

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ataxin-1 (Homo sapiens (Human)) | BDBM446120 ((E)-1-((3aR,8aS)-2-(1H-benzo[d][1,2,3]triazole-5-c...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10669268 (2020) BindingDB Entry DOI: 10.7270/Q2R78J83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM477064 (US10882857, Example 4.02 | US11673888, Example 4.0...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2NV9P52 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM446431 (1-[(3aS,6aS)-5-[(5R)-4,5,6,7-tetrahydro-1H-benzotr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10669268 (2020) BindingDB Entry DOI: 10.7270/Q2R78J83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM477062 (US10882857, Example 4 | US11673888, Example 4 | [3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2NV9P52 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM477058 (US10882857, Example 2.06 | US11673888, Example 2.0...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2NV9P52 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM511524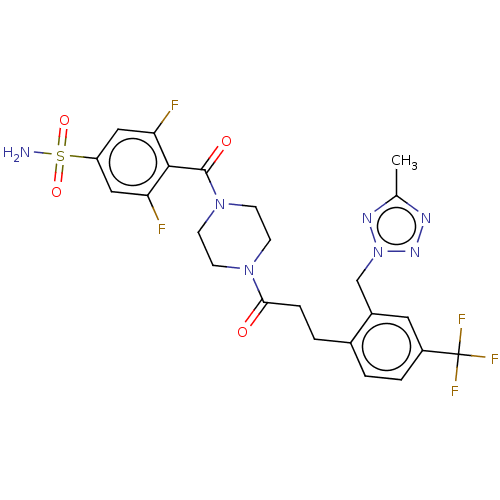 (US11059794, Example 4.00) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM511585 (US11059794, Example 6.45 | tert-butyl 4-[2-[(5-sul...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM511581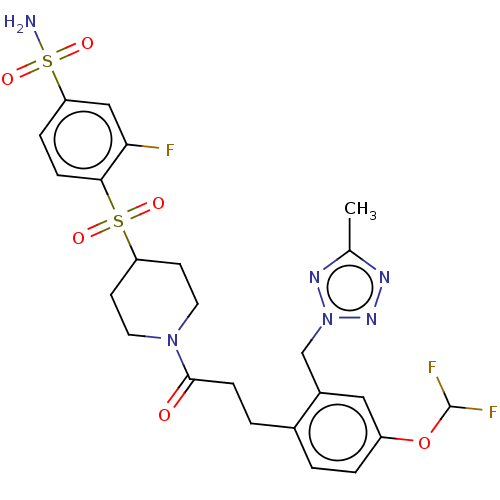 (US11059794, Example 6.41 | tert-butyl 4-(2-fluoro-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM511579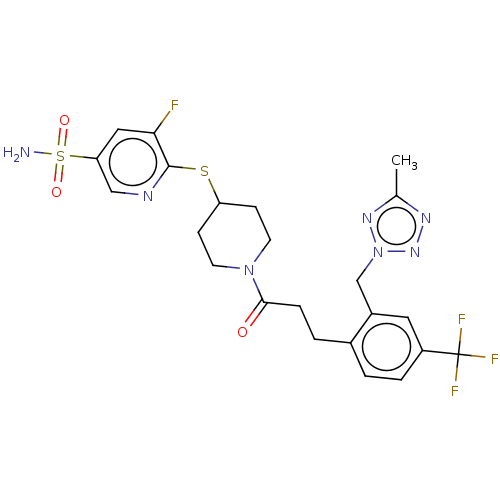 (US11059794, Example 6.39 | tert-butyl 4-(3-fluoro-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM511578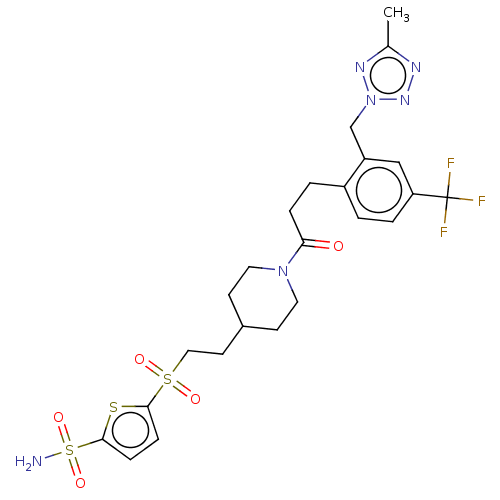 (US11059794, Example 6.38 | tert-butyl 4-[2-(5- sul...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM511567 (US11059794, Example 6.27 | tert-butyl 4-(2,3-diflu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM511558 (US11059794, Example 6.18 | tert-butyl 4-[2-(2-fluo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM475790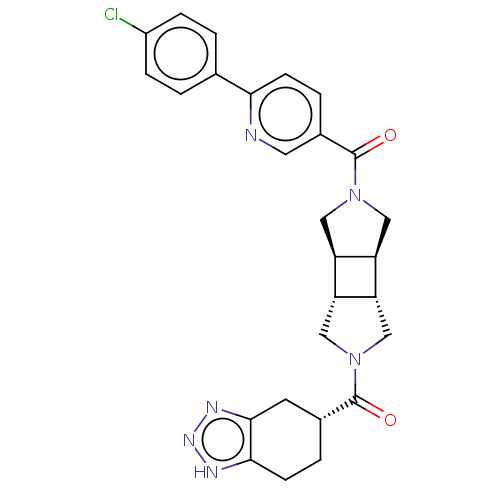 (US10849881, Example 1.063 | [6-(4-chloro-phenyl)-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10849881 (2020) BindingDB Entry DOI: 10.7270/Q26976PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM475791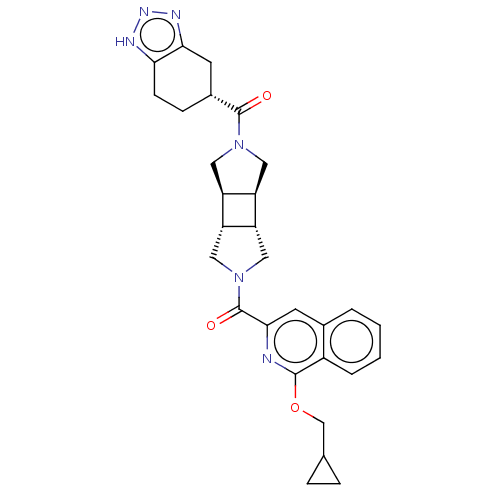 ((1-cyclopropylmethoxy-isoquinolin-3-yl)- [(3aR,3bS...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10849881 (2020) BindingDB Entry DOI: 10.7270/Q26976PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM467326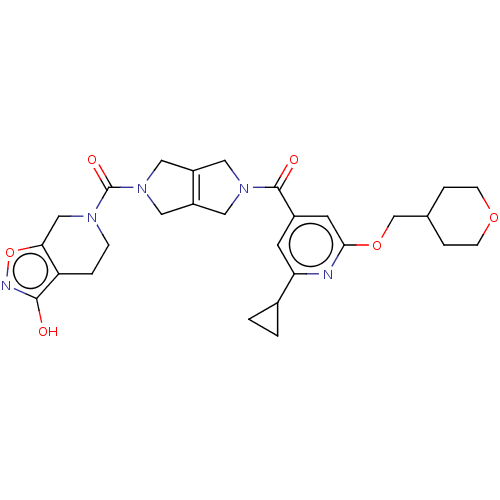 (US10800786, Example 2.01) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffman-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10800786 (2020) BindingDB Entry DOI: 10.7270/Q2RN3BZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM467343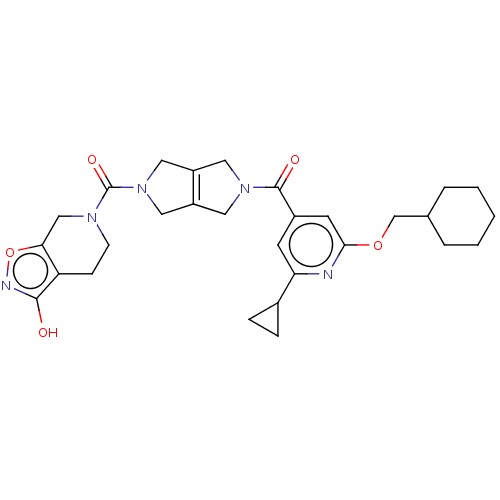 (US10800786, Example 2.15) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffman-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10800786 (2020) BindingDB Entry DOI: 10.7270/Q2RN3BZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM467346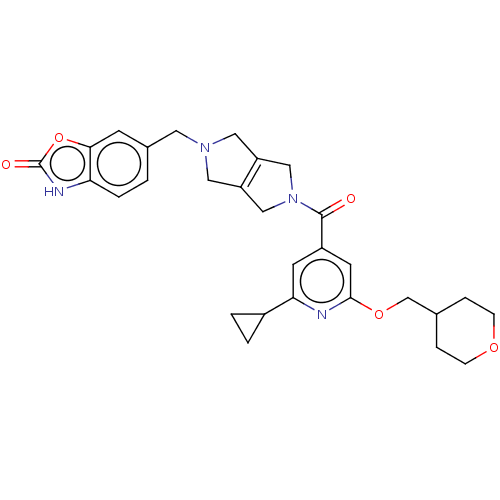 (US10800786, Example 5.00) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffman-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10800786 (2020) BindingDB Entry DOI: 10.7270/Q2RN3BZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM50187688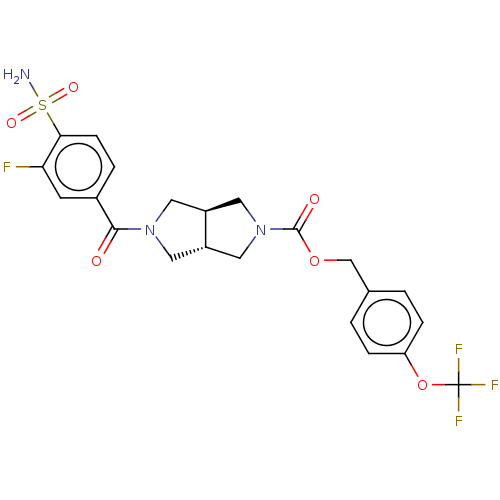 (CHEMBL3828650 | US10913745, Example 1.11) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Assay buffer (50 mM Tris-HCl, 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Triton-X-100, pH 8.0;ATX solution: ATX (human His-tagged) stock so... | US Patent US10913745 (2021) BindingDB Entry DOI: 10.7270/Q2NP27HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM481899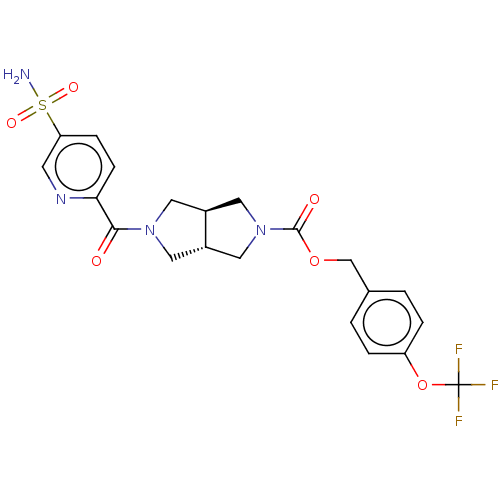 (US10913745, Example 1.13 | US10913745, Example 1.1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Assay buffer (50 mM Tris-HCl, 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Triton-X-100, pH 8.0;ATX solution: ATX (human His-tagged) stock so... | US Patent US10913745 (2021) BindingDB Entry DOI: 10.7270/Q2NP27HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM481899 (US10913745, Example 1.13 | US10913745, Example 1.1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Assay buffer (50 mM Tris-HCl, 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Triton-X-100, pH 8.0;ATX solution: ATX (human His-tagged) stock so... | US Patent US10913745 (2021) BindingDB Entry DOI: 10.7270/Q2NP27HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM481920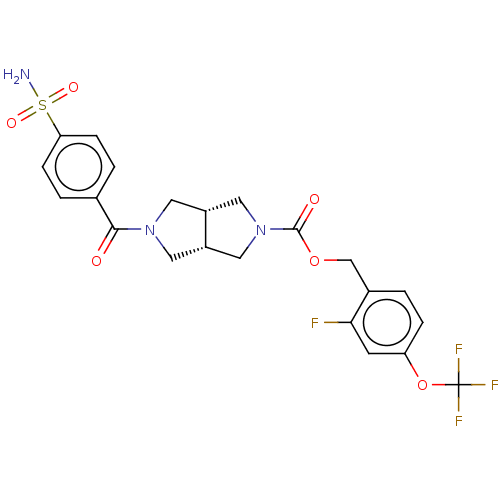 (US10913745, Example 7.01) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Assay buffer (50 mM Tris-HCl, 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Triton-X-100, pH 8.0;ATX solution: ATX (human His-tagged) stock so... | US Patent US10913745 (2021) BindingDB Entry DOI: 10.7270/Q2NP27HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM481930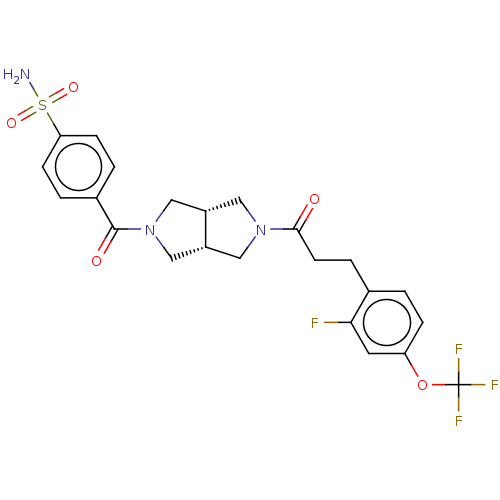 (US10913745, Example 8.07) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Assay buffer (50 mM Tris-HCl, 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Triton-X-100, pH 8.0;ATX solution: ATX (human His-tagged) stock so... | US Patent US10913745 (2021) BindingDB Entry DOI: 10.7270/Q2NP27HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM481932 (US10913745, Example 8.09) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Assay buffer (50 mM Tris-HCl, 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Triton-X-100, pH 8.0;ATX solution: ATX (human His-tagged) stock so... | US Patent US10913745 (2021) BindingDB Entry DOI: 10.7270/Q2NP27HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM511530 (4-(piperidin-4- ylmethyl)benzenesulfonamide hydroc...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM511538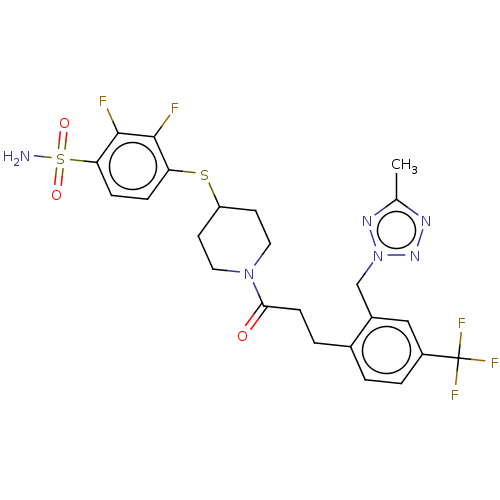 (US11059794, Example 6.00) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM511541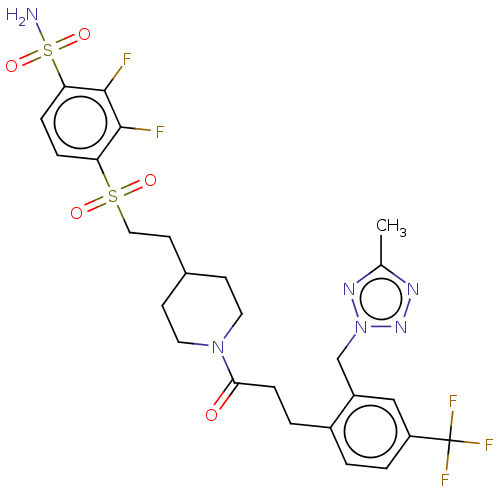 (US11059794, Example 6.03 | tert-butyl 4-[2-(2,3-di...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM511551 (US11059794, Example 6.13 | tert-butyl 4-(2-fluoro-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM475786 ((4-isopropoxy-naphthalen-2-yl)- [(3aR,3bS,6aR,6bS)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10849881 (2020) BindingDB Entry DOI: 10.7270/Q26976PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM475941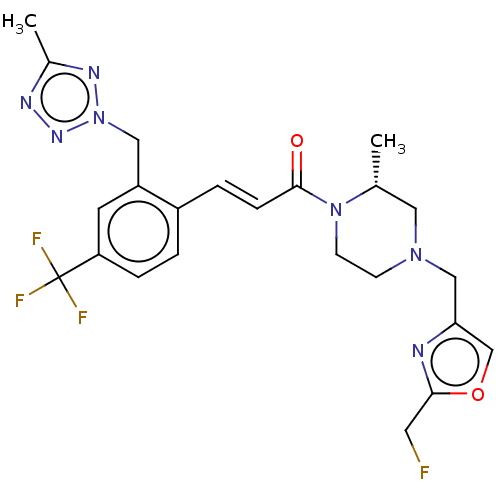 (US10865195, Compound 6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description ATX activity was determined by measurement of released choline in reactions containing ATX (10 nM), choline oxidase (0.1 U/ml), HRP (100 U/ml), ample... | US Patent US10865195 (2020) BindingDB Entry DOI: 10.7270/Q2BV7KQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM50557594 (CHEMBL4788435 | US11485727, Example 1.3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM recombinant ATX (Cayman Chemicals) was supplemented to 50 mM Tris buffer (pH 8.0) containing 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2 0.14 mM NaCl, and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X06BXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM481931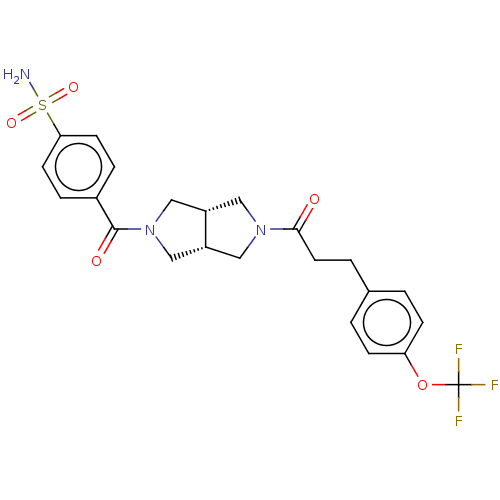 (US10913745, Example 8.08) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Assay buffer (50 mM Tris-HCl, 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Triton-X-100, pH 8.0;ATX solution: ATX (human His-tagged) stock so... | US Patent US10913745 (2021) BindingDB Entry DOI: 10.7270/Q2NP27HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM622503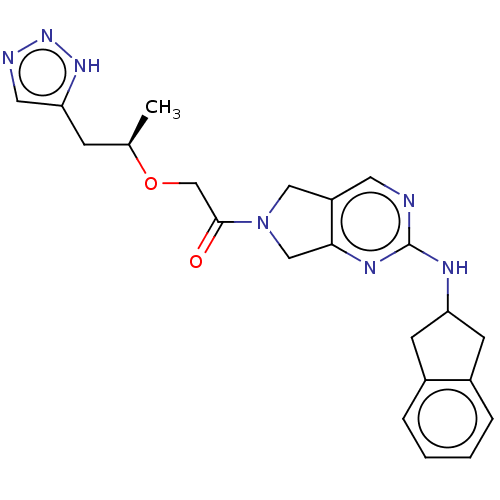 (US20230312582, Example Compound Formula (I)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1.59 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM50557595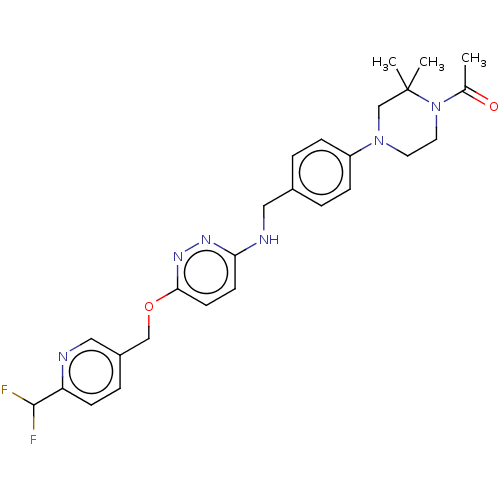 (CHEMBL4745566 | US11485727, Example 1.7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM recombinant ATX (Cayman Chemicals) was supplemented to 50 mM Tris buffer (pH 8.0) containing 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2 0.14 mM NaCl, and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X06BXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM50557599 (CHEMBL4751395 | US11485727, Example 2.10) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM recombinant ATX (Cayman Chemicals) was supplemented to 50 mM Tris buffer (pH 8.0) containing 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2 0.14 mM NaCl, and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X06BXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM50557597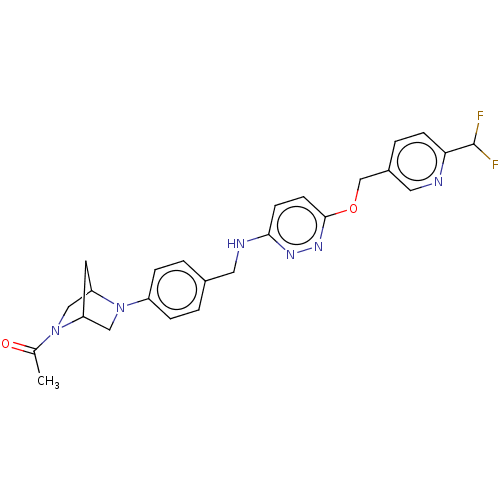 (CHEMBL4758133 | US11485727, Example 2.6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM recombinant ATX (Cayman Chemicals) was supplemented to 50 mM Tris buffer (pH 8.0) containing 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2 0.14 mM NaCl, and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X06BXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM50557598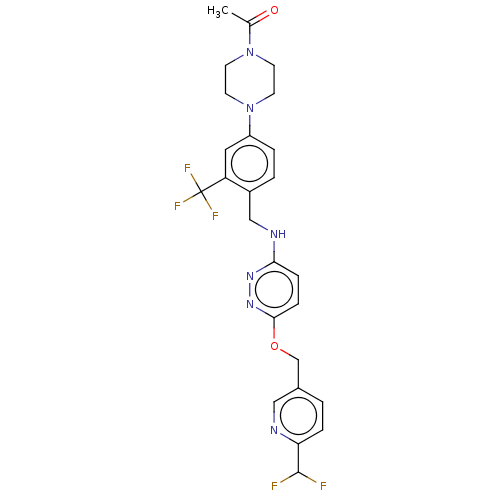 (CHEMBL4784419 | US11485727, Example 2.7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM recombinant ATX (Cayman Chemicals) was supplemented to 50 mM Tris buffer (pH 8.0) containing 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2 0.14 mM NaCl, and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X06BXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM50557596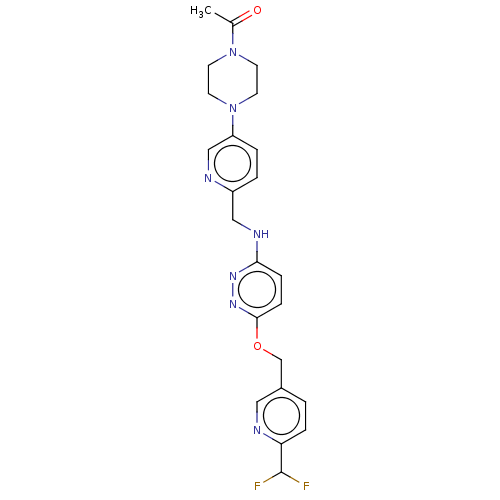 (CHEMBL4743845 | US11485727, Example 2.4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM recombinant ATX (Cayman Chemicals) was supplemented to 50 mM Tris buffer (pH 8.0) containing 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2 0.14 mM NaCl, and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X06BXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM321944 ((E)-1-(4-((1-Methyl-1H-pyrazol-4-yl)methyl)piperaz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Method All experimental measurements were performed in black 384 well polystyrene (low volume, round bottom, Corning (3676)) plates. PerkinElmer EnVi... | US Patent US9763957 (2017) BindingDB Entry DOI: 10.7270/Q2QF8W0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM477060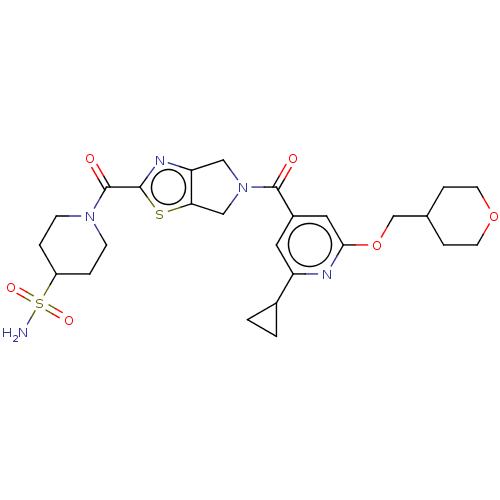 (US10882857, Example 3.01 | US11673888, Example 3.0...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2NV9P52 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM322065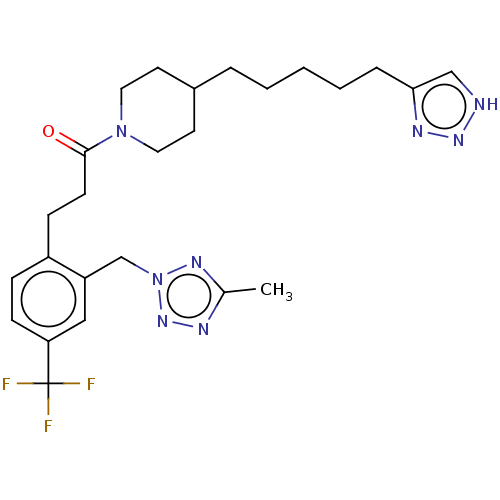 (1-(4-(5-(1H-1,2,3-Triazol-4-yl)pentyl)piperidin-1-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Method All experimental measurements were performed in black 384 well polystyrene (low volume, round bottom, Corning (3676)) plates. PerkinElmer EnVi... | US Patent US9763957 (2017) BindingDB Entry DOI: 10.7270/Q2QF8W0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM321962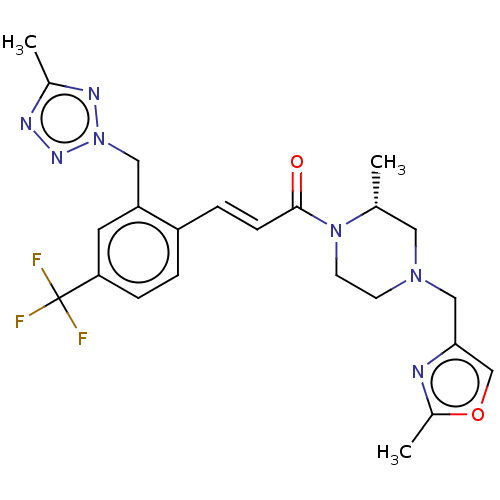 ((R,E)-3-(2-((5-Methyl-2H-tetrazol-2-yl)methyl)-4-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Method All experimental measurements were performed in black 384 well polystyrene (low volume, round bottom, Corning (3676)) plates. PerkinElmer EnVi... | US Patent US9763957 (2017) BindingDB Entry DOI: 10.7270/Q2QF8W0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM477049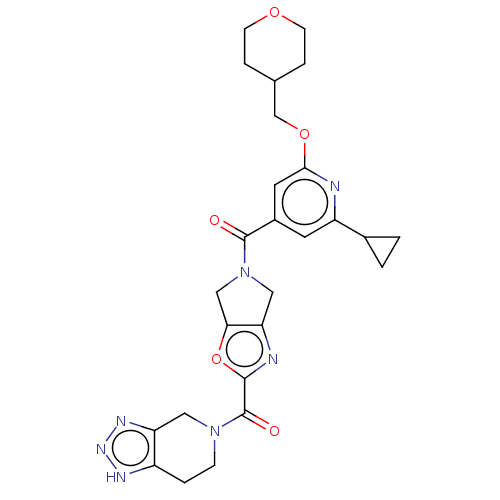 (US10882857, Example 1.01 | US11673888, Example 1.0...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2NV9P52 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM477042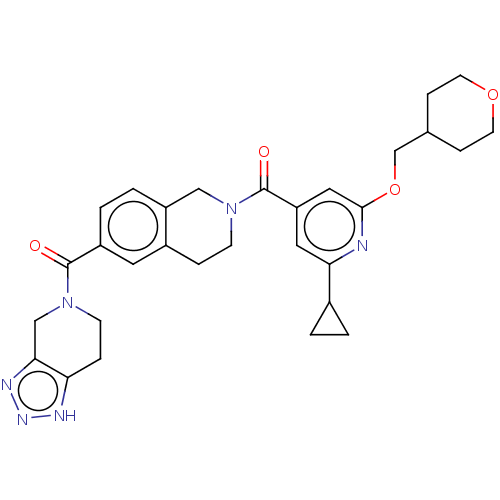 (US10882857, Example 1 | US11673888, Example 1 | [2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2NV9P52 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM191570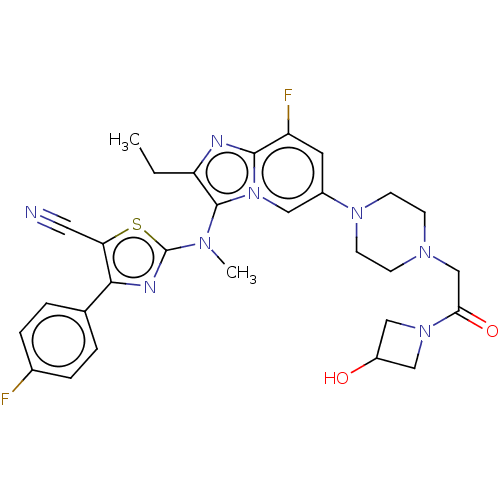 (US10526329, Compound 12 | US11072611, Compound 12 ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM recombinant ATX (Cayman Chemicals) was supplemented to 50 mM Tris buffer (pH 8.0) containing 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2 0.14 mM NaCl, and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X06BXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM579837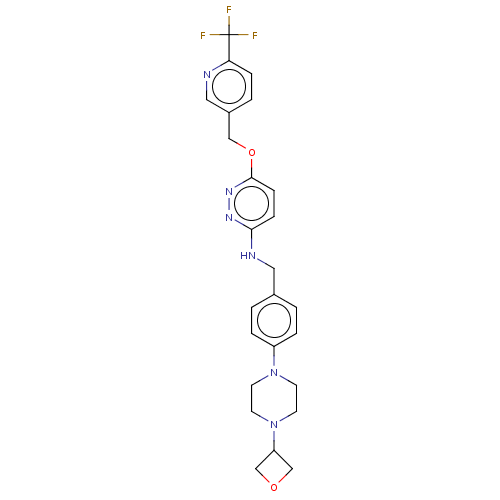 (US11485727, Example 2.8) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM recombinant ATX (Cayman Chemicals) was supplemented to 50 mM Tris buffer (pH 8.0) containing 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2 0.14 mM NaCl, and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X06BXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM511525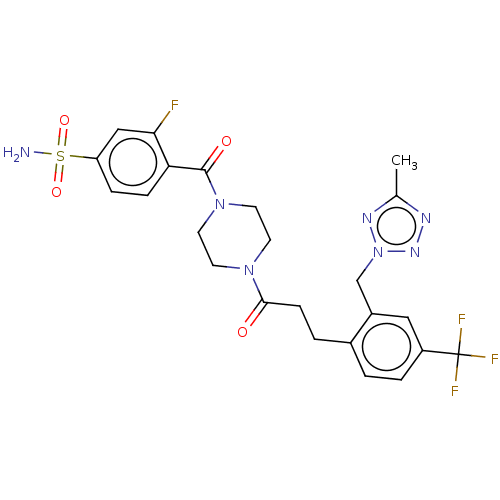 (2-fluoro-4-sulfamoylbenzoic acid (CAS-RN 714968-42...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM511523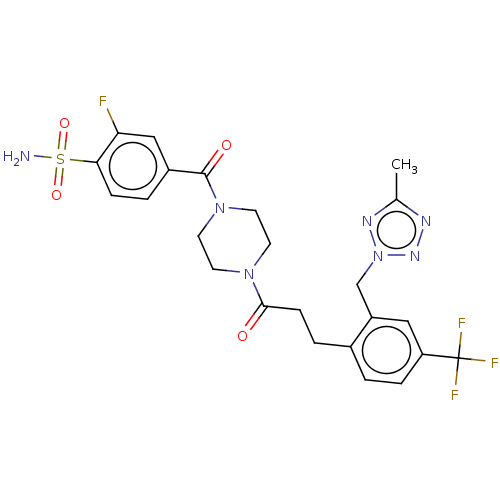 (US11059794, Example 3.00) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM511520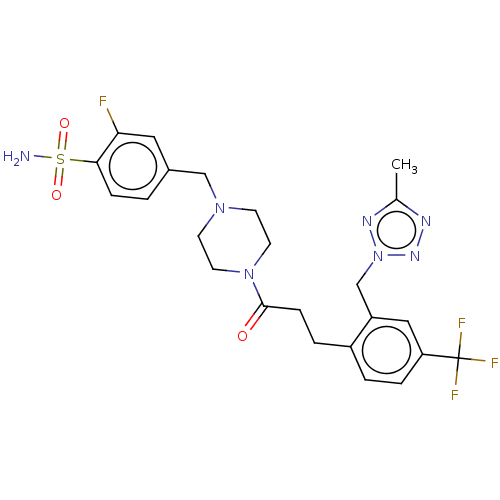 (US11059794, Example 2.00 | US11059794, Example 2.0...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM511559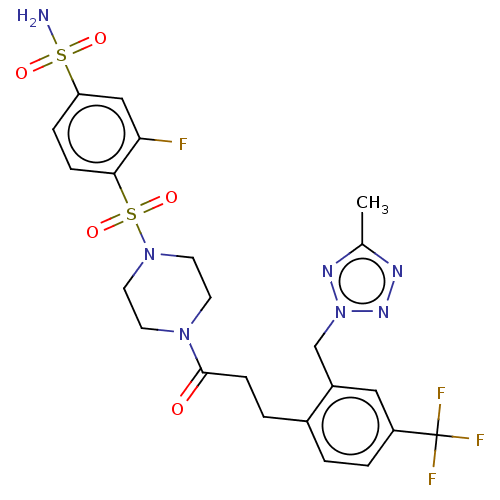 (US11059794, Example 6.19 | tert-butyl 4-(2-fluoro-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM511557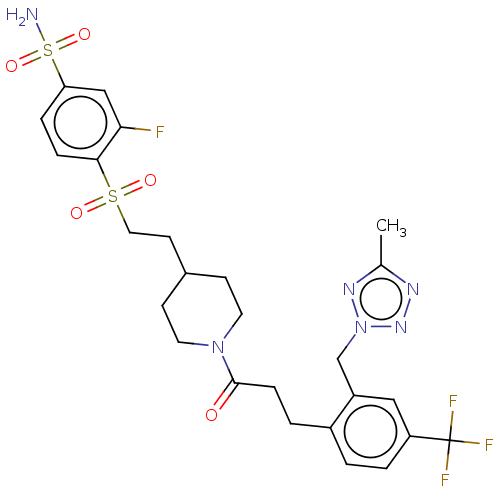 (US11059794, Example 6.17 | tert-butyl 4-[2-(2-fluo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1104 total ) | Next | Last >> |