

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM117016 (US8664258, 32 | US9987252, 32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Arena Pharmaceuticals, Inc. US Patent | Assay Description Radioligand binding assays for human 5-HT2A receptor was conducted using the 5-HT2 agonist [125]DOI as radioligand. To define nonspecific binding, 10... | US Patent US8664258 (2014) BindingDB Entry DOI: 10.7270/Q2WQ02G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM117016 (US8664258, 32 | US9987252, 32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia at Charlottesville | Assay Description Radioligand binding assays for human 5-HT2A receptor was conducted using the 5-HT2 agonist [125I]DOI as radioligand. To define nonspecific binding, 1... | Chem Biol 14: 1186-97 (2007) BindingDB Entry DOI: 10.7270/Q29K4DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50001775 ((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals Inc. | Assay Description R-SAT assays were performed as described previously (Weiner et al., 2001), with the following modifications. In brief, NIH-3T3 cells were grown to 80... | J Pharmacol Exp Ther 317: 910-8 (2006) Article DOI: 10.1124/jpet.105.097006 BindingDB Entry DOI: 10.7270/Q269728N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM119676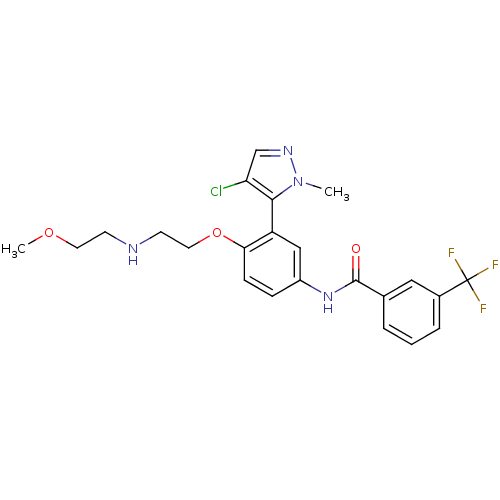 (US8680119, 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0820 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Arena Pharmaceuticals, Inc. US Patent | Assay Description Radioligand binding assays for human 5-HT2A receptor was conducted using the 5-HT2 agonist [125I]DOI as radioligand. To define nonspecific binding, 1... | US Patent US8680119 (2014) BindingDB Entry DOI: 10.7270/Q2RV0MB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM117019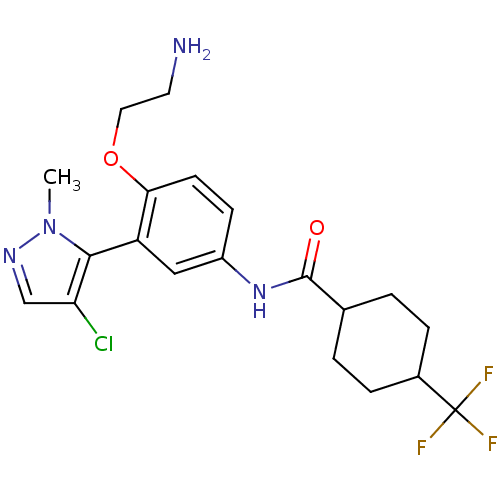 (US8664258, 303 | US9987252, 303) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Arena Pharmaceuticals, Inc. US Patent | Assay Description Radioligand binding assays for human 5-HT2A receptor was conducted using the 5-HT2 agonist [125]DOI as radioligand. To define nonspecific binding, 10... | US Patent US8664258 (2014) BindingDB Entry DOI: 10.7270/Q2WQ02G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM117019 (US8664258, 303 | US9987252, 303) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia at Charlottesville | Assay Description Radioligand binding assays for human 5-HT2A receptor was conducted using the 5-HT2 agonist [125I]DOI as radioligand. To define nonspecific binding, 1... | Chem Biol 14: 1186-97 (2007) BindingDB Entry DOI: 10.7270/Q29K4DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM86525 (AMI-193 | CAS_77445 | CHEMBL79834 | NSC_77445) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals A/S Curated by ChEMBL | Assay Description Compound was evaluated for its inverse agonist activity against 5-hydroxytryptamine 2A receptor | Bioorg Med Chem Lett 10: 2435-9 (2001) BindingDB Entry DOI: 10.7270/Q2V69HTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM21395 (3-(2-(4-(4-Fluorobenzoyl)piperidinol)ethyl)-2,4(1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human recombinant 5HT2A receptor expressed in HEK293 cells measured after 60 mins by scintillation counting metho... | Bioorg Med Chem 25: 471-482 (2017) Article DOI: 10.1016/j.bmc.2016.11.014 BindingDB Entry DOI: 10.7270/Q2CF9S3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM21395 (3-(2-(4-(4-Fluorobenzoyl)piperidinol)ethyl)-2,4(1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in HEK293 cells | Bioorg Med Chem 24: 1793-810 (2016) Article DOI: 10.1016/j.bmc.2016.03.006 BindingDB Entry DOI: 10.7270/Q2J67JS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50540335 (CHEMBL4635010) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at 5HT2A receptor (unknown origin) expressed in HEK293 cells assessed as inhibition of serotonin hydrochloride-induced calcium fl... | J Med Chem 63: 4171-4182 (2020) Article DOI: 10.1021/acs.jmedchem.0c00002 BindingDB Entry DOI: 10.7270/Q2CZ3BPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50048803 (5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against 5-hydroxytryptamine 2 receptor | J Med Chem 48: 6523-43 (2005) Article DOI: 10.1021/jm058225d BindingDB Entry DOI: 10.7270/Q2SF2WZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM616185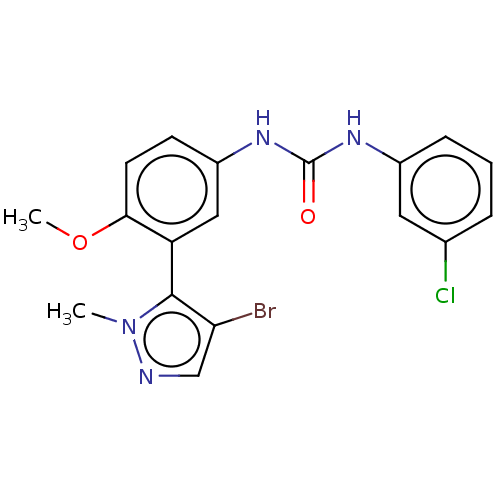 (1-[3-(4-Bromo-2-methyl-2H- pyrazol-3-yl)-4-methoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8CS2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50306837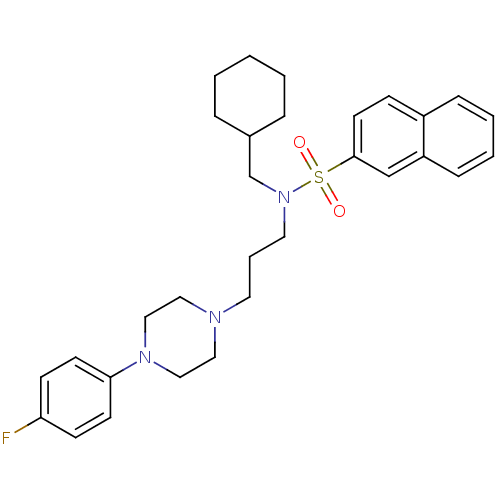 (CHEMBL602284 | N-(Cyclohexylmethyl)-N-(3-(4-(4-flu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human recombinant 5HT2A receptor expressed in avCHO-K1 cells | Bioorg Med Chem 18: 1665-75 (2010) Article DOI: 10.1016/j.bmc.2009.12.067 BindingDB Entry DOI: 10.7270/Q2R78F99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM119679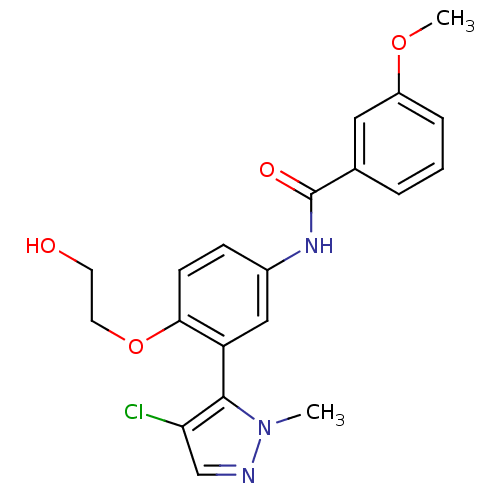 (US8680119, 68) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Arena Pharmaceuticals, Inc. US Patent | Assay Description Radioligand binding assays for human 5-HT2A receptor was conducted using the 5-HT2 agonist [125I]DOI as radioligand. To define nonspecific binding, 1... | US Patent US8680119 (2014) BindingDB Entry DOI: 10.7270/Q2RV0MB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM21395 (3-(2-(4-(4-Fluorobenzoyl)piperidinol)ethyl)-2,4(1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor in HEK293 cells after 60 mins by scintillation counting | J Med Chem 60: 349-361 (2017) Article DOI: 10.1021/acs.jmedchem.6b01422 BindingDB Entry DOI: 10.7270/Q27M0B6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50556204 (ITI 007 | ITI-007 | ITI-722 | ITI007 | Lumateperon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at 5HT2A receptor (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112667 BindingDB Entry DOI: 10.7270/Q2HD80BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50596723 (CHEMBL5205903 | US20230348421, Compound 59) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50596723 (CHEMBL5205903 | US20230348421, Compound 59) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114246 BindingDB Entry DOI: 10.7270/Q20P142F | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50464898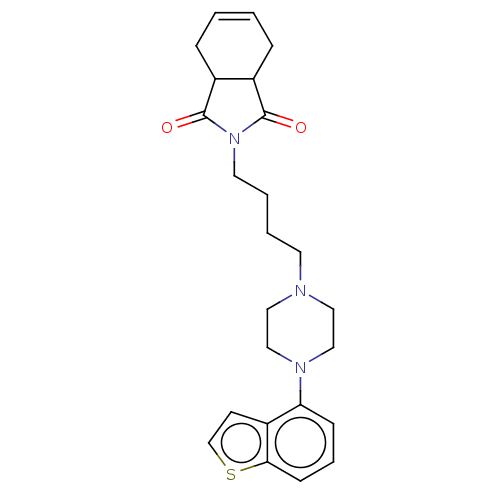 (CHEMBL4287980) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2A receptor (unknown origin) after 10 mins by calcium 5 dye based FLIPR assay | Eur J Med Chem 145: 74-85 (2018) Article DOI: 10.1016/j.ejmech.2017.12.099 BindingDB Entry DOI: 10.7270/Q2Z03BVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50540327 (CHEMBL4644559) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at 5HT2A receptor (unknown origin) expressed in HEK293 cells assessed as inhibition of serotonin hydrochloride-induced calcium fl... | J Med Chem 63: 4171-4182 (2020) Article DOI: 10.1021/acs.jmedchem.0c00002 BindingDB Entry DOI: 10.7270/Q2CZ3BPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50172414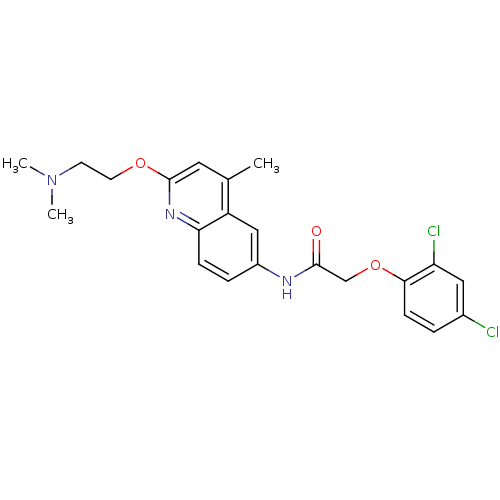 (2-(2,4-Dichloro-phenoxy)-N-[2-(2-dimethylamino-eth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
7TM Pharma A/S Curated by ChEMBL | Assay Description Inhibitory concentration against 5-hydroxytryptamine 2A receptor | J Med Chem 48: 5684-97 (2005) Article DOI: 10.1021/jm050103y BindingDB Entry DOI: 10.7270/Q2H41QZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM392076 (US10301272, Example 7/9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin human recombinant 5-HT2A receptor after 60 mins by scintillation counting analysis | Bioorg Med Chem Lett 28: 1446-1455 (2018) Article DOI: 10.1016/j.bmcl.2018.03.093 BindingDB Entry DOI: 10.7270/Q27W6FPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50306834 (CHEMBL600012 | N-(Cyclohexylmethyl)-N-(3-(4-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human recombinant 5HT2A receptor expressed in avCHO-K1 cells | Bioorg Med Chem 18: 1665-75 (2010) Article DOI: 10.1016/j.bmc.2009.12.067 BindingDB Entry DOI: 10.7270/Q2R78F99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.708 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lublin Curated by ChEMBL | Assay Description Antagonist activity at human 5HT2A receptor expressed in CHOK1 cells assessed as inhibition of 5-HT induced inositol phosphate production incubated f... | Eur J Med Chem 180: 673-689 (2019) Article DOI: 10.1016/j.ejmech.2019.07.050 BindingDB Entry DOI: 10.7270/Q2XK8JXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lublin Curated by ChEMBL | Assay Description Antagonist activity at human 5HT2A receptor expressed in CHOK1 cells assessed as inhibition of 5-HT induced inositol phosphate production incubated f... | Eur J Med Chem 180: 673-689 (2019) Article DOI: 10.1016/j.ejmech.2019.07.050 BindingDB Entry DOI: 10.7270/Q2XK8JXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50194847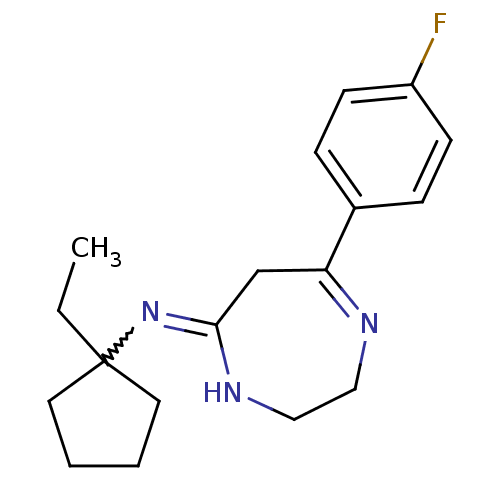 (CHEMBL221250 | N-(1-ethylcyclopentyl)-7-(4-fluorop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2A receptor expressed in CHOK1 cells | Bioorg Med Chem Lett 16: 6058-62 (2006) Article DOI: 10.1016/j.bmcl.2006.08.108 BindingDB Entry DOI: 10.7270/Q2D79B26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50544628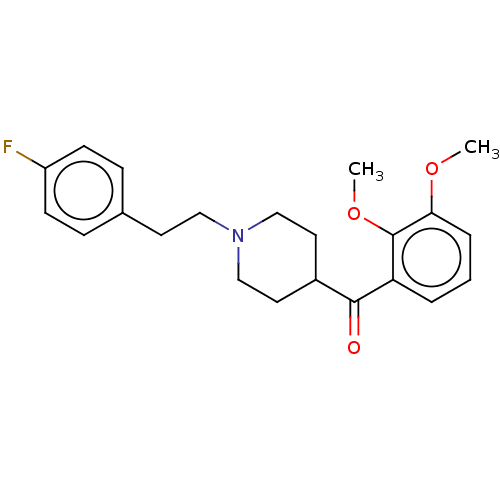 (CHEMBL4638006) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Antagonist activity at human 5HT2A receptor expressed in HEK293 cells assessed as inhibition of 5HT-induced intracellular Ca2+ mobilization incubated... | J Med Chem 63: 9928-9949 (2020) Article DOI: 10.1021/acs.jmedchem.0c01058 BindingDB Entry DOI: 10.7270/Q2ZC86GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50544628 (CHEMBL4638006) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.813 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Antagonist activity at human 5HT2A receptor expressed in HEK293 cells assessed as inhibition of 5HT-induced intracellular Ca2+ mobilization incubated... | J Med Chem 63: 9928-9949 (2020) Article DOI: 10.1021/acs.jmedchem.0c01058 BindingDB Entry DOI: 10.7270/Q2ZC86GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM21395 (3-(2-(4-(4-Fluorobenzoyl)piperidinol)ethyl)-2,4(1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Ireland Galway Curated by ChEMBL | Assay Description Displacement of [3H] ketanserin from human recombinant 5-HT2A receptor measured after 60 mins by scintillation counter method | Eur J Med Chem 176: 292-309 (2019) Article DOI: 10.1016/j.ejmech.2019.04.064 BindingDB Entry DOI: 10.7270/Q2NP27V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50540334 (CHEMBL4647697) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at 5HT2A receptor (unknown origin) expressed in HEK293 cells assessed as inhibition of serotonin hydrochloride-induced calcium fl... | J Med Chem 63: 4171-4182 (2020) Article DOI: 10.1021/acs.jmedchem.0c00002 BindingDB Entry DOI: 10.7270/Q2CZ3BPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM117018 (US8664258, 197 | US9987252, 197) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia at Charlottesville | Assay Description Radioligand binding assays for human 5-HT2A receptor was conducted using the 5-HT2 agonist [125I]DOI as radioligand. To define nonspecific binding, 1... | Chem Biol 14: 1186-97 (2007) BindingDB Entry DOI: 10.7270/Q29K4DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM117018 (US8664258, 197 | US9987252, 197) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Arena Pharmaceuticals, Inc. US Patent | Assay Description Radioligand binding assays for human 5-HT2A receptor was conducted using the 5-HT2 agonist [125]DOI as radioligand. To define nonspecific binding, 10... | US Patent US8664258 (2014) BindingDB Entry DOI: 10.7270/Q2WQ02G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM139371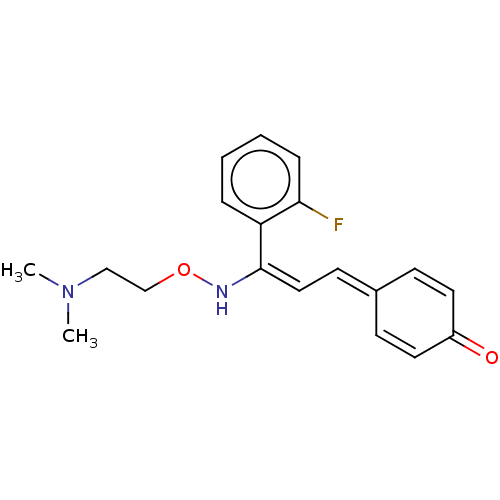 (eplivanserin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals Inc. | Assay Description R-SAT assays were performed as described previously (Weiner et al., 2001), with the following modifications. In brief, NIH-3T3 cells were grown to 80... | J Pharmacol Exp Ther 317: 910-8 (2006) Article DOI: 10.1124/jpet.105.097006 BindingDB Entry DOI: 10.7270/Q269728N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50194829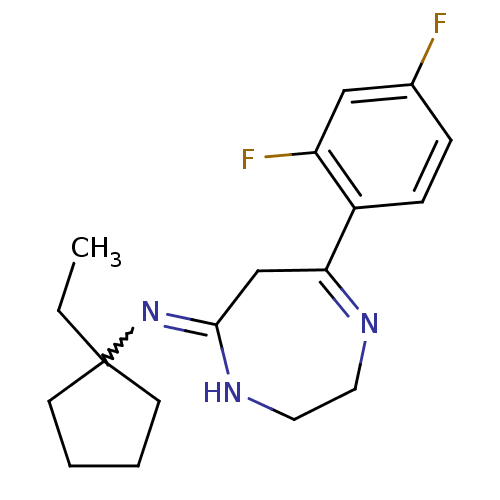 (7-(2,4-difluorophenyl)-N-(1-ethylcyclopentyl)-2,3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2A receptor expressed in CHOK1 cells | Bioorg Med Chem Lett 16: 6058-62 (2006) Article DOI: 10.1016/j.bmcl.2006.08.108 BindingDB Entry DOI: 10.7270/Q2D79B26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50232153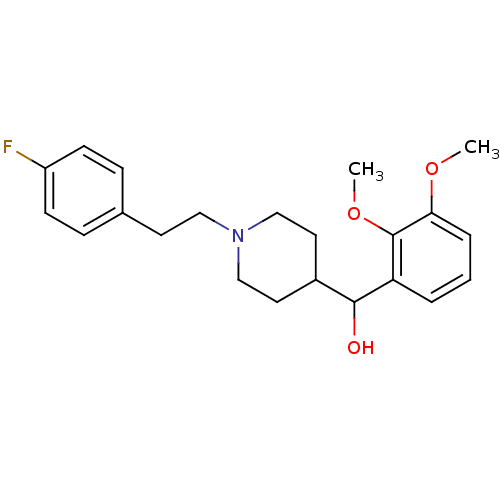 ((1-(4-fluorophenethyl)piperidin-4-yl)(2,3-dimethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals Inc. | Assay Description R-SAT assays were performed as described previously (Weiner et al., 2001), with the following modifications. In brief, NIH-3T3 cells were grown to 80... | J Pharmacol Exp Ther 317: 910-8 (2006) Article DOI: 10.1124/jpet.105.097006 BindingDB Entry DOI: 10.7270/Q269728N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50403974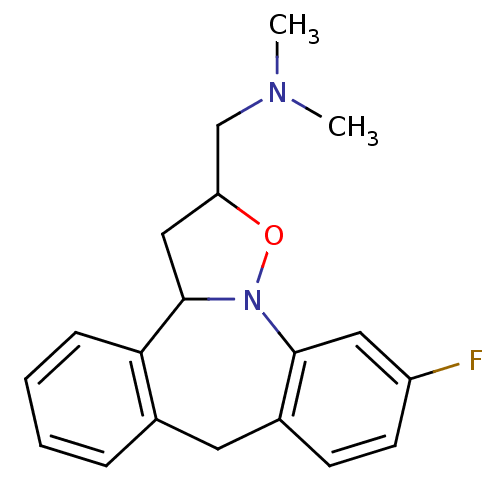 (CHEMBL84931) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen-Cilag Curated by ChEMBL | Assay Description Binding affinity at human cloned 5-hydroxytryptamine 2A receptor expressed in L929 cells by [125I]R91150 displacement. | Bioorg Med Chem Lett 12: 249-53 (2001) Article DOI: 10.1016/S0960-894X(01)00722-3 BindingDB Entry DOI: 10.7270/Q2ZW1N3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM616186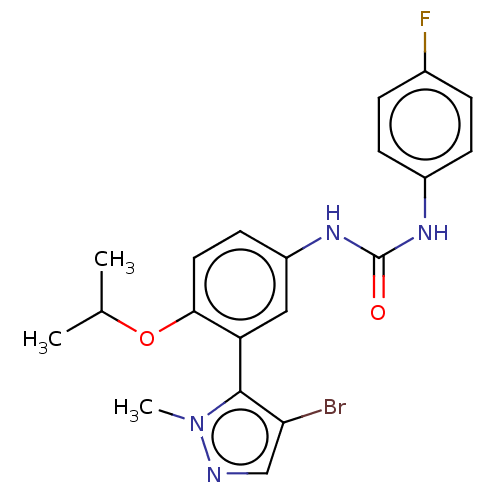 (1-[3-(4-Bromo-2-methyl-2H- pyrazol-3-yl)-4-isoprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8CS2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50464897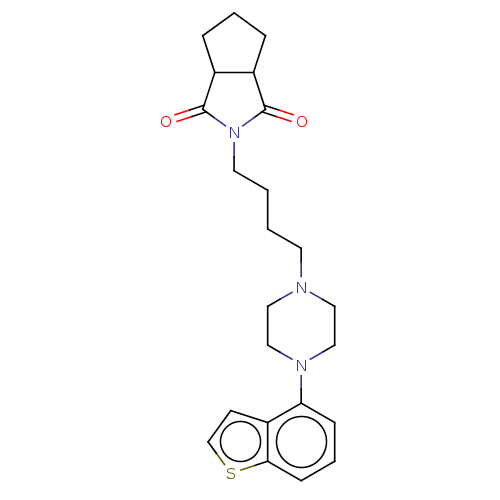 (CHEMBL4291387) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2A receptor (unknown origin) after 10 mins by calcium 5 dye based FLIPR assay | Eur J Med Chem 145: 74-85 (2018) Article DOI: 10.1016/j.ejmech.2017.12.099 BindingDB Entry DOI: 10.7270/Q2Z03BVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM463328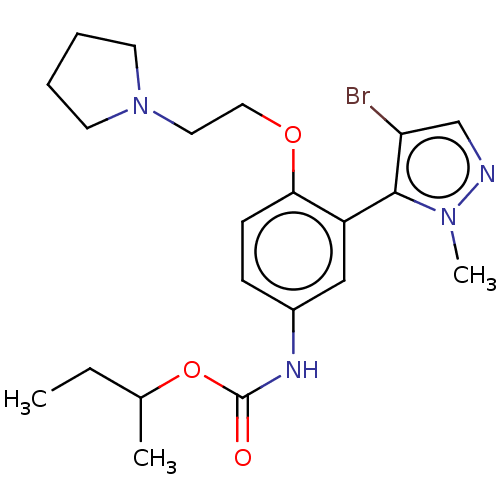 ( [3-(4-Bromo-2-methyl-2H-pyrazol-3-yl)-4-(2-pyrrol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc. US Patent | Assay Description Radioligand binding assays for human 5-HT2A receptor was conducted using the 5-HT2 agonist [125I]DOI as radioligand. To define nonspecific binding, 1... | US Patent US10781180 (2020) BindingDB Entry DOI: 10.7270/Q2SX6H84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM631393 (US20230348421, Compound 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1.12 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50246480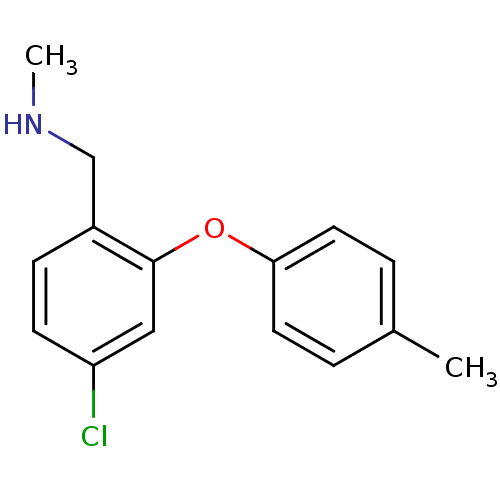 (1-(4-chloro-2-(p-tolyloxy)phenyl)-N-methylmethanam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.18 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from 5HT2A receptor (unknown origin) | Bioorg Med Chem Lett 18: 6088-92 (2008) Article DOI: 10.1016/j.bmcl.2008.10.028 BindingDB Entry DOI: 10.7270/Q2RJ4JBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM578155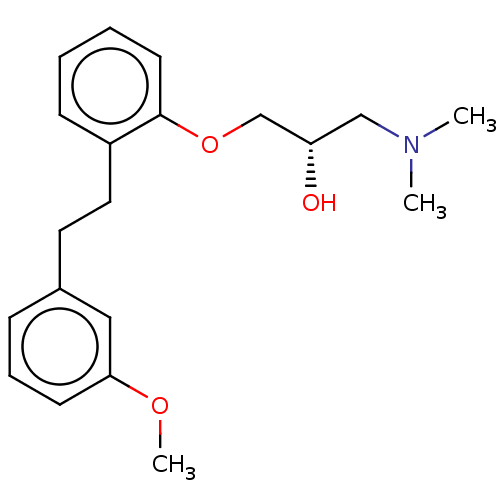 (US11478467, Compound (+)-147) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Evaluation of the affinity of compounds for the human 5-HT2A receptor in transfected HEK-293 cells determined in a radioligand binding assay. Cell me... | Citation and Details BindingDB Entry DOI: 10.7270/Q2862KPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM117020 (US8664258, 77) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Arena Pharmaceuticals, Inc. US Patent | Assay Description Radioligand binding assays for human 5-HT2A receptor was conducted using the 5-HT2 agonist [125]DOI as radioligand. To define nonspecific binding, 10... | US Patent US8664258 (2014) BindingDB Entry DOI: 10.7270/Q2WQ02G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM397088 (4-Bromo-thiophene-2- carboxylic acid [4-(2- amino-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia at Charlottesville | Assay Description Radioligand binding assays for human 5-HT2A receptor was conducted using the 5-HT2 agonist [125I]DOI as radioligand. To define nonspecific binding, 1... | Chem Biol 14: 1186-97 (2007) BindingDB Entry DOI: 10.7270/Q29K4DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM319608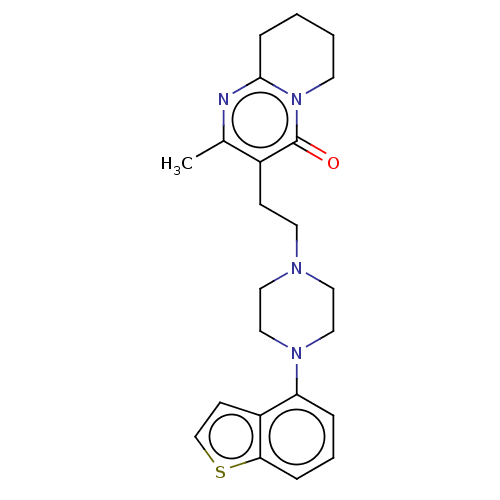 (3-(2-(4-(benzo[b]thiophen-4-yl)piperazin-1-yl)ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Xinjiang Technical Institute of Physics and Chemistry Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2A receptor (unknown origin) after 10 mins by calcium 5 dye based FLIPR assay | Bioorg Med Chem 25: 4904-4916 (2017) Article DOI: 10.1016/j.bmc.2017.07.040 BindingDB Entry DOI: 10.7270/Q2Z3222T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM578158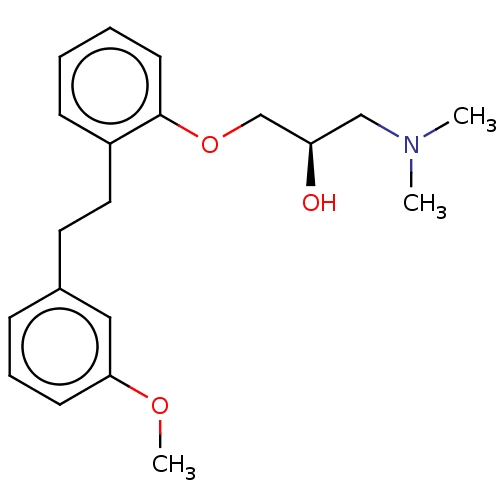 (US11478467, Compound (-)-148) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Evaluation of the affinity of compounds for the human 5-HT2A receptor in transfected HEK-293 cells determined in a radioligand binding assay. Cell me... | Citation and Details BindingDB Entry DOI: 10.7270/Q2862KPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50194836 (CHEMBL436513 | N-(2-cyclobutylpropan-2-yl)-7-(2,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2A receptor expressed in CHOK1 cells | Bioorg Med Chem Lett 16: 6058-62 (2006) Article DOI: 10.1016/j.bmcl.2006.08.108 BindingDB Entry DOI: 10.7270/Q2D79B26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50113332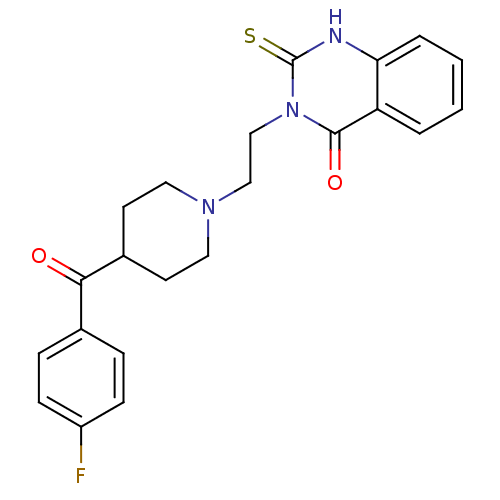 (3-(2-(4-(4-fluorobenzoyl)piperidin-1-yl)ethyl)-2-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dominican College Curated by ChEMBL | Assay Description Antagonist activity at recombinant human 5-HT2A expressed in human U2OS cells by pathhunter beta-arrestin assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127358 BindingDB Entry DOI: 10.7270/Q26113XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50194832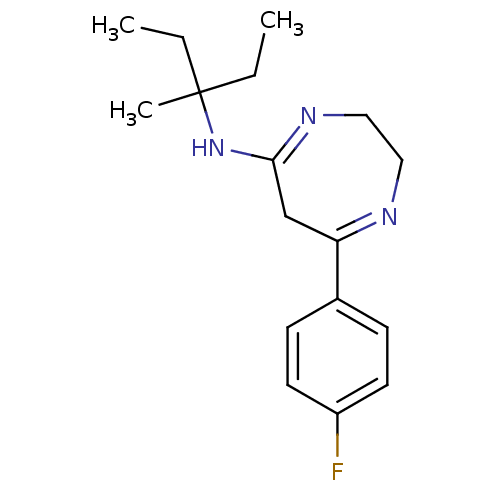 (7-(4-fluorophenyl)-N-(3-methylpentan-3-yl)-2,3-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2A receptor expressed in CHOK1 cells | Bioorg Med Chem Lett 16: 6058-62 (2006) Article DOI: 10.1016/j.bmcl.2006.08.108 BindingDB Entry DOI: 10.7270/Q2D79B26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50194850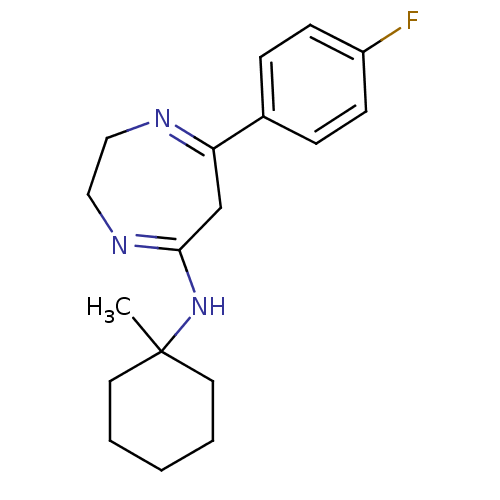 (7-(4-fluorophenyl)-N-(1-methylcyclohexyl)-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2A receptor expressed in CHOK1 cells | Bioorg Med Chem Lett 16: 6058-62 (2006) Article DOI: 10.1016/j.bmcl.2006.08.108 BindingDB Entry DOI: 10.7270/Q2D79B26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1465 total ) | Next | Last >> |