

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50240713 (CHEMBL4066318) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation Curated by ChEMBL | Assay Description Inhibition constants against ATP substrate site of M-2 (kidney form) variant of rat Methionine adenosyltransferase | J Med Chem 60: 8691-8705 (2017) Article DOI: 10.1021/acs.jmedchem.7b00510 BindingDB Entry DOI: 10.7270/Q2TT4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50240713 (CHEMBL4066318) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation Curated by ChEMBL | Assay Description Inhibition constants against Met (L-methionine)P substrate site of M-T (Novikoff Ascitic Hepatoma form) variant of rat Methionine adenosyltransferase | J Med Chem 60: 8691-8705 (2017) Article DOI: 10.1021/acs.jmedchem.7b00510 BindingDB Entry DOI: 10.7270/Q2TT4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50363928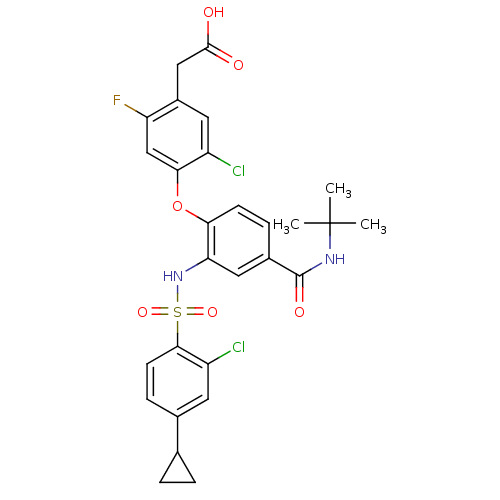 (CHEMBL1951575) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc Curated by ChEMBL | Assay Description Competitive inhibition of CYP2C8 in human liver microsomes assessed as paclitaxel 6-hydroxylation after 20 mins by LC-MS/MS analysis | Drug Metab Dispos 40: 2239-49 (2012) Article DOI: 10.1124/dmd.112.047928 BindingDB Entry DOI: 10.7270/Q2Z039WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50105417 (CHEMBL1089 | Nardil | PHENELZINE | Phenethyl-hydra...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Mechanism based inhibition of human cytochrome P450 2C8 measured by paclitaxel hydroxylation using a recombinant system | Curr Drug Metab 6: 413-54 (2005) BindingDB Entry DOI: 10.7270/Q2VQ33X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM18957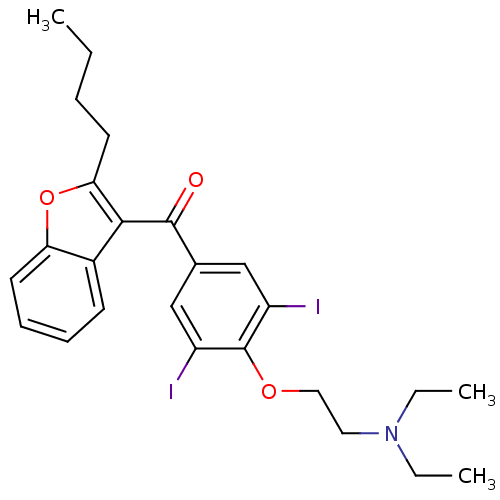 ((2-{4-[(2-butyl-1-benzofuran-3-yl)carbonyl]-2,6-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Mechanism based inhibition of human cytochrome P450 2C8 measured by paclitaxel hydroxylation using a recombinant system | Curr Drug Metab 6: 413-54 (2005) BindingDB Entry DOI: 10.7270/Q2VQ33X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50363928 (CHEMBL1951575) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc Curated by ChEMBL | Assay Description Biphasic inhibition of CYP2C8 in human liver microsomes assessed as montelukast 36-hydroxylation after 20 mins by LC-MS/MS analysis | Drug Metab Dispos 40: 2239-49 (2012) Article DOI: 10.1124/dmd.112.047928 BindingDB Entry DOI: 10.7270/Q2Z039WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50088490 (CHEMBL3526979) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc Curated by ChEMBL | Assay Description Linear mixed inhibition of CYP2C8 in human liver microsomes assessed as paclitaxel 6-hydroxylation after 20 mins by LC-MS/MS analysis | Drug Metab Dispos 40: 2239-49 (2012) Article DOI: 10.1124/dmd.112.047928 BindingDB Entry DOI: 10.7270/Q2Z039WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50363928 (CHEMBL1951575) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc Curated by ChEMBL | Assay Description Competitive inhibition of CYP2C8 in human liver microsomes assessed as rosiglitazone demethylation after 20 mins by LC-MS/MS analysis | Drug Metab Dispos 40: 2239-49 (2012) Article DOI: 10.1124/dmd.112.047928 BindingDB Entry DOI: 10.7270/Q2Z039WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50088490 (CHEMBL3526979) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc Curated by ChEMBL | Assay Description Linear mixed inhibition of CYP2C8 in human liver microsomes assessed as rosiglitazone demethylation after 20 mins by LC-MS/MS analysis | Drug Metab Dispos 40: 2239-49 (2012) Article DOI: 10.1124/dmd.112.047928 BindingDB Entry DOI: 10.7270/Q2Z039WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50088490 (CHEMBL3526979) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc Curated by ChEMBL | Assay Description Biphasic inhibition of CYP2C8 in human liver microsomes assessed as montelukast 36-hydroxylation after 20 mins by LC-MS/MS analysis | Drug Metab Dispos 40: 2239-49 (2012) Article DOI: 10.1124/dmd.112.047928 BindingDB Entry DOI: 10.7270/Q2Z039WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50088513 (CHEMBL3526940) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation Curated by ChEMBL | Assay Description Inhibition of CYP2C8 in human liver microsomes preincubated for 30 mins followed by substrate addition measured after 2 mins | J Med Chem 60: 8691-8705 (2017) Article DOI: 10.1021/acs.jmedchem.7b00510 BindingDB Entry DOI: 10.7270/Q2TT4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM58922 (MLS001304727 | MONTELUKAST Na | SMR000469188 | cid...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP2C8 expressed in microsomes isolated from baculovirus infected insect cell after 3 mins | Drug Metab Dispos 39: 904-11 (2011) Article DOI: 10.1124/dmd.110.037689 BindingDB Entry DOI: 10.7270/Q2XW4MJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM81939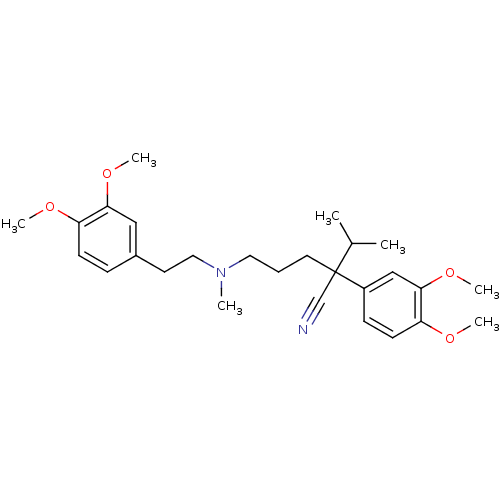 (CAS_52-53-9 | NSC_62969 | VERAPAMIL) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | DrugBank PubMed | 1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Mechanism based inhibition of human cytochrome P450 2C8 measured by paclitaxel hydroxylation using a recombinant system | Curr Drug Metab 6: 413-54 (2005) BindingDB Entry DOI: 10.7270/Q2VQ33X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50397663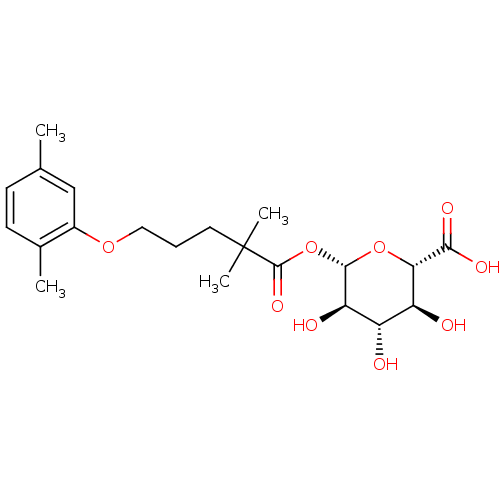 (CHEMBL2181817) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem | Article PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Time dependent inhibition of CYP2C8 in human liver microsomes | J Med Chem 55: 4896-933 (2012) Article DOI: 10.1021/jm300065h BindingDB Entry DOI: 10.7270/Q2PG1SVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50397663 (CHEMBL2181817) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem | Article PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation Curated by ChEMBL | Assay Description Inhibition constants with Met (L-methionine) substrate site of M-2 (kidney form) variant of rat Methionine adenosyltransferase | J Med Chem 60: 8691-8705 (2017) Article DOI: 10.1021/acs.jmedchem.7b00510 BindingDB Entry DOI: 10.7270/Q2TT4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50088489 (CHEMBL3527324) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc Curated by ChEMBL | Assay Description Inhibition of recombinant CYP2C8 (unknown origin) treated with AMG 853 | Drug Metab Dispos 40: 2239-49 (2012) Article DOI: 10.1124/dmd.112.047928 BindingDB Entry DOI: 10.7270/Q2Z039WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM112777 (NORTRIPTYLINE | US8629135, SW-02) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PubMed | 4.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Mechanism based inhibition of human cytochrome P450 2C8 measured by paclitaxel hydroxylation using a recombinant system | Curr Drug Metab 6: 413-54 (2005) BindingDB Entry DOI: 10.7270/Q2VQ33X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM18957 ((2-{4-[(2-butyl-1-benzofuran-3-yl)carbonyl]-2,6-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | 5.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Mechanism based inhibition of human cytochrome P450 2C8 measured by paclitaxel hydroxylation using human liver microsomes | Curr Drug Metab 6: 413-54 (2005) BindingDB Entry DOI: 10.7270/Q2VQ33X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50397663 (CHEMBL2181817) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem | Article PubMed | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation Curated by ChEMBL | Assay Description Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate preincubated for 2 to 40 mins followed by NADPH-generating system additi... | J Med Chem 60: 8691-8705 (2017) Article DOI: 10.1021/acs.jmedchem.7b00510 BindingDB Entry DOI: 10.7270/Q2TT4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50105417 (CHEMBL1089 | Nardil | PHENELZINE | Phenethyl-hydra...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank PubMed | 5.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Mechanism based inhibition of human cytochrome P450 2C8 measured by paclitaxel hydroxylation using human liver microsomes | Curr Drug Metab 6: 413-54 (2005) BindingDB Entry DOI: 10.7270/Q2VQ33X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50336507 (4-pyridinecarbohydrazide(Isoniazid) | CHEMBL64 | D...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | DrugBank PubMed | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Mechanism based inhibition of human cytochrome P450 2C8 measured by paclitaxel hydroxylation using human liver microsomes | Curr Drug Metab 6: 413-54 (2005) BindingDB Entry DOI: 10.7270/Q2VQ33X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM30130 (CHEMBL1201082 | CHEMBL41 | Fluoxetin | Fluoxetine ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 2.94E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Mechanism based inhibition of human cytochrome P450 2C8 measured by paclitaxel hydroxylation using a recombinant system | Curr Drug Metab 6: 413-54 (2005) BindingDB Entry DOI: 10.7270/Q2VQ33X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50336507 (4-pyridinecarbohydrazide(Isoniazid) | CHEMBL64 | D...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | DrugBank PubMed | 3.74E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Mechanism based inhibition of human cytochrome P450 2C8 measured by paclitaxel hydroxylation using a recombinant system | Curr Drug Metab 6: 413-54 (2005) BindingDB Entry DOI: 10.7270/Q2VQ33X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||