-HTRF a... |
J Med Chem 63: 4349-4369 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00297
BindingDB Entry DOI: 10.7270/Q2CR5XWB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50538205

(CHEMBL4636528)Show SMILES Fc1ccc(NC(=O)OCC2CCNCC2)c(c1)-c1ccc(F)c(Cl)c1 Show InChI InChI=1S/C19H19ClF2N2O2/c20-16-9-13(1-3-17(16)22)15-10-14(21)2-4-18(15)24-19(25)26-11-12-5-7-23-8-6-12/h1-4,9-10,12,23H,5-8,11H2,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg
Curated by ChEMBL
| Assay Description
Antagonist activity at human muscarinic M3 receptor expressed in HEK293 assessed as inhibition of carbachol-induced IP1 accumulation preincubated for... |
J Med Chem 63: 4349-4369 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00297
BindingDB Entry DOI: 10.7270/Q2CR5XWB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50109647
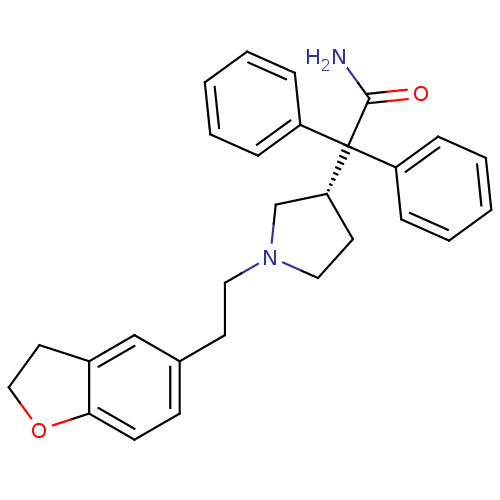
(2-{1-[2-(2,3-Dihydro-benzofuran-5-yl)-ethyl]-pyrro...)Show SMILES NC(=O)C([C@@H]1CCN(CCc2ccc3OCCc3c2)C1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C28H30N2O2/c29-27(31)28(23-7-3-1-4-8-23,24-9-5-2-6-10-24)25-14-17-30(20-25)16-13-21-11-12-26-22(19-21)15-18-32-26/h1-12,19,25H,13-18,20H2,(H2,29,31)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg
Curated by ChEMBL
| Assay Description
Antagonist activity at muscarinic M3 receptor (unknown origin) expressed in HEK293 cells coexpressing EA tagged beta-arrestin2 assessed as inhibition... |
J Med Chem 63: 4349-4369 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00297
BindingDB Entry DOI: 10.7270/Q2CR5XWB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50538206

(CHEMBL4640703)Show InChI InChI=1S/C19H20ClFN2O2/c20-15-3-1-2-14(10-15)17-11-16(21)4-5-18(17)23-19(24)25-12-13-6-8-22-9-7-13/h1-5,10-11,13,22H,6-9,12H2,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg
Curated by ChEMBL
| Assay Description
Antagonist activity at human muscarinic M3 receptor expressed in HEK293 assessed as inhibition of carbachol-induced IP1 accumulation preincubated for... |
J Med Chem 63: 4349-4369 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00297
BindingDB Entry DOI: 10.7270/Q2CR5XWB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50538208

(CHEMBL4648071)Show SMILES Fc1ccc(NC(=O)O[C@H]2CN3CCC2CC3)c(c1)-c1cccc(Cl)c1 |r,wU:9.8,(59.08,-25.57,;59.08,-24.06,;60.42,-23.29,;60.41,-21.74,;59.07,-20.98,;59.07,-19.44,;60.41,-18.66,;60.4,-17.12,;61.74,-19.43,;63.07,-18.66,;63.07,-17.12,;64.4,-16.35,;65.74,-17.12,;65.74,-18.66,;64.41,-19.43,;64.89,-18.24,;63.9,-17.85,;57.75,-21.75,;57.75,-23.29,;56.42,-20.98,;55.09,-21.75,;53.75,-20.99,;53.75,-19.44,;55.09,-18.67,;55.09,-17.16,;56.42,-19.44,)| Show InChI InChI=1S/C20H20ClFN2O2/c21-15-3-1-2-14(10-15)17-11-16(22)4-5-18(17)23-20(25)26-19-12-24-8-6-13(19)7-9-24/h1-5,10-11,13,19H,6-9,12H2,(H,23,25)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg
Curated by ChEMBL
| Assay Description
Antagonist activity at human muscarinic M3 receptor expressed in HEK293 assessed as inhibition of carbachol-induced IP1 accumulation preincubated for... |
J Med Chem 63: 4349-4369 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00297
BindingDB Entry DOI: 10.7270/Q2CR5XWB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50538207
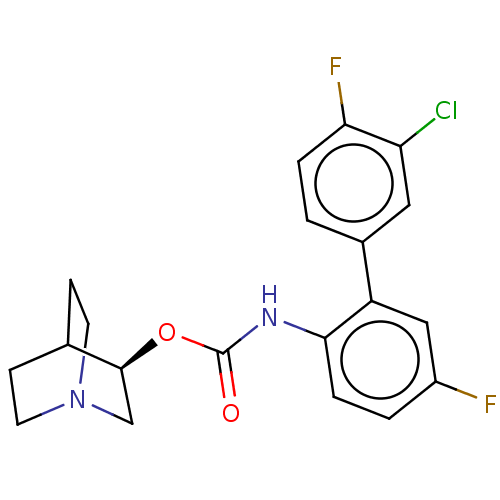
(CHEMBL4640324)Show SMILES Fc1ccc(NC(=O)O[C@H]2CN3CCC2CC3)c(c1)-c1ccc(F)c(Cl)c1 |r,wU:9.8,(12.23,-40.25,;12.23,-38.74,;13.58,-37.97,;13.57,-36.42,;12.23,-35.65,;12.23,-34.11,;13.56,-33.34,;13.56,-31.8,;14.89,-34.11,;16.23,-33.33,;16.23,-31.8,;17.56,-31.03,;18.9,-31.8,;18.9,-33.34,;17.57,-34.11,;18.04,-32.92,;17.05,-32.52,;10.91,-36.42,;10.9,-37.97,;9.57,-35.66,;8.25,-36.42,;6.91,-35.66,;6.91,-34.11,;5.6,-33.36,;8.25,-33.34,;8.25,-31.83,;9.57,-34.11,)| Show InChI InChI=1S/C20H19ClF2N2O2/c21-16-9-13(1-3-17(16)23)15-10-14(22)2-4-18(15)24-20(26)27-19-11-25-7-5-12(19)6-8-25/h1-4,9-10,12,19H,5-8,11H2,(H,24,26)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg
Curated by ChEMBL
| Assay Description
Antagonist activity at human muscarinic M3 receptor expressed in HEK293 assessed as inhibition of carbachol-induced IP1 accumulation preincubated for... |
J Med Chem 63: 4349-4369 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00297
BindingDB Entry DOI: 10.7270/Q2CR5XWB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50004656

((2-Carbamoyloxy-ethyl)-trimethyl-ammonium | (2-Car...)Show InChI InChI=1S/C6H14N2O2/c1-8(2,3)4-5-10-6(7)9/h4-5H2,1-3H3,(H-,7,9)/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50004656

((2-Carbamoyloxy-ethyl)-trimethyl-ammonium | (2-Car...)Show InChI InChI=1S/C6H14N2O2/c1-8(2,3)4-5-10-6(7)9/h4-5H2,1-3H3,(H-,7,9)/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg
Curated by ChEMBL
| Assay Description
Agonist activity at muscarinic M3 receptor (unknown origin) expressed in HEK293 cells coexpressing EA tagged beta-arrestin2 assessed as beta-arrestin... |
J Med Chem 63: 4349-4369 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00297
BindingDB Entry DOI: 10.7270/Q2CR5XWB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM10759

(2-acetoxyethyl(trimethyl)ammonium;bromide | 2-acet...)Show InChI InChI=1S/C7H16NO2/c1-7(9)10-6-5-8(2,3)4/h5-6H2,1-4H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human muscarinic M3 receptor expressed in CHO cells assessed as upregulation in ERK1/2 phosphorylation after 5 mins b... |
J Med Chem 60: 9239-9250 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01113
BindingDB Entry DOI: 10.7270/Q2445PW2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM10759

(2-acetoxyethyl(trimethyl)ammonium;bromide | 2-acet...)Show InChI InChI=1S/C7H16NO2/c1-7(9)10-6-5-8(2,3)4/h5-6H2,1-4H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human muscarinic M3 receptor expressed in CHO cells assessed as upregulation in ERK1/2 phosphorylation after 5 mins b... |
J Med Chem 60: 9239-9250 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01113
BindingDB Entry DOI: 10.7270/Q2445PW2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50004656

((2-Carbamoyloxy-ethyl)-trimethyl-ammonium | (2-Car...)Show InChI InChI=1S/C6H14N2O2/c1-8(2,3)4-5-10-6(7)9/h4-5H2,1-3H3,(H-,7,9)/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50370682
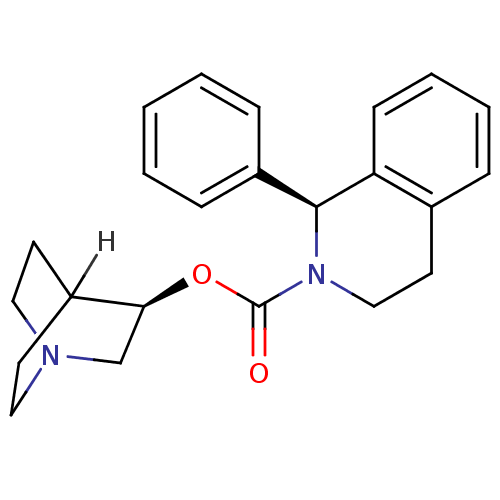
(SOLIFENACIN)Show SMILES [H]C12CCN(CC1)C[C@@H]2OC(=O)N1CCc2ccccc2[C@@H]1c1ccccc1 |wD:8.10,21.25,TLB:9:8:2.3:6.5,(23.54,-9.18,;23.57,-7.64,;22.04,-8.3,;21.84,-6.92,;23.31,-6.28,;23.38,-4.63,;23.82,-5.74,;24.65,-6.88,;24.94,-8.28,;24.93,-9.81,;23.59,-10.56,;22.26,-9.79,;23.59,-12.1,;24.92,-12.9,;24.88,-14.45,;23.53,-15.19,;23.52,-16.72,;22.17,-17.47,;20.85,-16.68,;20.87,-15.14,;22.21,-14.39,;22.23,-12.84,;20.91,-12.06,;19.57,-12.81,;18.25,-12.02,;18.28,-10.47,;19.62,-9.73,;20.94,-10.52,)| Show InChI InChI=1S/C23H26N2O2/c26-23(27-21-16-24-13-10-18(21)11-14-24)25-15-12-17-6-4-5-9-20(17)22(25)19-7-2-1-3-8-19/h1-9,18,21-22H,10-16H2/t21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| DrugBank
| n/a | n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50538207

(CHEMBL4640324)Show SMILES Fc1ccc(NC(=O)O[C@H]2CN3CCC2CC3)c(c1)-c1ccc(F)c(Cl)c1 |r,wU:9.8,(12.23,-40.25,;12.23,-38.74,;13.58,-37.97,;13.57,-36.42,;12.23,-35.65,;12.23,-34.11,;13.56,-33.34,;13.56,-31.8,;14.89,-34.11,;16.23,-33.33,;16.23,-31.8,;17.56,-31.03,;18.9,-31.8,;18.9,-33.34,;17.57,-34.11,;18.04,-32.92,;17.05,-32.52,;10.91,-36.42,;10.9,-37.97,;9.57,-35.66,;8.25,-36.42,;6.91,-35.66,;6.91,-34.11,;5.6,-33.36,;8.25,-33.34,;8.25,-31.83,;9.57,-34.11,)| Show InChI InChI=1S/C20H19ClF2N2O2/c21-16-9-13(1-3-17(16)23)15-10-14(22)2-4-18(15)24-20(26)27-19-11-25-7-5-12(19)6-8-25/h1-4,9-10,12,19H,5-8,11H2,(H,24,26)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg
Curated by ChEMBL
| Assay Description
Antagonist activity at muscarinic M3 receptor (unknown origin) expressed in HEK293 cells coexpressing EA tagged beta-arrestin2 assessed as inhibition... |
J Med Chem 63: 4349-4369 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00297
BindingDB Entry DOI: 10.7270/Q2CR5XWB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50004656

((2-Carbamoyloxy-ethyl)-trimethyl-ammonium | (2-Car...)Show InChI InChI=1S/C6H14N2O2/c1-8(2,3)4-5-10-6(7)9/h4-5H2,1-3H3,(H-,7,9)/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M3 receptor expressed in HEK293 cells assessed as increase in IP1 accumulation incubated for 90 min by TR-HTRF a... |
J Med Chem 63: 4349-4369 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00297
BindingDB Entry DOI: 10.7270/Q2CR5XWB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50538206

(CHEMBL4640703)Show InChI InChI=1S/C19H20ClFN2O2/c20-15-3-1-2-14(10-15)17-11-16(21)4-5-18(17)23-19(24)25-12-13-6-8-22-9-7-13/h1-5,10-11,13,22H,6-9,12H2,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg
Curated by ChEMBL
| Assay Description
Antagonist activity at muscarinic M3 receptor (unknown origin) expressed in HEK293 cells coexpressing EA tagged beta-arrestin2 assessed as inhibition... |
J Med Chem 63: 4349-4369 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00297
BindingDB Entry DOI: 10.7270/Q2CR5XWB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50475443

(CHEMBL364454)Show SMILES CCCCCCC(=O)OC1CC2CC(OC(C)=O)C(C1)N2C |TLB:8:9:20:13.12,THB:14:13:20:19.10.9| Show InChI InChI=1S/C17H29NO4/c1-4-5-6-7-8-17(20)22-14-9-13-10-16(21-12(2)19)15(11-14)18(13)3/h13-16H,4-11H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 26.9 | n/a | n/a | n/a | n/a |
Shanghai Second Medical University
Curated by ChEMBL
| Assay Description
Agonistic activity against Muscarinic acetylcholine receptor of guinea pig ileal longitudinal muscle |
Bioorg Med Chem Lett 15: 4814-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.045
BindingDB Entry DOI: 10.7270/Q23T9KZT |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50538208

(CHEMBL4648071)Show SMILES Fc1ccc(NC(=O)O[C@H]2CN3CCC2CC3)c(c1)-c1cccc(Cl)c1 |r,wU:9.8,(59.08,-25.57,;59.08,-24.06,;60.42,-23.29,;60.41,-21.74,;59.07,-20.98,;59.07,-19.44,;60.41,-18.66,;60.4,-17.12,;61.74,-19.43,;63.07,-18.66,;63.07,-17.12,;64.4,-16.35,;65.74,-17.12,;65.74,-18.66,;64.41,-19.43,;64.89,-18.24,;63.9,-17.85,;57.75,-21.75,;57.75,-23.29,;56.42,-20.98,;55.09,-21.75,;53.75,-20.99,;53.75,-19.44,;55.09,-18.67,;55.09,-17.16,;56.42,-19.44,)| Show InChI InChI=1S/C20H20ClFN2O2/c21-15-3-1-2-14(10-15)17-11-16(22)4-5-18(17)23-20(25)26-19-12-24-8-6-13(19)7-9-24/h1-5,10-11,13,19H,6-9,12H2,(H,23,25)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg
Curated by ChEMBL
| Assay Description
Antagonist activity at muscarinic M3 receptor (unknown origin) expressed in HEK293 cells coexpressing EA tagged beta-arrestin2 assessed as inhibition... |
J Med Chem 63: 4349-4369 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00297
BindingDB Entry DOI: 10.7270/Q2CR5XWB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50046728
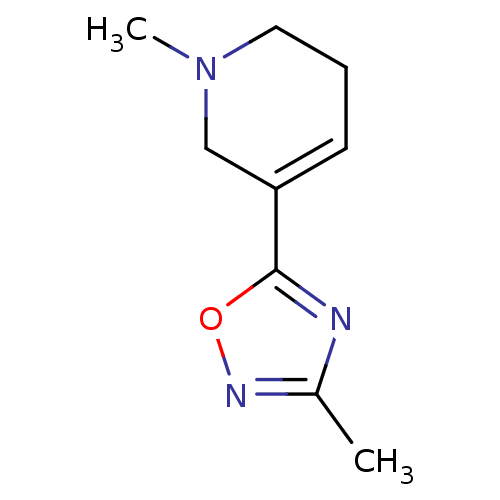
(1-Methyl-5-(3-methyl-[1,2,4]oxadiazol-5-yl)-1,2,3,...)Show InChI InChI=1S/C9H13N3O/c1-7-10-9(13-11-7)8-4-3-5-12(2)6-8/h4H,3,5-6H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a |
Ferrosan A/S
Curated by ChEMBL
| Assay Description
Agonist activity at muscarinic acetylcholine receptor in isolated guinea pig ileum. |
J Med Chem 34: 687-92 (1991)
BindingDB Entry DOI: 10.7270/Q2RN3B25 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50109647

(2-{1-[2-(2,3-Dihydro-benzofuran-5-yl)-ethyl]-pyrro...)Show SMILES NC(=O)C([C@@H]1CCN(CCc2ccc3OCCc3c2)C1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C28H30N2O2/c29-27(31)28(23-7-3-1-4-8-23,24-9-5-2-6-10-24)25-14-17-30(20-25)16-13-21-11-12-26-22(19-21)15-18-32-26/h1-12,19,25H,13-18,20H2,(H2,29,31)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M3 receptor expressed in HEK293 cells assessed as increase in IP1 accumulation incubated for 90 min by TR-HTRF a... |
J Med Chem 63: 4349-4369 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00297
BindingDB Entry DOI: 10.7270/Q2CR5XWB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM193787

(US9670209, Compound 304 1-((R)-3-(3-(Cyclopropylme...)Show SMILES C[C@H](CN1[C@H]2CC[C@@H]1C[C@@H](C2)OCC1CC1)Cn1ncc2ccc(C)cc12 |r,THB:2:3:8.9.10:5.6| Show InChI InChI=1S/C23H33N3O/c1-16-3-6-19-12-24-26(23(19)9-16)14-17(2)13-25-20-7-8-21(25)11-22(10-20)27-15-18-4-5-18/h3,6,9,12,17-18,20-22H,4-5,7-8,10-11,13-15H2,1-2H3/t17-,20-,21+,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
ACADIA PHARMACEUTICALS INC.; ALLERGAN, INC.
US Patent
| Assay Description
NIH-3T3 cells were grown in 96-well tissue culture plates to 70% to 80% confluence. Cells were transfected with plasmid DNAs using Superfect Reagent ... |
US Patent US9670209 (2017)
BindingDB Entry DOI: 10.7270/Q2MS3QX2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM193787

(US9670209, Compound 304 1-((R)-3-(3-(Cyclopropylme...)Show SMILES C[C@H](CN1[C@H]2CC[C@@H]1C[C@@H](C2)OCC1CC1)Cn1ncc2ccc(C)cc12 |r,THB:2:3:8.9.10:5.6| Show InChI InChI=1S/C23H33N3O/c1-16-3-6-19-12-24-26(23(19)9-16)14-17(2)13-25-20-7-8-21(25)11-22(10-20)27-15-18-4-5-18/h3,6,9,12,17-18,20-22H,4-5,7-8,10-11,13-15H2,1-2H3/t17-,20-,21+,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
ACADIA PHARMACEUTICALS INC.; ALLERGAN, INC.
US Patent
| Assay Description
NIH-3T3 cells were grown in 96-well tissue culture plates to 70% to 80% confluence. Cells were transfected with plasmid DNAs using Superfect Reagent ... |
US Patent US9670209 (2017)
BindingDB Entry DOI: 10.7270/Q2MS3QX2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50475458
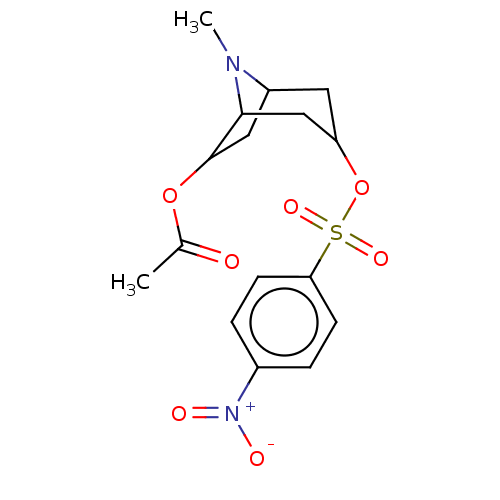
(CHEMBL194793)Show SMILES CN1C2CC(OC(C)=O)C1CC(C2)OS(=O)(=O)c1ccc(cc1)[N+]([O-])=O |TLB:5:4:1:11.10.12,THB:13:11:1:4.3| Show InChI InChI=1S/C16H20N2O7S/c1-10(19)24-16-8-12-7-13(9-15(16)17(12)2)25-26(22,23)14-5-3-11(4-6-14)18(20)21/h3-6,12-13,15-16H,7-9H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 44.7 | n/a | n/a | n/a | n/a |
Shanghai Second Medical University
Curated by ChEMBL
| Assay Description
Agonistic activity against Muscarinic acetylcholine receptor of guinea pig ileal longitudinal muscle |
Bioorg Med Chem Lett 15: 4814-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.045
BindingDB Entry DOI: 10.7270/Q23T9KZT |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50109647

(2-{1-[2-(2,3-Dihydro-benzofuran-5-yl)-ethyl]-pyrro...)Show SMILES NC(=O)C([C@@H]1CCN(CCc2ccc3OCCc3c2)C1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C28H30N2O2/c29-27(31)28(23-7-3-1-4-8-23,24-9-5-2-6-10-24)25-14-17-30(20-25)16-13-21-11-12-26-22(19-21)15-18-32-26/h1-12,19,25H,13-18,20H2,(H2,29,31)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | n/a | 45 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg
Curated by ChEMBL
| Assay Description
Antagonist activity at human muscarinic M3 receptor expressed in HEK293 assessed as inhibition of carbachol-induced IP1 accumulation preincubated for... |
J Med Chem 63: 4349-4369 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00297
BindingDB Entry DOI: 10.7270/Q2CR5XWB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM193789

(US9670209, Compound 307 1-((R)-3-(3-(Cyclopropylme...)Show SMILES C[C@H](CN1[C@H]2CC[C@@H]1C[C@@H](C2)OCC1CC1)Cn1ncc2ccc(F)cc12 |r,THB:2:3:8.9.10:5.6| Show InChI InChI=1S/C22H30FN3O/c1-15(13-26-22-8-18(23)5-4-17(22)11-24-26)12-25-19-6-7-20(25)10-21(9-19)27-14-16-2-3-16/h4-5,8,11,15-16,19-21H,2-3,6-7,9-10,12-14H2,1H3/t15-,19-,20+,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
ACADIA PHARMACEUTICALS INC.; ALLERGAN, INC.
US Patent
| Assay Description
NIH-3T3 cells were grown in 96-well tissue culture plates to 70% to 80% confluence. Cells were transfected with plasmid DNAs using Superfect Reagent ... |
US Patent US9670209 (2017)
BindingDB Entry DOI: 10.7270/Q2MS3QX2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50475453

(CHEMBL372536)Show SMILES CN1C2CC(OC(C)=O)C1CC(C2)OS(=O)(=O)c1ccccc1 |TLB:5:4:1:11.10.12,THB:13:11:1:4.3| Show InChI InChI=1S/C16H21NO5S/c1-11(18)21-16-9-12-8-13(10-15(16)17(12)2)22-23(19,20)14-6-4-3-5-7-14/h3-7,12-13,15-16H,8-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 50.1 | n/a | n/a | n/a | n/a |
Shanghai Second Medical University
Curated by ChEMBL
| Assay Description
Agonistic activity against Muscarinic acetylcholine receptor of guinea pig ileal longitudinal muscle |
Bioorg Med Chem Lett 15: 4814-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.045
BindingDB Entry DOI: 10.7270/Q23T9KZT |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50416733

(CHEMBL1223803)Show SMILES CCCO[C@H]1CC[C@@](C)(CC1)N1CCC(CC1)N1C(=O)Cc2ccc(C)cc12 |r,wD:4.3,7.7,(20.54,1.43,;19,1.41,;18.25,.07,;16.71,.05,;15.96,-1.29,;14.42,-1.31,;13.66,-2.66,;14.45,-3.97,;12.91,-3.97,;15.99,-3.95,;16.74,-2.62,;13.7,-5.32,;14.49,-6.64,;13.74,-7.98,;12.2,-8.01,;11.41,-6.69,;12.16,-5.34,;11.47,-9.36,;12.38,-10.62,;13.92,-10.62,;11.47,-11.88,;9.99,-11.4,;8.65,-12.17,;7.32,-11.4,;7.32,-9.84,;5.99,-9.07,;8.65,-9.08,;9.98,-9.84,)| Show InChI InChI=1S/C24H36N2O2/c1-4-15-28-21-7-11-24(3,12-8-21)25-13-9-20(10-14-25)26-22-16-18(2)5-6-19(22)17-23(26)27/h5-6,16,20-21H,4,7-15,17H2,1-3H3/t21-,24- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 63.1 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M3 receptor expressed in CHO cells assessed as effect on calcium mobilization by FLIPR assay |
Bioorg Med Chem Lett 20: 5434-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.097
BindingDB Entry DOI: 10.7270/Q2MG7QR7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50004656

((2-Carbamoyloxy-ethyl)-trimethyl-ammonium | (2-Car...)Show InChI InChI=1S/C6H14N2O2/c1-8(2,3)4-5-10-6(7)9/h4-5H2,1-3H3,(H-,7,9)/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Contraction of guinea pig ileum by muscarinic AChR activation, which could be inhibited by application of atropine |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM46858
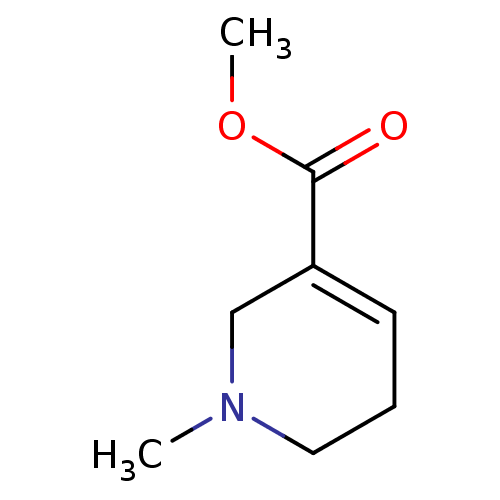
(1-methyl-3,6-dihydro-2H-pyridine-5-carboxylic acid...)Show InChI InChI=1S/C8H13NO2/c1-9-5-3-4-7(6-9)8(10)11-2/h4H,3,5-6H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 73 | n/a | n/a | n/a | n/a |
Ferrosan A/S
Curated by ChEMBL
| Assay Description
Agonist activity at muscarinic acetylcholine receptor in isolated guinea pig ileum. |
J Med Chem 34: 687-92 (1991)
BindingDB Entry DOI: 10.7270/Q2RN3B25 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM193834

(US9670209, Comparative compound 2)Show SMILES CC(C)(C)OC(=O)N1C2CC[C@H]1C[C@@H](O)C2 |r,$_AV:13;11;14;15;4;;7;1;2;9;10;3;5;8;12;6$,THB:14:13:7:9.10| Show InChI InChI=1S/C12H21NO3/c1-12(2,3)16-11(15)13-8-4-5-9(13)7-10(14)6-8/h8-10,14H,4-7H2,1-3H3/t8-,9?,10+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 79 | n/a | n/a | n/a | n/a |
ACADIA PHARMACEUTICALS INC.; ALLERGAN, INC.
US Patent
| Assay Description
NIH-3T3 cells were grown in 96-well tissue culture plates to 70% to 80% confluence. Cells were transfected with plasmid DNAs using Superfect Reagent ... |
US Patent US9670209 (2017)
BindingDB Entry DOI: 10.7270/Q2MS3QX2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50475449
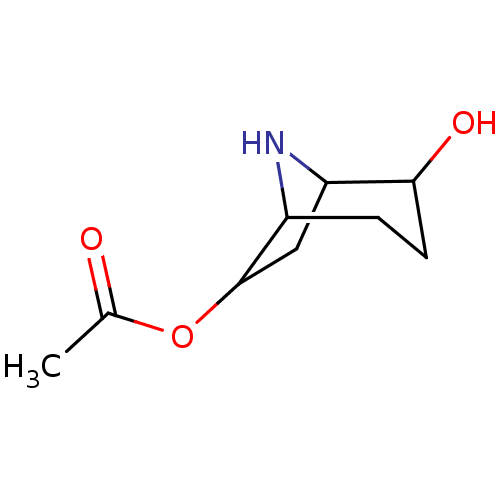
(CHEMBL197092)Show SMILES CC(=O)OC1CC2NC1CCC2O |TLB:3:4:7:11.9.10,THB:12:11:7:4.5| Show InChI InChI=1S/C9H15NO3/c1-5(11)13-9-4-7-8(12)3-2-6(9)10-7/h6-10,12H,2-4H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 79.4 | n/a | n/a | n/a | n/a |
Shanghai Second Medical University
Curated by ChEMBL
| Assay Description
Agonistic activity against Muscarinic acetylcholine receptor of guinea pig ileal longitudinal muscle |
Bioorg Med Chem Lett 15: 4814-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.045
BindingDB Entry DOI: 10.7270/Q23T9KZT |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50416732

(CHEMBL1223754)Show SMILES CCCO[C@H]1CC[C@@](C)(CC1)N1CCC(CC1)n1c2cc(C)c(F)cc2[nH]c1=O |r,wD:4.3,7.7,(20.55,1.43,;19.01,1.41,;18.26,.07,;16.72,.05,;15.96,-1.29,;14.42,-1.31,;13.66,-2.66,;14.46,-3.98,;12.91,-3.97,;16,-3.95,;16.75,-2.62,;13.7,-5.32,;14.49,-6.64,;13.75,-7.98,;12.21,-8.01,;11.41,-6.69,;12.16,-5.34,;11.47,-9.36,;9.99,-9.84,;8.65,-9.08,;7.32,-9.85,;5.99,-9.07,;7.32,-11.41,;5.99,-12.18,;8.66,-12.17,;9.99,-11.41,;11.47,-11.88,;12.38,-10.62,;13.92,-10.63,)| Show InChI InChI=1S/C23H34FN3O2/c1-4-13-29-18-5-9-23(3,10-6-18)26-11-7-17(8-12-26)27-21-14-16(2)19(24)15-20(21)25-22(27)28/h14-15,17-18H,4-13H2,1-3H3,(H,25,28)/t18-,23- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 79.4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M3 receptor expressed in CHO cells assessed as effect on calcium mobilization by FLIPR assay |
Bioorg Med Chem Lett 20: 5434-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.097
BindingDB Entry DOI: 10.7270/Q2MG7QR7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50475450
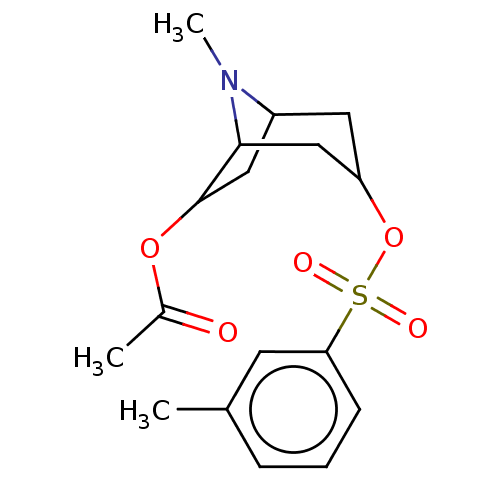
(CHEMBL194196)Show SMILES CN1C2CC(OC(C)=O)C1CC(C2)OS(=O)(=O)c1cccc(C)c1 |TLB:5:4:1:11.10.12,THB:13:11:1:4.3| Show InChI InChI=1S/C17H23NO5S/c1-11-5-4-6-15(7-11)24(20,21)23-14-8-13-9-17(22-12(2)19)16(10-14)18(13)3/h4-7,13-14,16-17H,8-10H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 89.1 | n/a | n/a | n/a | n/a |
Shanghai Second Medical University
Curated by ChEMBL
| Assay Description
Agonistic activity against Muscarinic acetylcholine receptor of guinea pig ileal longitudinal muscle |
Bioorg Med Chem Lett 15: 4814-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.045
BindingDB Entry DOI: 10.7270/Q23T9KZT |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50475446

(CHEMBL196151)Show SMILES COc1ccc(cc1)S(=O)(=O)OC1CC2CC(OC(C)=O)C(C1)N2C |TLB:11:12:23:16.15,THB:17:16:23:12.22.13| Show InChI InChI=1S/C17H23NO6S/c1-11(19)23-17-9-12-8-14(10-16(17)18(12)2)24-25(20,21)15-6-4-13(22-3)5-7-15/h4-7,12,14,16-17H,8-10H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 93.3 | n/a | n/a | n/a | n/a |
Shanghai Second Medical University
Curated by ChEMBL
| Assay Description
Agonistic activity against Muscarinic acetylcholine receptor of guinea pig ileal longitudinal muscle |
Bioorg Med Chem Lett 15: 4814-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.045
BindingDB Entry DOI: 10.7270/Q23T9KZT |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50228570
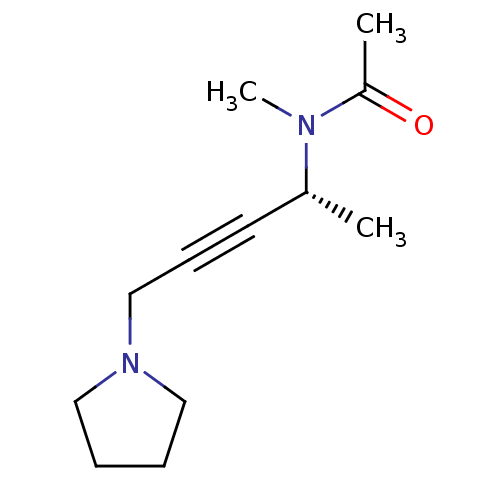
(CHEMBL1788202)Show InChI InChI=1S/C12H20N2O/c1-11(13(3)12(2)15)7-6-10-14-8-4-5-9-14/h11H,4-5,8-10H2,1-3H3/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 94 | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Compound was tested for its potency against Muscarinic acetylcholine receptor in guinea pig ileum |
J Med Chem 33: 3182-9 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3PR1 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50229049
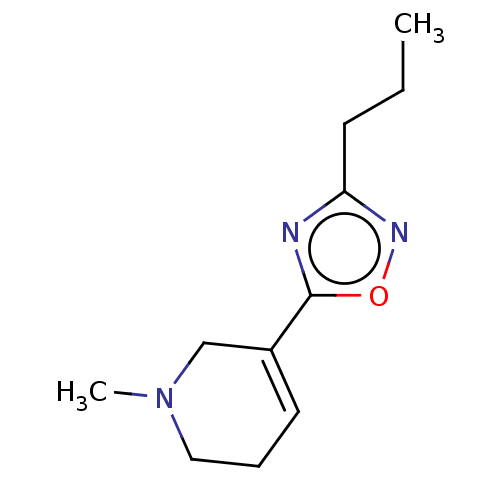
(CHEMBL351934)Show SMILES OC(=O)C(O)=O.CCCc1noc(n1)C1=CCCN(C)C1 |t:14| Show InChI InChI=1S/C11H17N3O/c1-3-5-10-12-11(15-13-10)9-6-4-7-14(2)8-9/h6H,3-5,7-8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Ferrosan A/S
Curated by ChEMBL
| Assay Description
Agonist activity at muscarinic acetylcholine receptor in isolated guinea pig ileum. |
J Med Chem 34: 687-92 (1991)
BindingDB Entry DOI: 10.7270/Q2RN3B25 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data