 Found 47 hits of ic50 for UniProtKB: P61431
Found 47 hits of ic50 for UniProtKB: P61431 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM119080
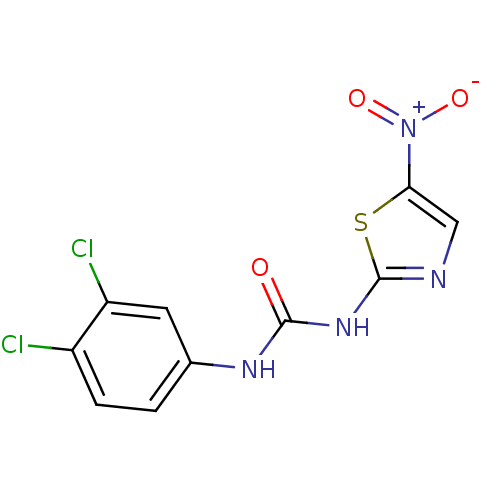
(MurB inhibitor (compound 19))Show SMILES [O-][N+](=O)c1cnc(NC(=O)Nc2ccc(Cl)c(Cl)c2)s1 Show InChI InChI=1S/C10H6Cl2N4O3S/c11-6-2-1-5(3-7(6)12)14-9(17)15-10-13-4-8(20-10)16(18)19/h1-4H,(H2,13,14,15,17) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana, A?kerceva 7, 1000 Ljubljana, Slovenia
| |
Bioorg Chem 55: 2-15 (2014)
Article DOI: 10.1016/j.bioorg.2014.03.008
BindingDB Entry DOI: 10.7270/Q2ZG6QWR |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM119079

(MurB inhibitor (compound 18))Show SMILES CC1(CC(=O)Nc2ccc(Cl)cc2Cl)C(=O)N(N(C1=O)c1ccc(Cl)cc1)c1ccc(Cl)cc1 Show InChI InChI=1S/C24H17Cl4N3O3/c1-24(13-21(32)29-20-11-6-16(27)12-19(20)28)22(33)30(17-7-2-14(25)3-8-17)31(23(24)34)18-9-4-15(26)5-10-18/h2-12H,13H2,1H3,(H,29,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana, A?kerceva 7, 1000 Ljubljana, Slovenia
| |
Bioorg Chem 55: 2-15 (2014)
Article DOI: 10.1016/j.bioorg.2014.03.008
BindingDB Entry DOI: 10.7270/Q2ZG6QWR |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50166493

(4-(2-Benzylsulfanyl-ethyl)-1,2-bis-(4-chloro-pheny...)Show SMILES Oc1c(CCSCc2ccccc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H20Cl2N2O2S/c25-18-6-10-20(11-7-18)27-23(29)22(14-15-31-16-17-4-2-1-3-5-17)24(30)28(27)21-12-8-19(26)9-13-21/h1-13,29H,14-16H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Staphylococcus aureus |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50166485
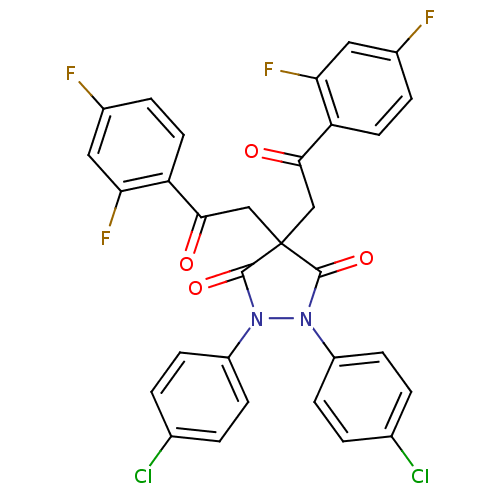
(1,2-Bis-(4-chloro-phenyl)-4,4-bis-[2-(2,4-difluoro...)Show SMILES Fc1ccc(C(=O)CC2(CC(=O)c3ccc(F)cc3F)C(=O)N(N(C2=O)c2ccc(Cl)cc2)c2ccc(Cl)cc2)c(F)c1 Show InChI InChI=1S/C31H18Cl2F4N2O4/c32-17-1-7-21(8-2-17)38-29(42)31(15-27(40)23-11-5-19(34)13-25(23)36,16-28(41)24-12-6-20(35)14-26(24)37)30(43)39(38)22-9-3-18(33)4-10-22/h1-14H,15-16H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana, A?kerceva 7, 1000 Ljubljana, Slovenia
| |
Bioorg Chem 55: 2-15 (2014)
Article DOI: 10.1016/j.bioorg.2014.03.008
BindingDB Entry DOI: 10.7270/Q2ZG6QWR |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50166485

(1,2-Bis-(4-chloro-phenyl)-4,4-bis-[2-(2,4-difluoro...)Show SMILES Fc1ccc(C(=O)CC2(CC(=O)c3ccc(F)cc3F)C(=O)N(N(C2=O)c2ccc(Cl)cc2)c2ccc(Cl)cc2)c(F)c1 Show InChI InChI=1S/C31H18Cl2F4N2O4/c32-17-1-7-21(8-2-17)38-29(42)31(15-27(40)23-11-5-19(34)13-25(23)36,16-28(41)24-12-6-20(35)14-26(24)37)30(43)39(38)22-9-3-18(33)4-10-22/h1-14H,15-16H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Staphylococcus aureus |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50166480
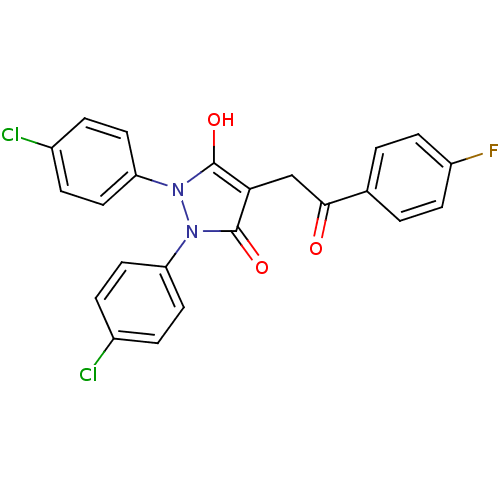
(1,2-Bis-(4-chloro-phenyl)-4-[2-(4-fluoro-phenyl)-2...)Show SMILES Oc1c(CC(=O)c2ccc(F)cc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C23H15Cl2FN2O3/c24-15-3-9-18(10-4-15)27-22(30)20(13-21(29)14-1-7-17(26)8-2-14)23(31)28(27)19-11-5-16(25)6-12-19/h1-12,30H,13H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Staphylococcus aureus |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50166500
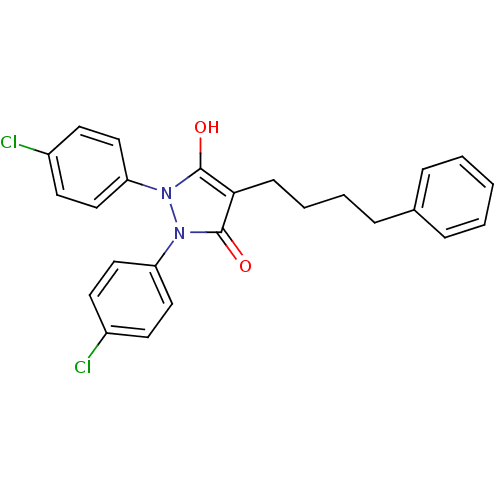
(1,2-Bis-(4-chloro-phenyl)-4-(4-phenyl-butyl)-pyraz...)Show SMILES Oc1c(CCCCc2ccccc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C25H22Cl2N2O2/c26-19-10-14-21(15-11-19)28-24(30)23(9-5-4-8-18-6-2-1-3-7-18)25(31)29(28)22-16-12-20(27)13-17-22/h1-3,6-7,10-17,30H,4-5,8-9H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Staphylococcus aureus |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50166499

(4,4-Bis-(2-benzyloxy-ethyl)-1,2-bis-(4-chloro-phen...)Show SMILES Clc1ccc(cc1)N1N(C(=O)C(CCOCc2ccccc2)(CCOCc2ccccc2)C1=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C33H30Cl2N2O4/c34-27-11-15-29(16-12-27)36-31(38)33(19-21-40-23-25-7-3-1-4-8-25,20-22-41-24-26-9-5-2-6-10-26)32(39)37(36)30-17-13-28(35)14-18-30/h1-18H,19-24H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Staphylococcus aureus |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50166475

(1,2-Bis-(4-chloro-phenyl)-4,4-bis-(6-phenyl-hexyl)...)Show SMILES Clc1ccc(cc1)N1N(C(=O)C(CCCCCCc2ccccc2)(CCCCCCc2ccccc2)C1=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C39H42Cl2N2O2/c40-33-21-25-35(26-22-33)42-37(44)39(38(45)43(42)36-27-23-34(41)24-28-36,29-13-3-1-7-15-31-17-9-5-10-18-31)30-14-4-2-8-16-32-19-11-6-12-20-32/h5-6,9-12,17-28H,1-4,7-8,13-16,29-30H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Staphylococcus aureus |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50166497
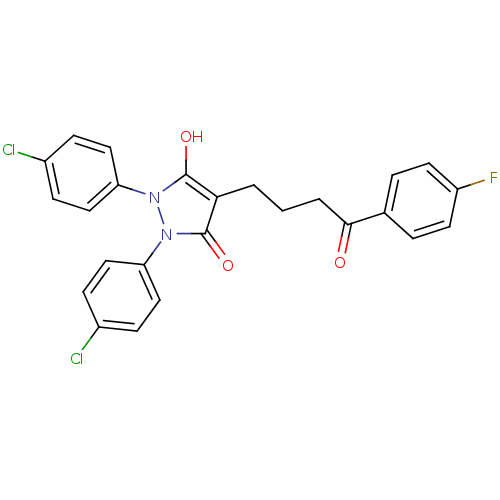
(1,2-Bis-(4-chloro-phenyl)-4-[4-(4-fluoro-phenyl)-4...)Show SMILES Oc1c(CCCC(=O)c2ccc(F)cc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C25H19Cl2FN2O3/c26-17-6-12-20(13-7-17)29-24(32)22(25(33)30(29)21-14-8-18(27)9-15-21)2-1-3-23(31)16-4-10-19(28)11-5-16/h4-15,32H,1-3H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Staphylococcus aureus |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50166483
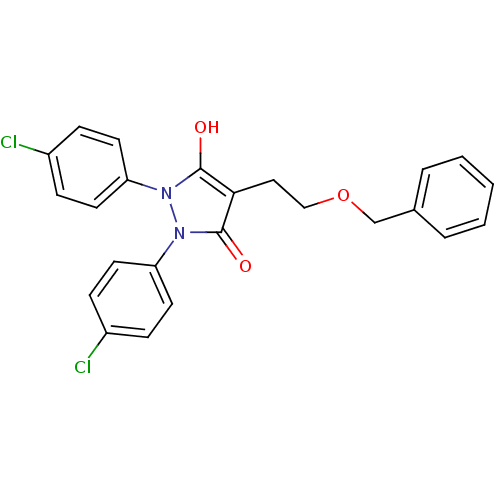
(4-(2-Benzyloxy-ethyl)-1,2-bis-(4-chloro-phenyl)-py...)Show SMILES Oc1c(CCOCc2ccccc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H20Cl2N2O3/c25-18-6-10-20(11-7-18)27-23(29)22(14-15-31-16-17-4-2-1-3-5-17)24(30)28(27)21-12-8-19(26)9-13-21/h1-13,29H,14-16H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Staphylococcus aureus |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50166478

(1,2-Bis-(4-chloro-phenyl)-4-(6-phenyl-hexyl)-pyraz...)Show SMILES Oc1c(CCCCCCc2ccccc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C27H26Cl2N2O2/c28-21-12-16-23(17-13-21)30-26(32)25(27(33)31(30)24-18-14-22(29)15-19-24)11-7-2-1-4-8-20-9-5-3-6-10-20/h3,5-6,9-10,12-19,32H,1-2,4,7-8,11H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Staphylococcus aureus |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50166496

(1-{4-[1,2-Bis-(4-chloro-phenyl)-3,5-dioxo-pyrazoli...)Show SMILES Oc1c(Cc2ccc(cc2)C2(CC=CC=C2)C#N)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 |c:13,15| Show InChI InChI=1S/C29H21Cl2N3O2/c30-22-8-12-24(13-9-22)33-27(35)26(28(36)34(33)25-14-10-23(31)11-15-25)18-20-4-6-21(7-5-20)29(19-32)16-2-1-3-17-29/h1-16,35H,17-18H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Staphylococcus aureus |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50221037
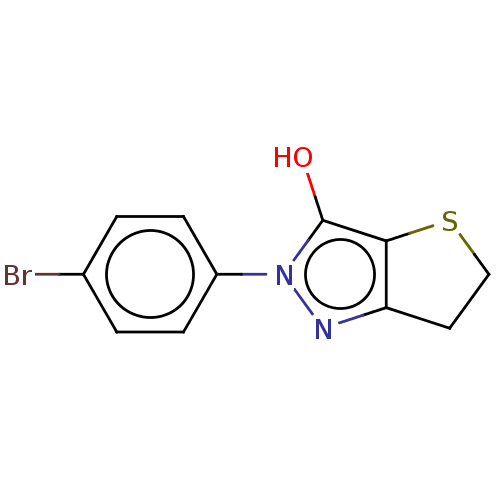
(CHEMBL87262)Show InChI InChI=1S/C11H9BrN2OS/c12-7-1-3-8(4-2-7)14-11(15)10-9(13-14)5-6-16-10/h1-4,15H,5-6H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Staphylococcus aureus UDP-N-acetylmuramate dehydrogenase |
Bioorg Med Chem Lett 13: 2591-4 (2003)
BindingDB Entry DOI: 10.7270/Q20P126D |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM119122
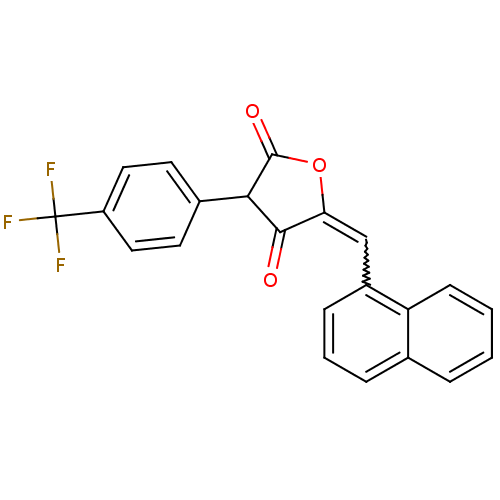
(MurA/B/C/D/E inhibitor (compound 67))Show SMILES FC(F)(F)c1ccc(cc1)C1C(=O)OC(=Cc2cccc3ccccc23)C1=O |w:15.16| Show InChI InChI=1S/C22H13F3O3/c23-22(24,25)16-10-8-14(9-11-16)19-20(26)18(28-21(19)27)12-15-6-3-5-13-4-1-2-7-17(13)15/h1-12,19H | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana, A?kerceva 7, 1000 Ljubljana, Slovenia
| |
Bioorg Chem 55: 2-15 (2014)
Article DOI: 10.1016/j.bioorg.2014.03.008
BindingDB Entry DOI: 10.7270/Q2ZG6QWR |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50166494
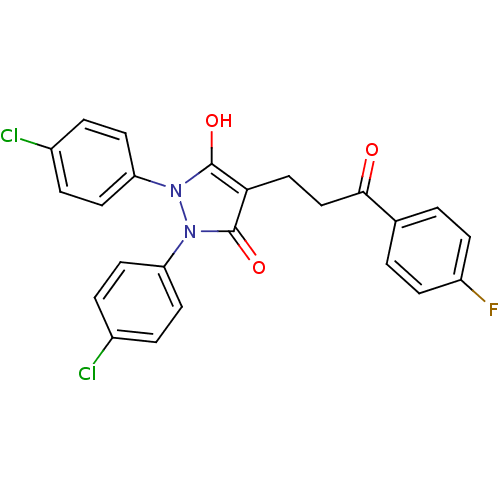
(1,2-Bis-(4-chloro-phenyl)-4-[3-(4-fluoro-phenyl)-3...)Show SMILES Oc1c(CCC(=O)c2ccc(F)cc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H17Cl2FN2O3/c25-16-3-9-19(10-4-16)28-23(31)21(13-14-22(30)15-1-7-18(27)8-2-15)24(32)29(28)20-11-5-17(26)6-12-20/h1-12,31H,13-14H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Staphylococcus aureus |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50221030
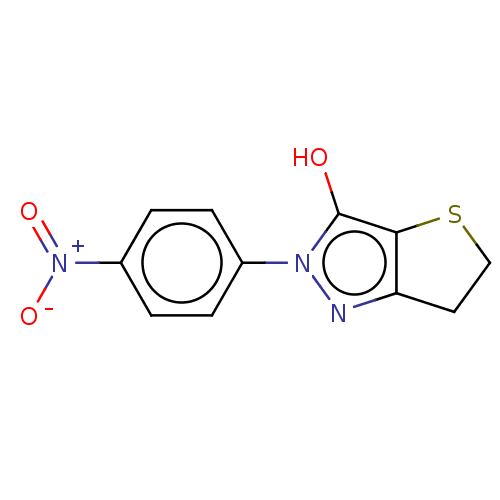
(CHEMBL313078)Show InChI InChI=1S/C11H9N3O3S/c15-11-10-9(5-6-18-10)12-13(11)7-1-3-8(4-2-7)14(16)17/h1-4,15H,5-6H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Staphylococcus aureus UDP-N-acetylmuramate dehydrogenase |
Bioorg Med Chem Lett 13: 2591-4 (2003)
BindingDB Entry DOI: 10.7270/Q20P126D |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50221032
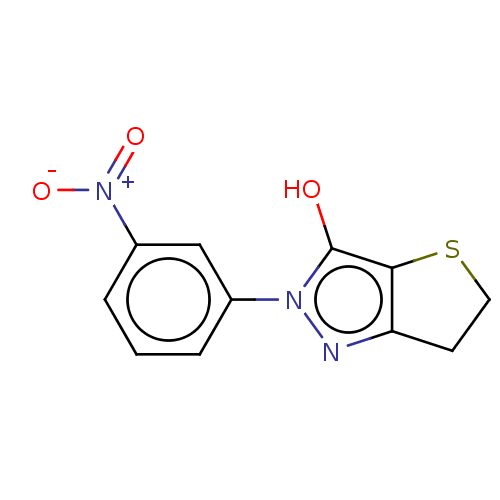
(CHEMBL86526)Show InChI InChI=1S/C11H9N3O3S/c15-11-10-9(4-5-18-10)12-13(11)7-2-1-3-8(6-7)14(16)17/h1-3,6,15H,4-5H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Staphylococcus aureus UDP-N-acetylmuramate dehydrogenase |
Bioorg Med Chem Lett 13: 2591-4 (2003)
BindingDB Entry DOI: 10.7270/Q20P126D |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50221018
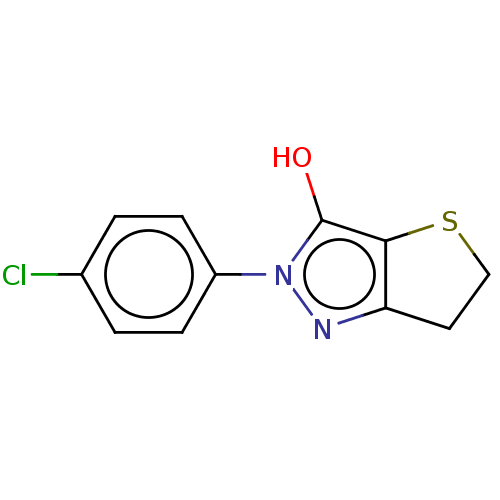
(CHEMBL85772)Show InChI InChI=1S/C11H9ClN2OS/c12-7-1-3-8(4-2-7)14-11(15)10-9(13-14)5-6-16-10/h1-4,15H,5-6H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Staphylococcus aureus UDP-N-acetylmuramate dehydrogenase |
Bioorg Med Chem Lett 13: 2591-4 (2003)
BindingDB Entry DOI: 10.7270/Q20P126D |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50166486
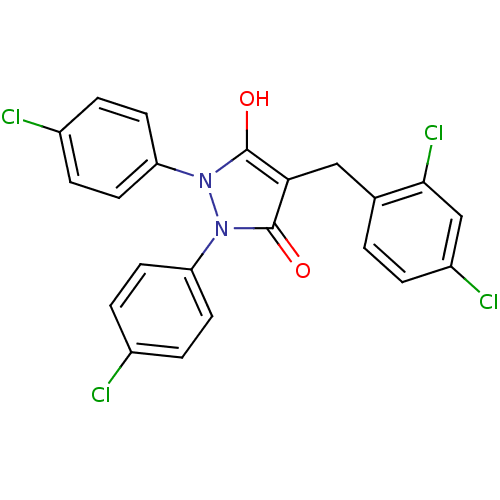
(1,2-Bis-(4-chloro-phenyl)-4-(2,4-dichloro-benzyl)-...)Show SMILES Oc1c(Cc2ccc(Cl)cc2Cl)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C22H14Cl4N2O2/c23-14-3-7-17(8-4-14)27-21(29)19(11-13-1-2-16(25)12-20(13)26)22(30)28(27)18-9-5-15(24)6-10-18/h1-10,12,29H,11H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Staphylococcus aureus |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50166495

(1,2-Bis-(4-chloro-phenyl)-4-(5-phenyl-pentyl)-pyra...)Show SMILES Oc1c(CCCCCc2ccccc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C26H24Cl2N2O2/c27-20-11-15-22(16-12-20)29-25(31)24(10-6-2-5-9-19-7-3-1-4-8-19)26(32)30(29)23-17-13-21(28)14-18-23/h1,3-4,7-8,11-18,31H,2,5-6,9-10H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Staphylococcus aureus |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50221025

(CHEMBL83021)Show InChI InChI=1S/C17H13ClN2OS/c18-12-6-8-13(9-7-12)20-17(21)16-14(19-20)10-15(22-16)11-4-2-1-3-5-11/h1-9,15,21H,10H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Staphylococcus aureus UDP-N-acetylmuramate dehydrogenase |
Bioorg Med Chem Lett 13: 2591-4 (2003)
BindingDB Entry DOI: 10.7270/Q20P126D |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50221038
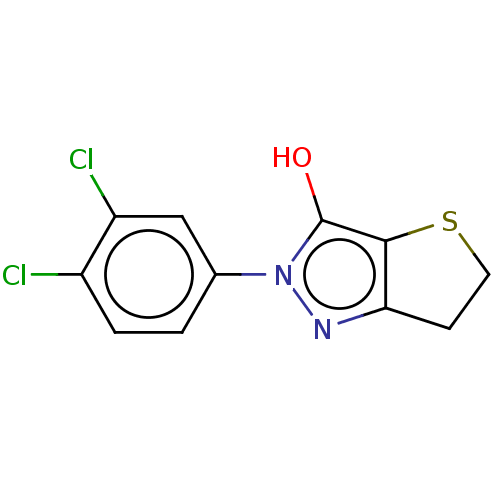
(CHEMBL87521)Show InChI InChI=1S/C11H8Cl2N2OS/c12-7-2-1-6(5-8(7)13)15-11(16)10-9(14-15)3-4-17-10/h1-2,5,16H,3-4H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Staphylococcus aureus UDP-N-acetylmuramate dehydrogenase |
Bioorg Med Chem Lett 13: 2591-4 (2003)
BindingDB Entry DOI: 10.7270/Q20P126D |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50221020

(CHEMBL87126)Show InChI InChI=1S/C12H9F3N2OS/c13-12(14,15)7-1-3-8(4-2-7)17-11(18)10-9(16-17)5-6-19-10/h1-4,18H,5-6H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Staphylococcus aureus UDP-N-acetylmuramate dehydrogenase |
Bioorg Med Chem Lett 13: 2591-4 (2003)
BindingDB Entry DOI: 10.7270/Q20P126D |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50166479
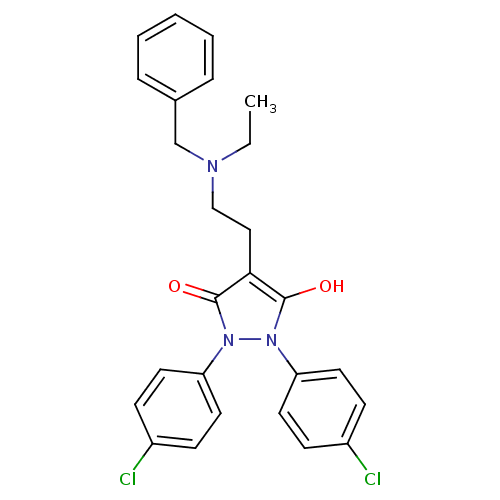
(4-[2-(Benzyl-ethyl-amino)-ethyl]-1,2-bis-(4-chloro...)Show SMILES CCN(CCc1c(O)n(-c2ccc(Cl)cc2)n(-c2ccc(Cl)cc2)c1=O)Cc1ccccc1 Show InChI InChI=1S/C26H25Cl2N3O2/c1-2-29(18-19-6-4-3-5-7-19)17-16-24-25(32)30(22-12-8-20(27)9-13-22)31(26(24)33)23-14-10-21(28)11-15-23/h3-15,32H,2,16-18H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Staphylococcus aureus |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50166484

(1,2-Bis-(4-chloro-phenyl)-4-methyl-4-(6-phenyl-hex...)Show SMILES CC1(CCCCCCc2ccccc2)C(=O)N(N(C1=O)c1ccc(Cl)cc1)c1ccc(Cl)cc1 Show InChI InChI=1S/C28H28Cl2N2O2/c1-28(20-8-3-2-5-9-21-10-6-4-7-11-21)26(33)31(24-16-12-22(29)13-17-24)32(27(28)34)25-18-14-23(30)15-19-25/h4,6-7,10-19H,2-3,5,8-9,20H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Staphylococcus aureus |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50166476

(4-(2-Chloro-benzyl)-1,2-bis-(4-chloro-phenyl)-pyra...)Show SMILES Oc1c(Cc2ccccc2Cl)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C22H15Cl3N2O2/c23-15-5-9-17(10-6-15)26-21(28)19(13-14-3-1-2-4-20(14)25)22(29)27(26)18-11-7-16(24)8-12-18/h1-12,28H,13H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Staphylococcus aureus |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50166502

(1,2-Bis-(4-chloro-phenyl)-5-[2-(2,4-difluoro-pheny...)Show SMILES CC1C(OCC(=O)c2ccc(F)cc2F)N(N(C1=O)c1ccc(Cl)cc1)c1ccc(Cl)cc1 Show InChI InChI=1S/C24H18Cl2F2N2O3/c1-14-23(32)29(18-7-2-15(25)3-8-18)30(19-9-4-16(26)5-10-19)24(14)33-13-22(31)20-11-6-17(27)12-21(20)28/h2-12,14,24H,13H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Staphylococcus aureus |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50166491
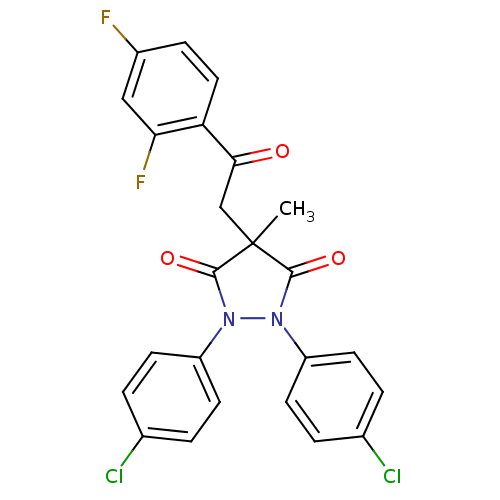
(1,2-Bis-(4-chloro-phenyl)-4-[2-(2,4-difluoro-pheny...)Show SMILES CC1(CC(=O)c2ccc(F)cc2F)C(=O)N(N(C1=O)c1ccc(Cl)cc1)c1ccc(Cl)cc1 Show InChI InChI=1S/C24H16Cl2F2N2O3/c1-24(13-21(31)19-11-6-16(27)12-20(19)28)22(32)29(17-7-2-14(25)3-8-17)30(23(24)33)18-9-4-15(26)5-10-18/h2-12H,13H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Staphylococcus aureus |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50221035
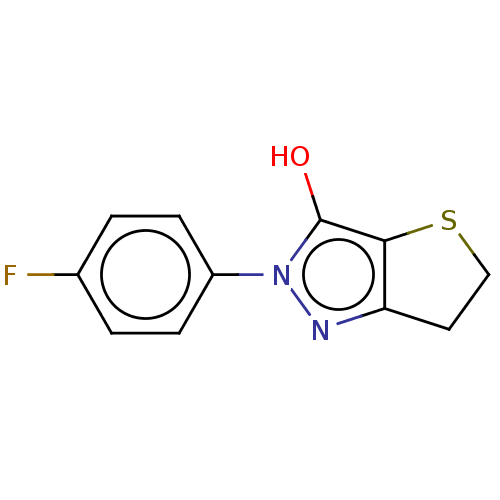
(CHEMBL86990)Show InChI InChI=1S/C11H9FN2OS/c12-7-1-3-8(4-2-7)14-11(15)10-9(13-14)5-6-16-10/h1-4,15H,5-6H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Staphylococcus aureus UDP-N-acetylmuramate dehydrogenase |
Bioorg Med Chem Lett 13: 2591-4 (2003)
BindingDB Entry DOI: 10.7270/Q20P126D |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50221027
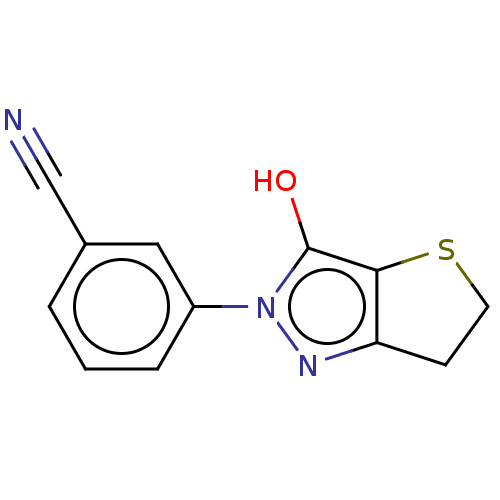
(CHEMBL327438)Show InChI InChI=1S/C12H9N3OS/c13-7-8-2-1-3-9(6-8)15-12(16)11-10(14-15)4-5-17-11/h1-3,6,16H,4-5H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Staphylococcus aureus UDP-N-acetylmuramate dehydrogenase |
Bioorg Med Chem Lett 13: 2591-4 (2003)
BindingDB Entry DOI: 10.7270/Q20P126D |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
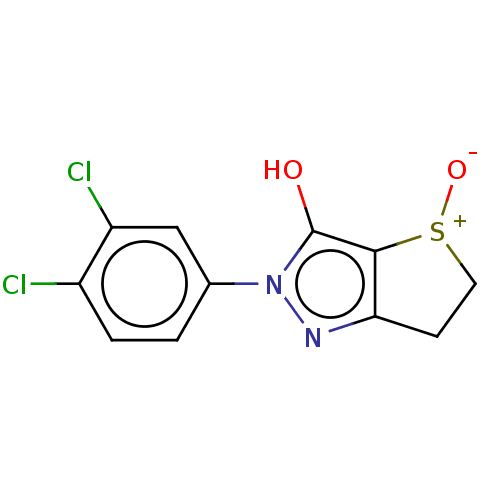
(Staphylococcus aureus (Firmicutes)) | BDBM50221034
(CHEMBL87428)Show InChI InChI=1S/C11H8Cl2N2O2S/c12-7-2-1-6(5-8(7)13)15-11(16)10-9(14-15)3-4-18(10)17/h1-2,5,16H,3-4H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Staphylococcus aureus UDP-N-acetylmuramate dehydrogenase |
Bioorg Med Chem Lett 13: 2591-4 (2003)
BindingDB Entry DOI: 10.7270/Q20P126D |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
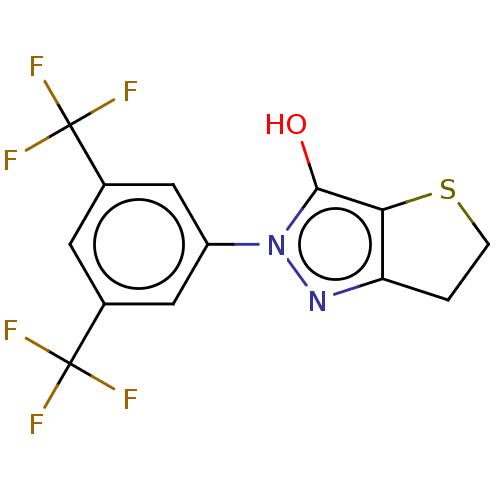
(Staphylococcus aureus (Firmicutes)) | BDBM50221026
(CHEMBL89482)Show InChI InChI=1S/C13H8F6N2OS/c14-12(15,16)6-3-7(13(17,18)19)5-8(4-6)21-11(22)10-9(20-21)1-2-23-10/h3-5,22H,1-2H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >7.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Staphylococcus aureus UDP-N-acetylmuramate dehydrogenase |
Bioorg Med Chem Lett 13: 2591-4 (2003)
BindingDB Entry DOI: 10.7270/Q20P126D |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50221036
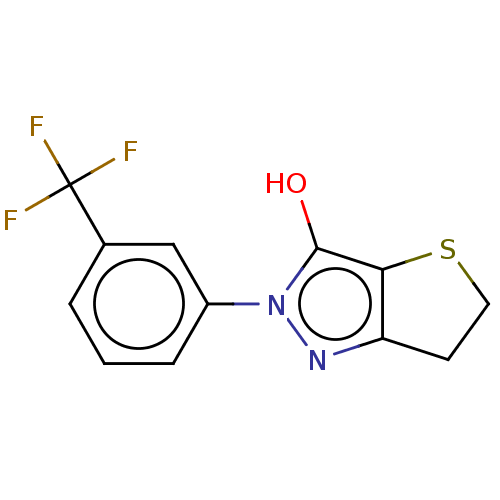
(CHEMBL315316)Show InChI InChI=1S/C12H9F3N2OS/c13-12(14,15)7-2-1-3-8(6-7)17-11(18)10-9(16-17)4-5-19-10/h1-3,6,18H,4-5H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Staphylococcus aureus UDP-N-acetylmuramate dehydrogenase |
Bioorg Med Chem Lett 13: 2591-4 (2003)
BindingDB Entry DOI: 10.7270/Q20P126D |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50221040

(CHEMBL83020)Show InChI InChI=1S/C11H8Cl2N2OS/c12-8-2-1-6(3-9(8)13)15-11(16)7-4-17-5-10(7)14-15/h1-3,16H,4-5H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >8.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Staphylococcus aureus UDP-N-acetylmuramate dehydrogenase |
Bioorg Med Chem Lett 13: 2591-4 (2003)
BindingDB Entry DOI: 10.7270/Q20P126D |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50221028
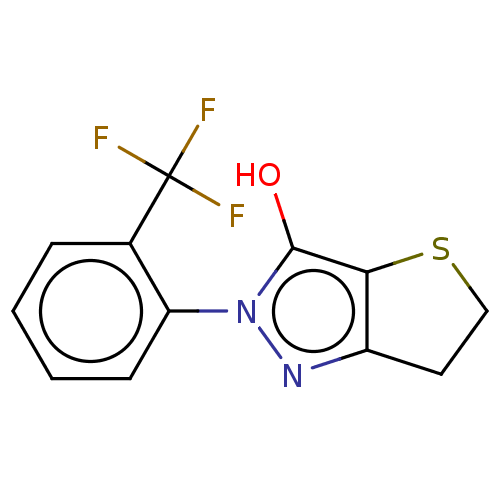
(CHEMBL87044)Show InChI InChI=1S/C12H9F3N2OS/c13-12(14,15)7-3-1-2-4-9(7)17-11(18)10-8(16-17)5-6-19-10/h1-4,18H,5-6H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >8.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Staphylococcus aureus UDP-N-acetylmuramate dehydrogenase |
Bioorg Med Chem Lett 13: 2591-4 (2003)
BindingDB Entry DOI: 10.7270/Q20P126D |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50221021
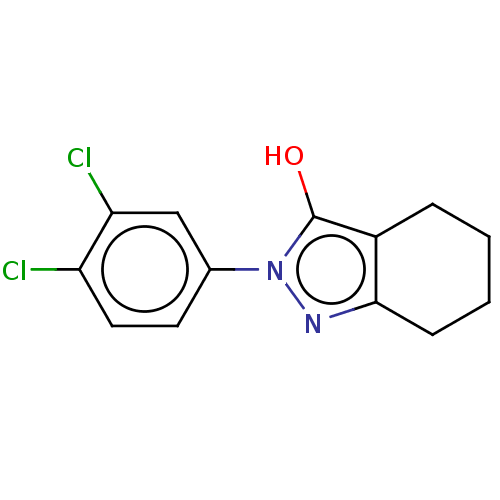
(CHEMBL86981)Show InChI InChI=1S/C13H12Cl2N2O/c14-10-6-5-8(7-11(10)15)17-13(18)9-3-1-2-4-12(9)16-17/h5-7,18H,1-4H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >8.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Staphylococcus aureus UDP-N-acetylmuramate dehydrogenase |
Bioorg Med Chem Lett 13: 2591-4 (2003)
BindingDB Entry DOI: 10.7270/Q20P126D |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50221039

(CHEMBL88099)Show InChI InChI=1S/C12H11N3O3S/c16-12-10-7-19-6-5-11(10)13-14(12)8-1-3-9(4-2-8)15(17)18/h1-4,16H,5-7H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >9.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Staphylococcus aureus UDP-N-acetylmuramate dehydrogenase |
Bioorg Med Chem Lett 13: 2591-4 (2003)
BindingDB Entry DOI: 10.7270/Q20P126D |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50221024
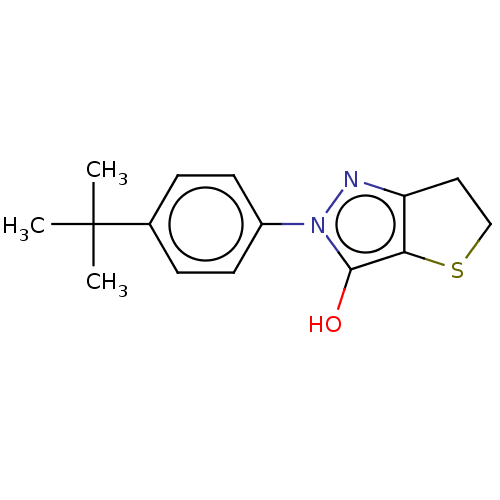
(CHEMBL86851)Show InChI InChI=1S/C15H18N2OS/c1-15(2,3)10-4-6-11(7-5-10)17-14(18)13-12(16-17)8-9-19-13/h4-7,18H,8-9H2,1-3H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >9.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Staphylococcus aureus UDP-N-acetylmuramate dehydrogenase |
Bioorg Med Chem Lett 13: 2591-4 (2003)
BindingDB Entry DOI: 10.7270/Q20P126D |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50221019
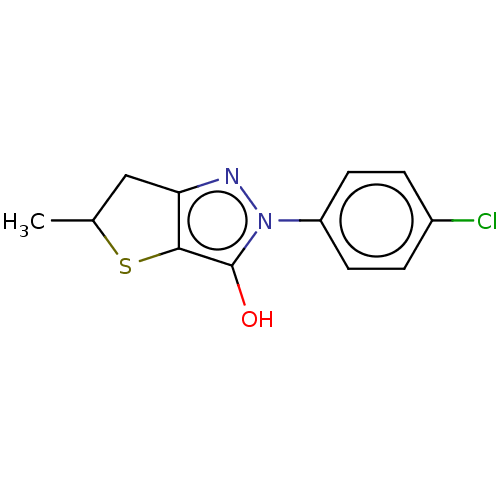
(CHEMBL87584)Show InChI InChI=1S/C12H11ClN2OS/c1-7-6-10-11(17-7)12(16)15(14-10)9-4-2-8(13)3-5-9/h2-5,7,16H,6H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >9.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Staphylococcus aureus UDP-N-acetylmuramate dehydrogenase |
Bioorg Med Chem Lett 13: 2591-4 (2003)
BindingDB Entry DOI: 10.7270/Q20P126D |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50221033
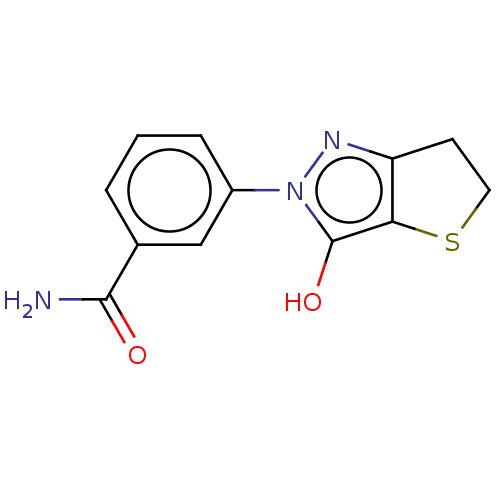
(CHEMBL420601)Show InChI InChI=1S/C12H11N3O2S/c13-11(16)7-2-1-3-8(6-7)15-12(17)10-9(14-15)4-5-18-10/h1-3,6,17H,4-5H2,(H2,13,16) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >9.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Staphylococcus aureus UDP-N-acetylmuramate dehydrogenase |
Bioorg Med Chem Lett 13: 2591-4 (2003)
BindingDB Entry DOI: 10.7270/Q20P126D |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50221031

(CHEMBL315456)Show InChI InChI=1S/C14H16N2OS/c1-9(2)10-3-5-11(6-4-10)16-14(17)13-12(15-16)7-8-18-13/h3-6,9,17H,7-8H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >9.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Staphylococcus aureus UDP-N-acetylmuramate dehydrogenase |
Bioorg Med Chem Lett 13: 2591-4 (2003)
BindingDB Entry DOI: 10.7270/Q20P126D |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50221022
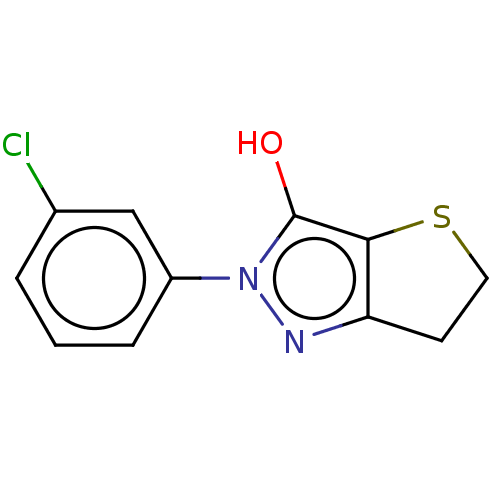
(CHEMBL86525)Show InChI InChI=1S/C11H9ClN2OS/c12-7-2-1-3-8(6-7)14-11(15)10-9(13-14)4-5-16-10/h1-3,6,15H,4-5H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >9.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Staphylococcus aureus UDP-N-acetylmuramate dehydrogenase |
Bioorg Med Chem Lett 13: 2591-4 (2003)
BindingDB Entry DOI: 10.7270/Q20P126D |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50221023
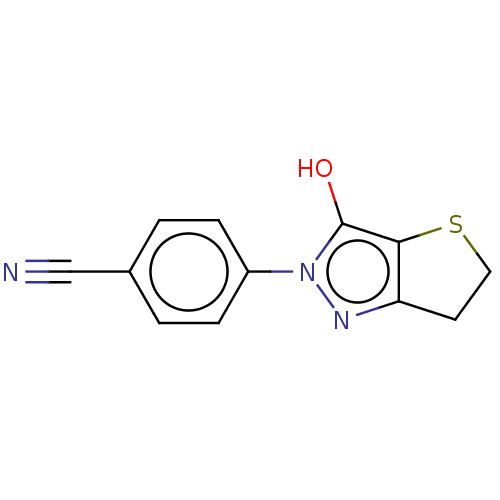
(CHEMBL84684)Show InChI InChI=1S/C12H9N3OS/c13-7-8-1-3-9(4-2-8)15-12(16)11-10(14-15)5-6-17-11/h1-4,16H,5-6H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Staphylococcus aureus UDP-N-acetylmuramate dehydrogenase |
Bioorg Med Chem Lett 13: 2591-4 (2003)
BindingDB Entry DOI: 10.7270/Q20P126D |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50221041
(CHEMBL83458)Show InChI InChI=1S/C11H9ClN2O2/c12-7-1-3-8(4-2-7)14-11(15)9-5-16-6-10(9)13-14/h1-4,15H,5-6H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Staphylococcus aureus UDP-N-acetylmuramate dehydrogenase |
Bioorg Med Chem Lett 13: 2591-4 (2003)
BindingDB Entry DOI: 10.7270/Q20P126D |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50221029

(CHEMBL87821)Show InChI InChI=1S/C11H10N2OS/c14-11-10-9(6-7-15-10)12-13(11)8-4-2-1-3-5-8/h1-5,14H,6-7H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.14E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Staphylococcus aureus UDP-N-acetylmuramate dehydrogenase |
Bioorg Med Chem Lett 13: 2591-4 (2003)
BindingDB Entry DOI: 10.7270/Q20P126D |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50221042

(CHEMBL83515)Show InChI InChI=1S/C11H9ClN2OS/c12-7-1-3-8(4-2-7)14-11(15)9-5-16-6-10(9)13-14/h1-4,15H,5-6H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Staphylococcus aureus UDP-N-acetylmuramate dehydrogenase |
Bioorg Med Chem Lett 13: 2591-4 (2003)
BindingDB Entry DOI: 10.7270/Q20P126D |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data