 Found 531 hits of ic50 for UniProtKB: P29317
Found 531 hits of ic50 for UniProtKB: P29317 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50345579

(5-((5-(4-((4-(2-hydroxyethyl)piperazin-1-yl)methyl...)Show SMILES CNC(=O)c1ncc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)n1C Show InChI InChI=1S/C30H33F3N6O3/c1-20-4-5-22(16-21(20)7-9-25-18-35-27(37(25)3)29(42)34-2)28(41)36-24-8-6-23(26(17-24)30(31,32)33)19-39-12-10-38(11-13-39)14-15-40/h4-6,8,16-18,40H,10-15,19H2,1-3H3,(H,34,42)(H,36,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human EPHA2 using poly[Glu:Tyr] peptide substrate by Hotspot assay |
Bioorg Med Chem Lett 21: 3743-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.060
BindingDB Entry DOI: 10.7270/Q21G0MM8 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2 [596-900]
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Refer to Reaction Biology Corps. |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-A receptor 2 [596-900]
(Homo sapiens (Human)) | BDBM50299218

(8-(2-Methoxyphenyl)-1-methyl-7-(2'-methyl-5'-hydro...)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(O)ccc1C |(16.89,-42.99,;16.89,-41.45,;18.23,-40.68,;19.55,-41.45,;20.88,-40.68,;20.88,-39.14,;19.55,-38.38,;18.23,-39.15,;16.9,-38.38,;16.91,-36.84,;15.44,-36.35,;14.53,-37.6,;15.43,-38.85,;14.53,-40.09,;13.06,-39.61,;11.74,-40.37,;11.73,-41.91,;10.41,-39.61,;9.07,-40.38,;10.41,-38.07,;11.74,-37.29,;11.74,-35.75,;13.06,-38.07,;18.24,-36.06,;19.58,-36.83,;20.9,-36.05,;22.24,-36.81,;20.9,-34.51,;19.55,-33.75,;18.23,-34.53,;16.89,-33.77,)| Show InChI InChI=1S/C22H19N5O4/c1-12-8-9-13(28)10-14(12)16-11-26-18-19(25(2)22(30)24-20(18)29)23-21(26)27(16)15-6-4-5-7-17(15)31-3/h4-11,28H,1-3H3,(H,24,29,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Refer to Reaction Biology Corps. |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-A receptor 2 [596-900]
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Refer to Reaction Biology Corps. |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2 [596-900]
(Homo sapiens (Human)) | BDBM50399540

(FORETINIB | US10464902, Foretinib | US10882853, Co...)Show SMILES COc1cc2c(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3F)ccnc2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C34H34F2N4O6/c1-43-30-20-25-27(21-31(30)45-16-2-13-40-14-17-44-18-15-40)37-12-9-28(25)46-29-8-7-24(19-26(29)36)39-33(42)34(10-11-34)32(41)38-23-5-3-22(35)4-6-23/h3-9,12,19-21H,2,10-11,13-18H2,1H3,(H,38,41)(H,39,42) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Refer to Reaction Biology Corps. |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-A receptor 2 [596-900]
(Homo sapiens (Human)) | BDBM4552

(4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-...)Show SMILES COc1cc(Nc2c(cnc3cc(OCCCN4CCN(C)CC4)c(OC)cc23)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C26H29Cl2N5O3/c1-32-6-8-33(9-7-32)5-4-10-36-25-13-21-18(11-24(25)35-3)26(17(15-29)16-30-21)31-22-14-23(34-2)20(28)12-19(22)27/h11-14,16H,4-10H2,1-3H3,(H,30,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Refer to Reaction Biology Corps. |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50299218

(8-(2-Methoxyphenyl)-1-methyl-7-(2'-methyl-5'-hydro...)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(O)ccc1C |(16.89,-42.99,;16.89,-41.45,;18.23,-40.68,;19.55,-41.45,;20.88,-40.68,;20.88,-39.14,;19.55,-38.38,;18.23,-39.15,;16.9,-38.38,;16.91,-36.84,;15.44,-36.35,;14.53,-37.6,;15.43,-38.85,;14.53,-40.09,;13.06,-39.61,;11.74,-40.37,;11.73,-41.91,;10.41,-39.61,;9.07,-40.38,;10.41,-38.07,;11.74,-37.29,;11.74,-35.75,;13.06,-38.07,;18.24,-36.06,;19.58,-36.83,;20.9,-36.05,;22.24,-36.81,;20.9,-34.51,;19.55,-33.75,;18.23,-34.53,;16.89,-33.77,)| Show InChI InChI=1S/C22H19N5O4/c1-12-8-9-13(28)10-14(12)16-11-26-18-19(25(2)22(30)24-20(18)29)23-21(26)27(16)15-6-4-5-7-17(15)31-3/h4-11,28H,1-3H3,(H,24,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphA2 by [gamma33-P]ATP based assay |
J Med Chem 52: 6433-46 (2009)
Article DOI: 10.1021/jm9009444
BindingDB Entry DOI: 10.7270/Q2BZ663G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-A receptor 2 [596-900]
(Homo sapiens (Human)) | BDBM209859
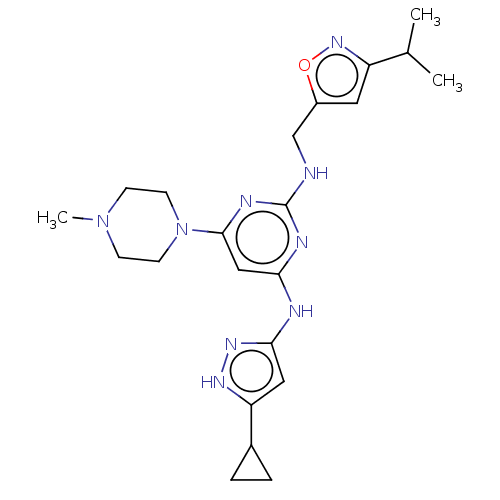
(4-N-(5-cyclopropyl-1H-pyrazol-3-yl)-6-(4-methylpip...)Show SMILES CC(C)c1cc(CNc2nc(Nc3cc([nH]n3)C3CC3)cc(n2)N2CCN(C)CC2)on1 Show InChI InChI=1S/C22H31N9O/c1-14(2)17-10-16(32-29-17)13-23-22-25-19(24-20-11-18(27-28-20)15-4-5-15)12-21(26-22)31-8-6-30(3)7-9-31/h10-12,14-15H,4-9,13H2,1-3H3,(H3,23,24,25,26,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Refer to Reaction Biology Corps. |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2 [596-900]
(Homo sapiens (Human)) | BDBM50311316
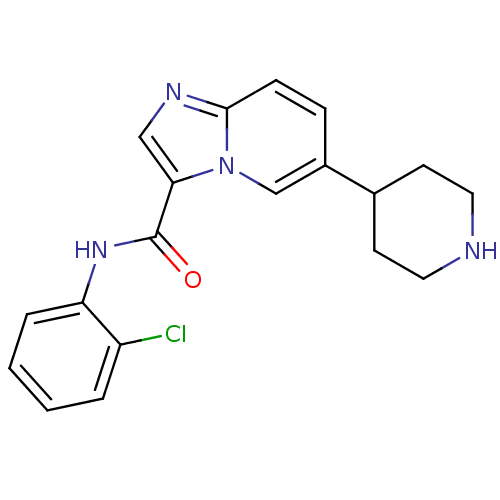
(CHEMBL1077739 | LDN-211904 | N-(2-chlorophenyl)-6-...)Show InChI InChI=1S/C19H19ClN4O/c20-15-3-1-2-4-16(15)23-19(25)17-11-22-18-6-5-14(12-24(17)18)13-7-9-21-10-8-13/h1-6,11-13,21H,7-10H2,(H,23,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Refer to Reaction Biology Corps. |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-A receptor 2 [596-900]
(Homo sapiens (Human)) | BDBM209858
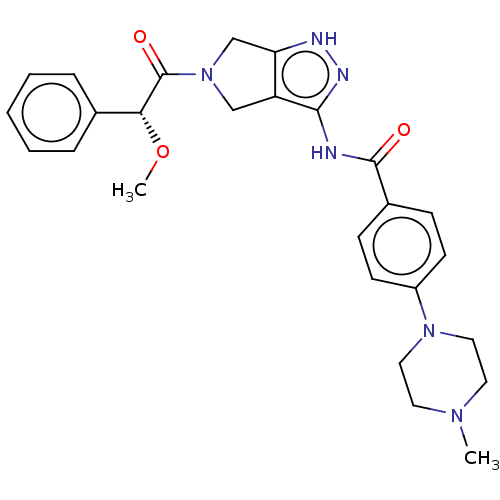
(Danusertib | N-[5-[(2R)-2-methoxy-2-phenylacetyl]-...)Show SMILES CO[C@@H](C(=O)N1Cc2[nH]nc(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2C1)c1ccccc1 Show InChI InChI=1S/C26H30N6O3/c1-30-12-14-31(15-13-30)20-10-8-19(9-11-20)25(33)27-24-21-16-32(17-22(21)28-29-24)26(34)23(35-2)18-6-4-3-5-7-18/h3-11,23H,12-17H2,1-2H3,(H2,27,28,29,33)/t23-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Refer to Reaction Biology Corps. |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-A receptor 2 [596-900]
(Homo sapiens (Human)) | BDBM209861
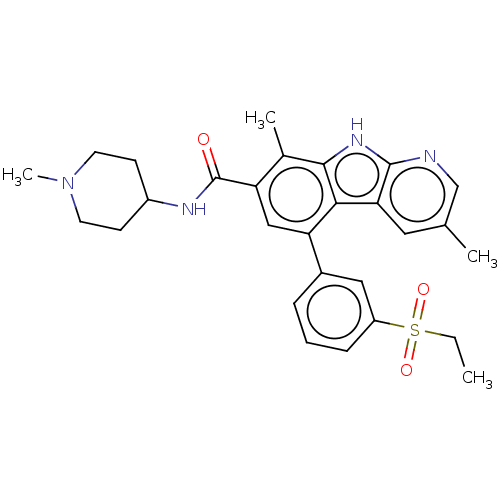
(5-(3-ethylsulfonylphenyl)-3,8-dimethyl-N-(1-methyl...)Show SMILES CCS(=O)(=O)c1cccc(c1)-c1cc(C(=O)NC2CCN(C)CC2)c(C)c2[nH]c3ncc(C)cc3c12 Show InChI InChI=1S/C28H32N4O3S/c1-5-36(34,35)21-8-6-7-19(14-21)23-15-22(28(33)30-20-9-11-32(4)12-10-20)18(3)26-25(23)24-13-17(2)16-29-27(24)31-26/h6-8,13-16,20H,5,9-12H2,1-4H3,(H,29,31)(H,30,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Refer to Reaction Biology Corps. |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50588613
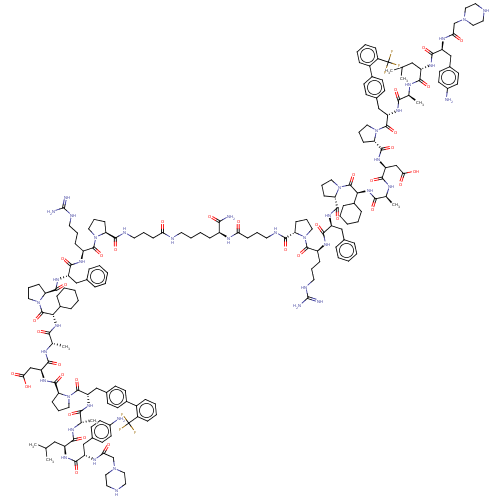
(CHEMBL5180264)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccc(N)cc1)NC(=O)CN1CCNCC1)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1C(F)(F)F)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](C1CCCCC1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)NCCCC(=O)NCCCC[C@H](NC(=O)CCCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(cc1)-c1ccccc1C(F)(F)F)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(N)cc1)NC(=O)CN1CCNCC1)C1CCCCC1)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01391
BindingDB Entry DOI: 10.7270/Q2V4105C |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50588611
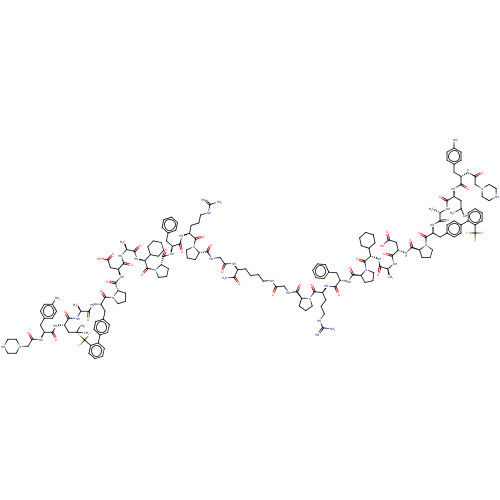
(CHEMBL5180519)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccc(N)cc1)NC(=O)CN1CCNCC1)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1C(F)(F)F)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](C1CCCCC1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)NCC(=O)NCCCC[C@H](NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(cc1)-c1ccccc1C(F)(F)F)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(N)cc1)NC(=O)CN1CCNCC1)C1CCCCC1)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01391
BindingDB Entry DOI: 10.7270/Q2V4105C |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50588612
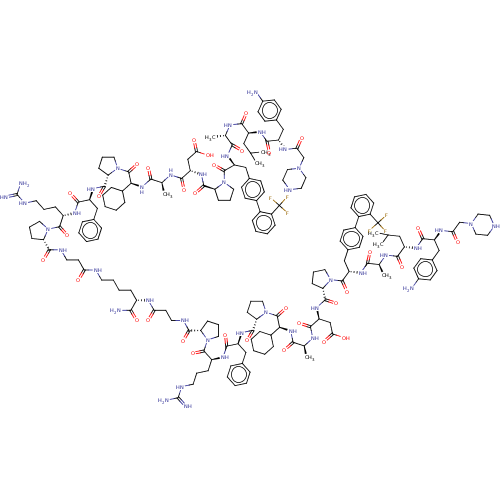
(CHEMBL5178807)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccc(N)cc1)NC(=O)CN1CCNCC1)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1C(F)(F)F)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](C1CCCCC1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)NCCC(=O)NCCCC[C@H](NC(=O)CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(cc1)-c1ccccc1C(F)(F)F)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(N)cc1)NC(=O)CN1CCNCC1)C1CCCCC1)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01391
BindingDB Entry DOI: 10.7270/Q2V4105C |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2 [596-900]
(Homo sapiens (Human)) | BDBM6568

(6-(2,6-dichlorophenyl)-8-methyl-2-{[3-(methylsulfa...)Show SMILES CSc1cccc(Nc2ncc3cc(-c4c(Cl)cccc4Cl)c(=O)n(C)c3n2)c1 |(-12.18,1.86,;-10.84,1.09,;-9.51,1.86,;-9.51,3.4,;-8.18,4.17,;-6.84,3.4,;-6.84,1.86,;-5.51,1.08,;-4.18,1.85,;-4.18,3.39,;-2.84,4.16,;-1.51,3.4,;-.18,4.17,;1.16,3.4,;2.49,4.17,;2.49,5.71,;1.16,6.48,;3.83,6.48,;5.16,5.71,;5.16,4.17,;3.83,3.39,;3.83,1.85,;1.16,1.86,;2.49,1.09,;-.18,1.09,;-.18,-.45,;-1.51,1.86,;-2.84,1.08,;-8.18,1.08,)| Show InChI InChI=1S/C21H16Cl2N4OS/c1-27-19-12(9-15(20(27)28)18-16(22)7-4-8-17(18)23)11-24-21(26-19)25-13-5-3-6-14(10-13)29-2/h3-11H,1-2H3,(H,24,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Refer to Reaction Biology Corps. |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50588607
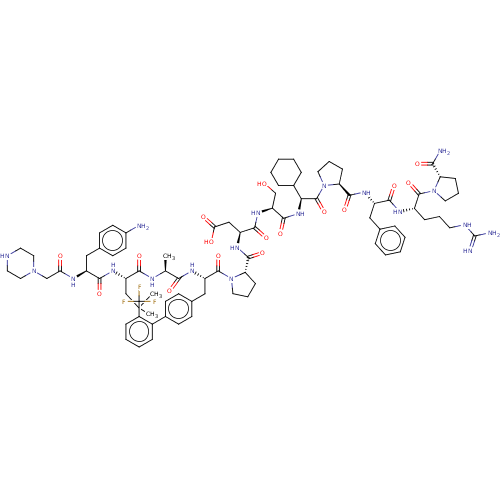
(CHEMBL5177810)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccc(N)cc1)NC(=O)CN1CCNCC1)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1C(F)(F)F)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C1CCCCC1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01391
BindingDB Entry DOI: 10.7270/Q2V4105C |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50240781
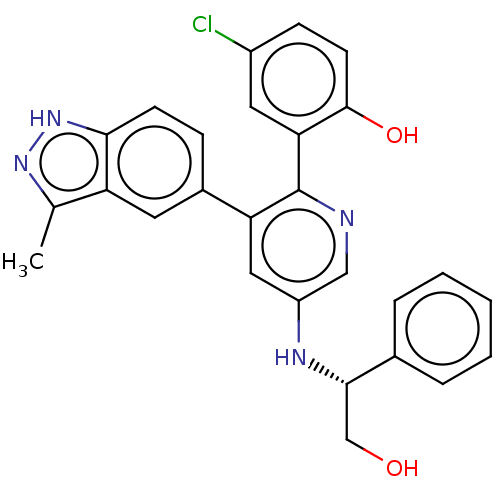
(CHEMBL4105329)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cc(N[C@@H](CO)c2ccccc2)cnc1-c1cc(Cl)ccc1O |r| Show InChI InChI=1S/C27H23ClN4O2/c1-16-21-11-18(7-9-24(21)32-31-16)22-13-20(30-25(15-33)17-5-3-2-4-6-17)14-29-27(22)23-12-19(28)8-10-26(23)34/h2-14,25,30,33-34H,15H2,1H3,(H,31,32)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant EPH-A2 (unknown origin) using poly (Glu,Tyr) 4:1 as substrate after 60 mins by ELISA |
J Med Chem 60: 6018-6035 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00076
BindingDB Entry DOI: 10.7270/Q2K939NC |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50240780
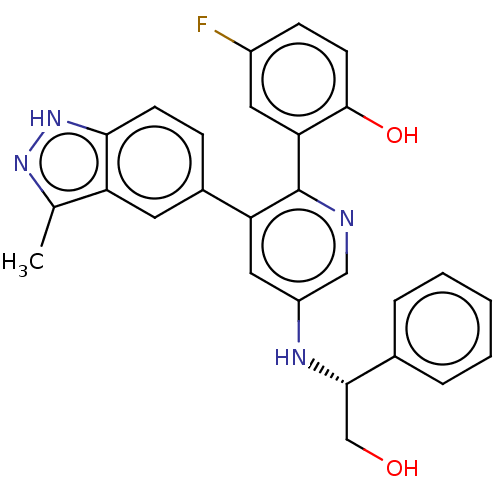
(CHEMBL4062877)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cc(N[C@@H](CO)c2ccccc2)cnc1-c1cc(F)ccc1O |r| Show InChI InChI=1S/C27H23FN4O2/c1-16-21-11-18(7-9-24(21)32-31-16)22-13-20(30-25(15-33)17-5-3-2-4-6-17)14-29-27(22)23-12-19(28)8-10-26(23)34/h2-14,25,30,33-34H,15H2,1H3,(H,31,32)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant EPH-A2 (unknown origin) using poly (Glu,Tyr) 4:1 as substrate after 60 mins by ELISA |
J Med Chem 60: 6018-6035 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00076
BindingDB Entry DOI: 10.7270/Q2K939NC |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50262685
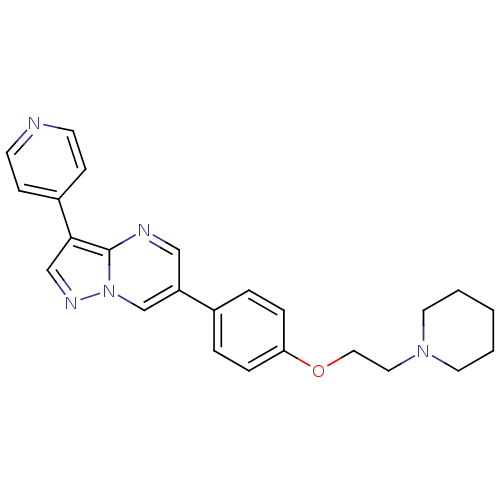
(6-(4-(2-(piperidin-1-yl)ethoxy)phenyl)-3-(pyridin-...)Show SMILES C(CN1CCCCC1)Oc1ccc(cc1)-c1cnc2c(cnn2c1)-c1ccncc1 Show InChI InChI=1S/C24H25N5O/c1-2-12-28(13-3-1)14-15-30-22-6-4-19(5-7-22)21-16-26-24-23(17-27-29(24)18-21)20-8-10-25-11-9-20/h4-11,16-18H,1-3,12-15H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of EphA2 by Hot Spot filtration binding assay |
Bioorg Med Chem Lett 20: 6394-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.088
BindingDB Entry DOI: 10.7270/Q2NP24NB |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50339608

(3-(1-Amino-1-methylethyl)-N-{(2R)-7-[(7-oxo-5,6,7,...)Show SMILES CC(C)(N)c1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C29H29F3N4O3/c1-28(2,33)19-11-18(12-20(15-19)29(30,31)32)27(38)35-21-5-3-16-4-6-22(14-17(16)13-21)39-24-9-10-34-26-23(24)7-8-25(37)36-26/h4,6,9-12,14-15,21H,3,5,7-8,13,33H2,1-2H3,(H,35,38)(H,34,36,37)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EPHA2 |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50588608
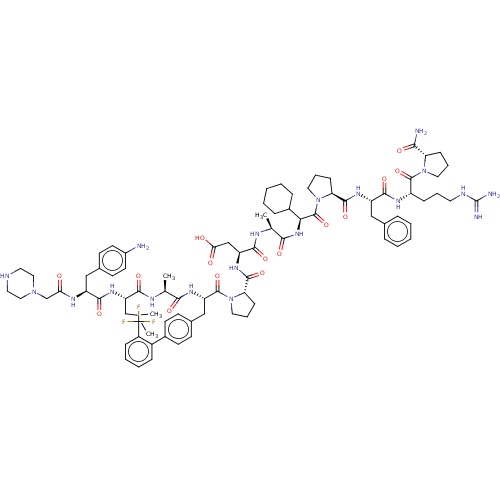
(CHEMBL5192123)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccc(N)cc1)NC(=O)CN1CCNCC1)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1C(F)(F)F)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](C1CCCCC1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01391
BindingDB Entry DOI: 10.7270/Q2V4105C |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2 [596-900]
(Homo sapiens (Human)) | BDBM50100615
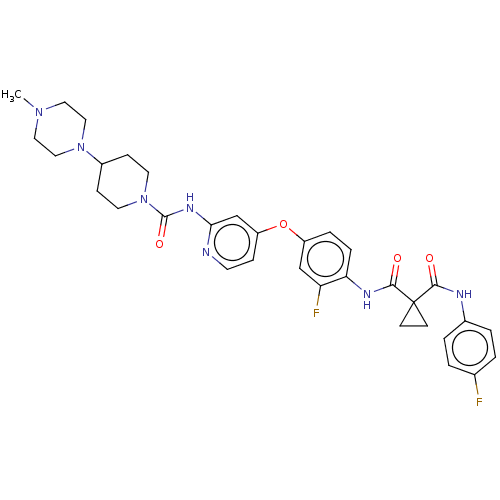
(E-7050 | E7050 | Golvatinib)Show SMILES CN1CCN(CC1)C1CCN(CC1)C(=O)Nc1cc(Oc2ccc(NC(=O)C3(CC3)C(=O)Nc3ccc(F)cc3)c(F)c2)ccn1 Show InChI InChI=1S/C33H37F2N7O4/c1-40-16-18-41(19-17-40)24-9-14-42(15-10-24)32(45)39-29-21-26(8-13-36-29)46-25-6-7-28(27(35)20-25)38-31(44)33(11-12-33)30(43)37-23-4-2-22(34)3-5-23/h2-8,13,20-21,24H,9-12,14-19H2,1H3,(H,37,43)(H,38,44)(H,36,39,45) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Refer to Reaction Biology Corps. |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM378888
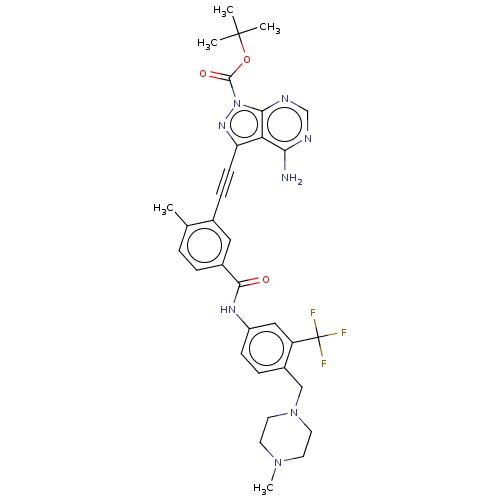
(Preparation of 3-(4-amino-1-(piperidin-4-yl)-1H-py...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3nn(C(=O)OC(C)(C)C)c4ncnc(N)c34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C33H35F3N8O3/c1-20-6-7-22(30(45)40-24-10-8-23(25(17-24)33(34,35)36)18-43-14-12-42(5)13-15-43)16-21(20)9-11-26-27-28(37)38-19-39-29(27)44(41-26)31(46)47-32(2,3)4/h6-8,10,16-17,19H,12-15,18H2,1-5H3,(H,40,45)(H2,37,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc.
| Assay Description
The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... |
Bioorg Med Chem Lett 17: 3562-9 (2007)
BindingDB Entry DOI: 10.7270/Q21C2065 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50507707
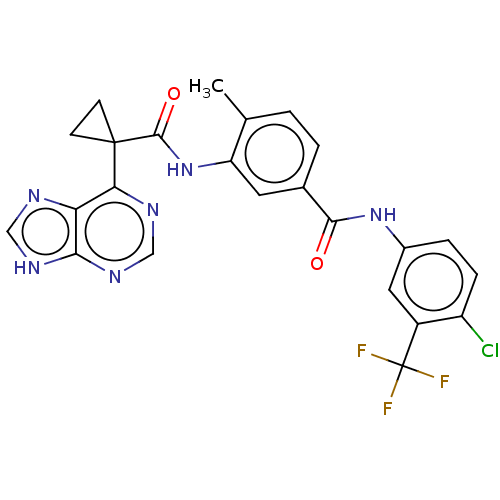
(CHEMBL4466555)Show SMILES Cc1ccc(cc1NC(=O)C1(CC1)c1ncnc2[nH]cnc12)C(=O)Nc1ccc(Cl)c(c1)C(F)(F)F Show InChI InChI=1S/C24H18ClF3N6O2/c1-12-2-3-13(21(35)33-14-4-5-16(25)15(9-14)24(26,27)28)8-17(12)34-22(36)23(6-7-23)19-18-20(31-10-29-18)32-11-30-19/h2-5,8-11H,6-7H2,1H3,(H,33,35)(H,34,36)(H,29,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human EPHA2 using poly[Glu:Tyr] (4:1) as substrate by [gamma-33P]-ATP assay |
Eur J Med Chem 163: 243-255 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.033
BindingDB Entry DOI: 10.7270/Q24B34M5 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50588609
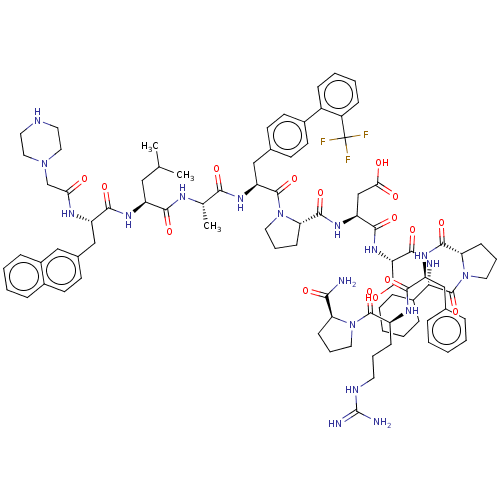
(CHEMBL5178830)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccc2ccccc2c1)NC(=O)CN1CCNCC1)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1C(F)(F)F)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C1CCCCC1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01391
BindingDB Entry DOI: 10.7270/Q2V4105C |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50086441

(CHEMBL3426225 | US10266537, Compound 3)Show SMILES CCn1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C30H31F3N8O/c1-4-41-28-26(27(34)35-18-36-28)25(38-41)10-8-20-15-21(6-5-19(20)2)29(42)37-23-9-7-22(24(16-23)30(31,32)33)17-40-13-11-39(3)12-14-40/h5-7,9,15-16,18H,4,11-14,17H2,1-3H3,(H,37,42)(H2,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc.
| Assay Description
The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... |
Bioorg Med Chem Lett 17: 3562-9 (2007)
BindingDB Entry DOI: 10.7270/Q21C2065 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50086441

(CHEMBL3426225 | US10266537, Compound 3)Show SMILES CCn1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C30H31F3N8O/c1-4-41-28-26(27(34)35-18-36-28)25(38-41)10-8-20-15-21(6-5-19(20)2)29(42)37-23-9-7-22(24(16-23)30(31,32)33)17-40-13-11-39(3)12-14-40/h5-7,9,15-16,18H,4,11-14,17H2,1-3H3,(H,37,42)(H2,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human EphA2 |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2 [596-900]
(Homo sapiens (Human)) | BDBM209860
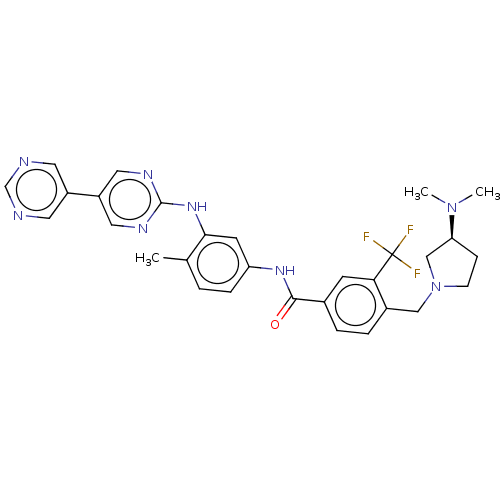
(4-[[(3S)-3-(dimethylamino)pyrrolidin-1-yl]methyl]-...)Show SMILES CN(C)[C@H]1CCN(Cc2ccc(cc2C(F)(F)F)C(=O)Nc2ccc(C)c(Nc3ncc(cn3)-c3cncnc3)c2)C1 Show InChI InChI=1S/C30H31F3N8O/c1-19-4-7-24(11-27(19)39-29-36-14-23(15-37-29)22-12-34-18-35-13-22)38-28(42)20-5-6-21(26(10-20)30(31,32)33)16-41-9-8-25(17-41)40(2)3/h4-7,10-15,18,25H,8-9,16-17H2,1-3H3,(H,38,42)(H,36,37,39)/t25-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Refer to Reaction Biology Corps. |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50339609

(3-[(Dimethylamino)methyl]-N-{(2R)-7-[(7-oxo-5,6,7,...)Show SMILES CN(C)Cc1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C29H29F3N4O3/c1-36(2)16-17-11-20(13-21(12-17)29(30,31)32)28(38)34-22-5-3-18-4-6-23(15-19(18)14-22)39-25-9-10-33-27-24(25)7-8-26(37)35-27/h4,6,9-13,15,22H,3,5,7-8,14,16H2,1-2H3,(H,34,38)(H,33,35,37)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EPHA2 |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM378887
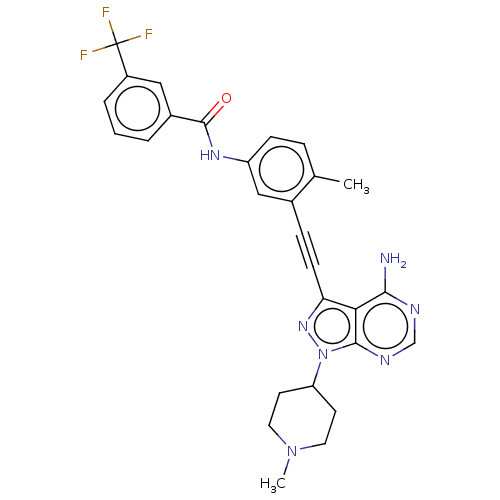
(US10266537, Compound 31)Show SMILES CN1CCC(CC1)n1nc(C#Cc2cc(NC(=O)c3cccc(c3)C(F)(F)F)ccc2C)c2c(N)ncnc12 Show InChI InChI=1S/C28H26F3N7O/c1-17-6-8-21(35-27(39)19-4-3-5-20(14-19)28(29,30)31)15-18(17)7-9-23-24-25(32)33-16-34-26(24)38(36-23)22-10-12-37(2)13-11-22/h3-6,8,14-16,22H,10-13H2,1-2H3,(H,35,39)(H2,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc.
| Assay Description
The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... |
Bioorg Med Chem Lett 17: 3562-9 (2007)
BindingDB Entry DOI: 10.7270/Q21C2065 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50096279
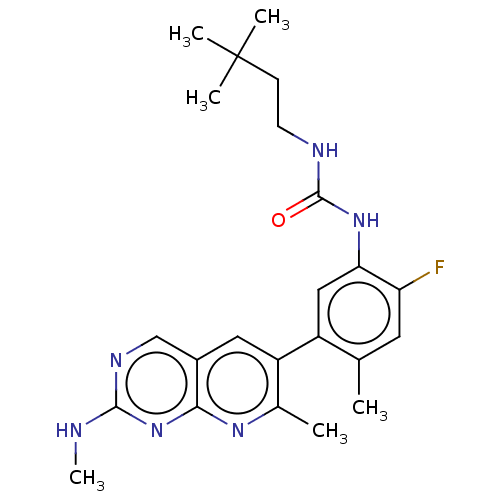
(CHEMBL3577124)Show SMILES CNc1ncc2cc(c(C)nc2n1)-c1cc(NC(=O)NCCC(C)(C)C)c(F)cc1C Show InChI InChI=1S/C18H13N3O5/c22-14(23)5-4-10-8-21-13-7-12-9(6-15(24)25)2-1-3-11(12)16(13)20-18(26)17(21)19-10/h1-5,8H,6-7H2,(H,20,26)(H,22,23)(H,24,25)/b5-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Competitive binding affinity to EphA2 in human A375 cells after 15 mins in presence of ATP analogue |
J Med Chem 58: 4165-79 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00067
BindingDB Entry DOI: 10.7270/Q2ZS2Z85 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50588606
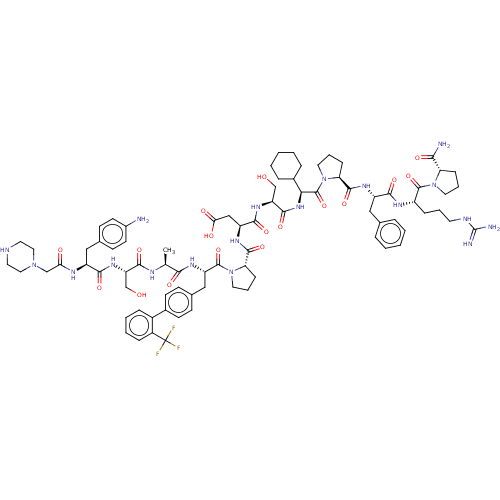
(CHEMBL5179135)Show SMILES C[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(N)cc1)NC(=O)CN1CCNCC1)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1C(F)(F)F)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C1CCCCC1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01391
BindingDB Entry DOI: 10.7270/Q2V4105C |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50588615
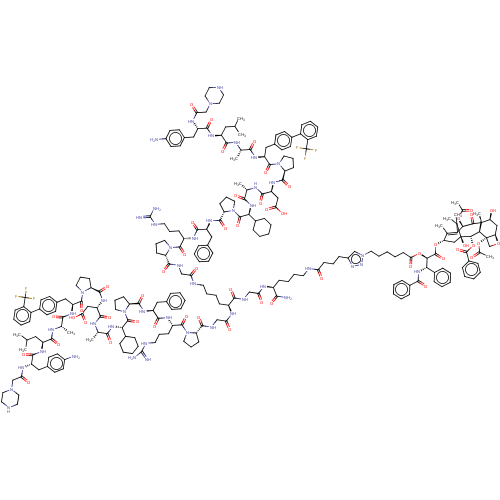
(CHEMBL5203641)Show SMILES [H][C@@]12C[C@H](O)[C@@]3(C)C(=O)[C@H](OC(C)=O)C4=C(C)[C@H](C[C@@](O)([C@@H](OC(=O)c5ccccc5)[C@]3([H])[C@@]1(CO2)OC(C)=O)C4(C)C)OC(=O)[C@H](OC(=O)CCCCCn1cc(CCCC(=O)NCCCC[C@H](NC(=O)CNC(=O)[C@H](CCCCNC(=O)CNC(=O)[C@@H]2CCCN2C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccc(cc2)-c2ccccc2C(F)(F)F)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc2ccc(N)cc2)NC(=O)CN2CCNCC2)C2CCCCC2)NC(=O)CNC(=O)[C@@H]2CCCN2C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccc(cc2)-c2ccccc2C(F)(F)F)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc2ccc(N)cc2)NC(=O)CN2CCNCC2)C2CCCCC2)C(N)=O)nn1)[C@@H](NC(=O)c1ccccc1)c1ccccc1 |r,c:14| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01391
BindingDB Entry DOI: 10.7270/Q2V4105C |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2 [596-900]
(Homo sapiens (Human)) | BDBM50382959

(CEP-32496 | CHEMBL2029988 | US9730937, Example 261)Show SMILES COc1cc2ncnc(Oc3cccc(NC(=O)Nc4cc(on4)C(C)(C)C(F)(F)F)c3)c2cc1OC Show InChI InChI=1S/C24H22F3N5O5/c1-23(2,24(25,26)27)19-11-20(32-37-19)31-22(33)30-13-6-5-7-14(8-13)36-21-15-9-17(34-3)18(35-4)10-16(15)28-12-29-21/h5-12H,1-4H3,(H2,30,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Refer to Reaction Biology Corps. |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50588616
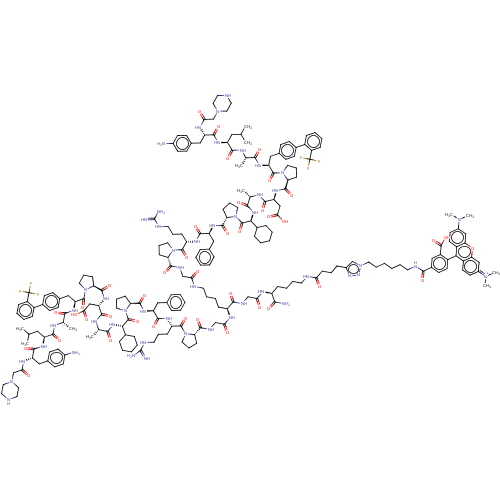
(CHEMBL5199267)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccc(N)cc1)NC(=O)CN1CCNCC1)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1C(F)(F)F)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](C1CCCCC1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)NCC(=O)NCCCC[C@H](NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(cc1)-c1ccccc1C(F)(F)F)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(N)cc1)NC(=O)CN1CCNCC1)C1CCCCC1)C(=O)NCC(=O)N[C@@H](CCCCNC(=O)CCCc1cn(CCCCCCNC(=O)c2ccc(c(c2)C(O)=O)-c2c3ccc(cc3oc3cc(ccc23)=[N+](C)C)N(C)C)nn1)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01391
BindingDB Entry DOI: 10.7270/Q2V4105C |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2 [596-900]
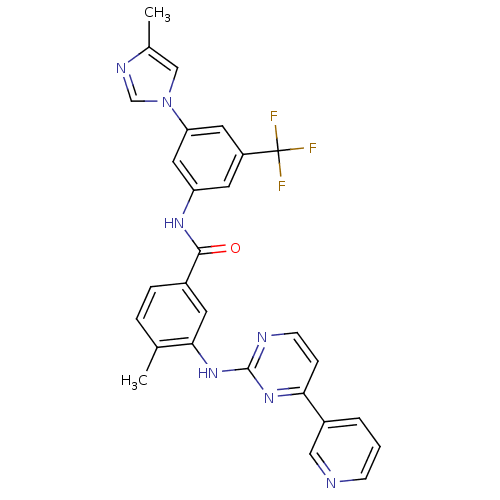
(Homo sapiens (Human)) | BDBM50237710
(4-methyl-N-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifl...)Show SMILES Cc1cn(cn1)-c1cc(NC(=O)c2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)cc(c1)C(F)(F)F Show InChI InChI=1S/C28H22F3N7O/c1-17-5-6-19(10-25(17)37-27-33-9-7-24(36-27)20-4-3-8-32-14-20)26(39)35-22-11-21(28(29,30)31)12-23(13-22)38-15-18(2)34-16-38/h3-16H,1-2H3,(H,35,39)(H,33,36,37) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Refer to Reaction Biology Corps. |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50312153

(1-(3-(8-(pyridin-4-ylmethylamino)imidazo[1,2-a]pyr...)Show SMILES FC(F)(F)c1cccc(NC(=O)Nc2cccc(c2)-c2cn3ccnc3c(NCc3ccncc3)n2)c1 Show InChI InChI=1S/C26H20F3N7O/c27-26(28,29)19-4-2-6-21(14-19)34-25(37)33-20-5-1-3-18(13-20)22-16-36-12-11-31-24(36)23(35-22)32-15-17-7-9-30-10-8-17/h1-14,16H,15H2,(H,32,35)(H2,33,34,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
CGI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EPHA2 after 1 hr |
Bioorg Med Chem Lett 19: 6991-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.037
BindingDB Entry DOI: 10.7270/Q2DR2VM3 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 39.9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human EPHA2 using poly[Glu:Tyr] (4:1) as substrate by [gamma-33P]-ATP assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2018.02.022
BindingDB Entry DOI: 10.7270/Q2DJ5KB8 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2 [596-900]
(Homo sapiens (Human)) | BDBM50277545

(4-(9-chloro-7-(2-fluoro-6-methoxyphenyl)-5H-benzo[...)Show SMILES COc1cc(Nc2ncc3CN=C(c4cc(Cl)ccc4-c3n2)c2c(F)cccc2OC)ccc1C(O)=O |c:11| Show InChI InChI=1S/C27H20ClFN4O4/c1-36-21-5-3-4-20(29)23(21)25-19-10-15(28)6-8-17(19)24-14(12-30-25)13-31-27(33-24)32-16-7-9-18(26(34)35)22(11-16)37-2/h3-11,13H,12H2,1-2H3,(H,34,35)(H,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Refer to Reaction Biology Corps. |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-A receptor 2
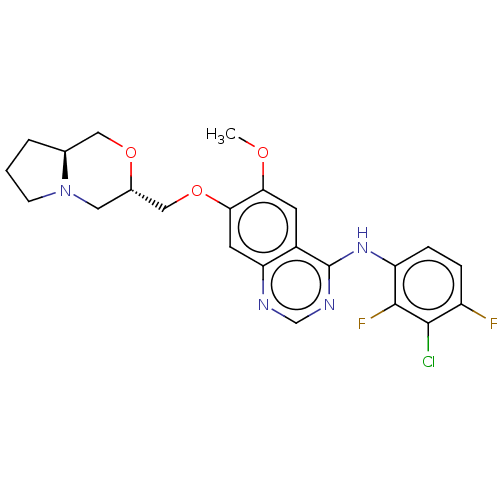
(Homo sapiens (Human)) | BDBM351307
(N-(3-chloro-2,4-difluorophenyl)-7-{[(3S,8aS)- hexa...)Show SMILES COc1cc2c(Nc3ccc(F)c(Cl)c3F)ncnc2cc1OC[C@@H]1CN2CCC[C@H]2CO1 |r| Show InChI InChI=1S/C23H23ClF2N4O3/c1-31-19-7-15-18(8-20(19)33-11-14-9-30-6-2-3-13(30)10-32-14)27-12-28-23(15)29-17-5-4-16(25)21(24)22(17)26/h4-5,7-8,12-14H,2-3,6,9-11H2,1H3,(H,27,28,29)/t13-,14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
SYMPHONY EVOLUTION, INC.
US Patent
| Assay Description
In AlphaScreen, when the donor and acceptor beads are close in proximity, a series of photochemical events will give rise to a fluorescent signal upo... |
US Patent US9796704 (2017)
BindingDB Entry DOI: 10.7270/Q28K7C6V |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM351309
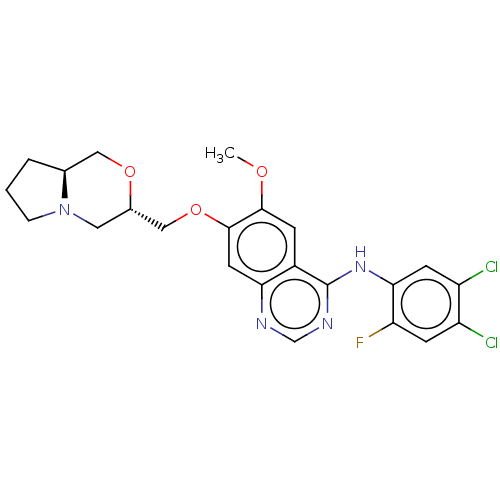
(N-(4,5-dichloro-2-fluorophenyl)-7-{[(3S,8aS)- hexa...)Show SMILES COc1cc2c(Nc3cc(Cl)c(Cl)cc3F)ncnc2cc1OC[C@@H]1CN2CCC[C@H]2CO1 |r| Show InChI InChI=1S/C23H23Cl2FN4O3/c1-31-21-5-15-19(8-22(21)33-11-14-9-30-4-2-3-13(30)10-32-14)27-12-28-23(15)29-20-7-17(25)16(24)6-18(20)26/h5-8,12-14H,2-4,9-11H2,1H3,(H,27,28,29)/t13-,14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
SYMPHONY EVOLUTION, INC.
US Patent
| Assay Description
In AlphaScreen, when the donor and acceptor beads are close in proximity, a series of photochemical events will give rise to a fluorescent signal upo... |
US Patent US9796704 (2017)
BindingDB Entry DOI: 10.7270/Q28K7C6V |
More data for this
Ligand-Target Pair | |
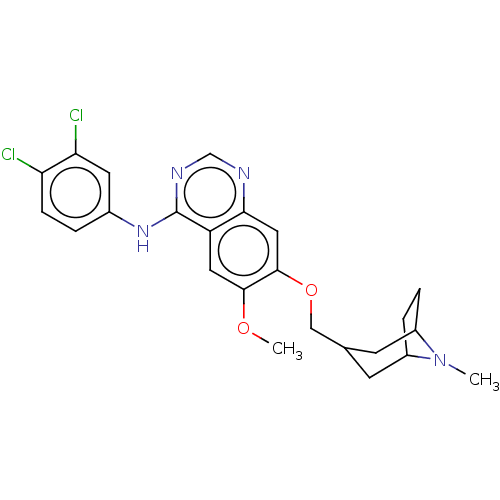
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM351323
(N-(3,4-dichlorophenyl)-7-{[(8-methyl-8- azabicyclo...)Show SMILES COc1cc2c(Nc3ccc(Cl)c(Cl)c3)ncnc2cc1OCC1CC2CCC(C1)N2C Show InChI InChI=1S/C24H26Cl2N4O2/c1-30-16-4-5-17(30)8-14(7-16)12-32-23-11-21-18(10-22(23)31-2)24(28-13-27-21)29-15-3-6-19(25)20(26)9-15/h3,6,9-11,13-14,16-17H,4-5,7-8,12H2,1-2H3,(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
SYMPHONY EVOLUTION, INC.
US Patent
| Assay Description
In AlphaScreen, when the donor and acceptor beads are close in proximity, a series of photochemical events will give rise to a fluorescent signal upo... |
US Patent US9796704 (2017)
BindingDB Entry DOI: 10.7270/Q28K7C6V |
More data for this
Ligand-Target Pair | |
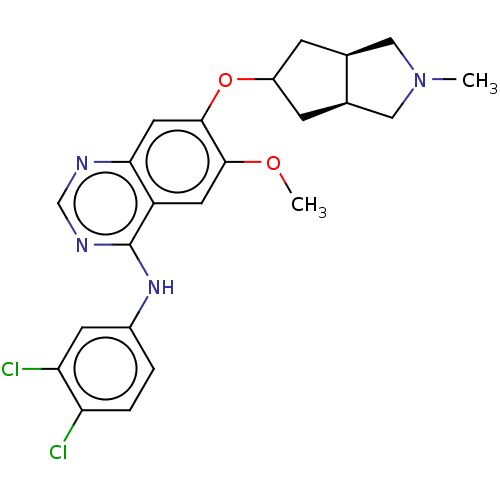
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM351324
(N-(3,4-dichlorophenyl)-7-{[(3aR,6aS)-2- methylocta...)Show SMILES COc1cc2c(Nc3ccc(Cl)c(Cl)c3)ncnc2cc1OC1C[C@H]2CN(C)C[C@H]2C1 |r| Show InChI InChI=1S/C23H24Cl2N4O2/c1-29-10-13-5-16(6-14(13)11-29)31-22-9-20-17(8-21(22)30-2)23(27-12-26-20)28-15-3-4-18(24)19(25)7-15/h3-4,7-9,12-14,16H,5-6,10-11H2,1-2H3,(H,26,27,28)/t13-,14+,16? | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
SYMPHONY EVOLUTION, INC.
US Patent
| Assay Description
In AlphaScreen, when the donor and acceptor beads are close in proximity, a series of photochemical events will give rise to a fluorescent signal upo... |
US Patent US9796704 (2017)
BindingDB Entry DOI: 10.7270/Q28K7C6V |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM351326

(1,4:3,6-dianhydro-2-O-[4-[(4-bromo-5-chloro- 2-flu...)Show SMILES CO[C@H]1CO[C@@H]2[C@H](CO[C@H]12)Oc1cc2ncnc(Nc3cc(Cl)c(Br)cc3F)c2cc1OC |r| Show InChI InChI=1S/C22H20BrClFN3O5/c1-29-16-3-10-14(6-17(16)33-19-8-32-20-18(30-2)7-31-21(19)20)26-9-27-22(10)28-15-5-12(24)11(23)4-13(15)25/h3-6,9,18-21H,7-8H2,1-2H3,(H,26,27,28)/t18-,19-,20+,21+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
SYMPHONY EVOLUTION, INC.
US Patent
| Assay Description
In AlphaScreen, when the donor and acceptor beads are close in proximity, a series of photochemical events will give rise to a fluorescent signal upo... |
US Patent US9796704 (2017)
BindingDB Entry DOI: 10.7270/Q28K7C6V |
More data for this
Ligand-Target Pair | |
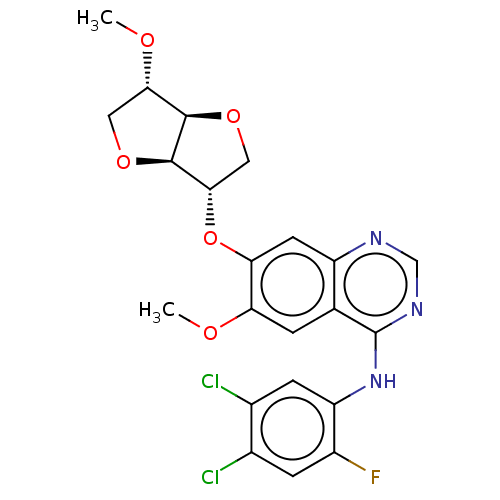
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM351349
(1,4:3,6-dianhydro-2-O-[4-[(4,5-dichloro-2- fluorop...)Show SMILES CO[C@H]1CO[C@@H]2[C@H](CO[C@H]12)Oc1cc2ncnc(Nc3cc(Cl)c(Cl)cc3F)c2cc1OC |r| Show InChI InChI=1S/C22H20Cl2FN3O5/c1-29-16-3-10-14(6-17(16)33-19-8-32-20-18(30-2)7-31-21(19)20)26-9-27-22(10)28-15-5-12(24)11(23)4-13(15)25/h3-6,9,18-21H,7-8H2,1-2H3,(H,26,27,28)/t18-,19-,20+,21+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
SYMPHONY EVOLUTION, INC.
US Patent
| Assay Description
In AlphaScreen, when the donor and acceptor beads are close in proximity, a series of photochemical events will give rise to a fluorescent signal upo... |
US Patent US9796704 (2017)
BindingDB Entry DOI: 10.7270/Q28K7C6V |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM351358

(1,4:3,6-dianhydro-2-O-[4-[(4-bromo-3- chlorophenyl...)Show SMILES CO[C@@H]1CO[C@@H]2[C@H](CO[C@H]12)Oc1cc2ncnc(Nc3ccc(Br)c(Cl)c3)c2cc1OC |r| Show InChI InChI=1S/C22H21BrClN3O5/c1-28-16-6-12-15(25-10-26-22(12)27-11-3-4-13(23)14(24)5-11)7-17(16)32-19-9-31-20-18(29-2)8-30-21(19)20/h3-7,10,18-21H,8-9H2,1-2H3,(H,25,26,27)/t18-,19+,20-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
SYMPHONY EVOLUTION, INC.
US Patent
| Assay Description
In AlphaScreen, when the donor and acceptor beads are close in proximity, a series of photochemical events will give rise to a fluorescent signal upo... |
US Patent US9796704 (2017)
BindingDB Entry DOI: 10.7270/Q28K7C6V |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM351359

(US9796704, Entry 79 | methyl 3,6-anhydro-5-O-[4-[(...)Show SMILES CO[C@@H]1O[C@@H]2[C@H](CO[C@@H]2[C@H]1OC)Oc1cc2ncnc(Nc3ccc(Br)c(Cl)c3)c2cc1OC |r| Show InChI InChI=1S/C23H23BrClN3O6/c1-29-16-7-12-15(26-10-27-22(12)28-11-4-5-13(24)14(25)6-11)8-17(16)33-18-9-32-20-19(18)34-23(31-3)21(20)30-2/h4-8,10,18-21,23H,9H2,1-3H3,(H,26,27,28)/t18-,19+,20-,21+,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
SYMPHONY EVOLUTION, INC.
US Patent
| Assay Description
In AlphaScreen, when the donor and acceptor beads are close in proximity, a series of photochemical events will give rise to a fluorescent signal upo... |
US Patent US9796704 (2017)
BindingDB Entry DOI: 10.7270/Q28K7C6V |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM351369
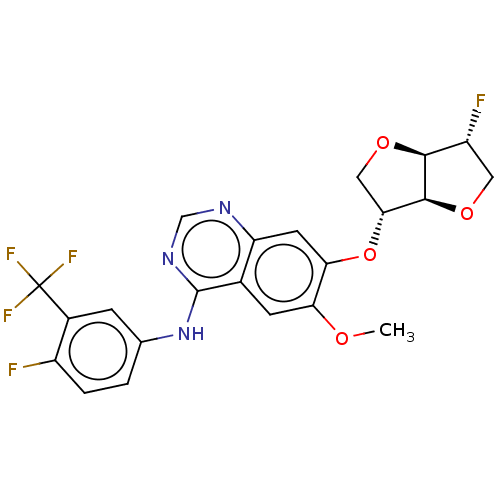
(1,4:3,6-dianhydro-2-deoxy-2-fluoro-5-O-[4- {[4-flu...)Show SMILES COc1cc2c(Nc3ccc(F)c(c3)C(F)(F)F)ncnc2cc1O[C@@H]1CO[C@H]2[C@H](F)CO[C@@H]12 |r| Show InChI InChI=1S/C22H18F5N3O4/c1-31-16-5-11-15(6-17(16)34-18-8-33-19-14(24)7-32-20(18)19)28-9-29-21(11)30-10-2-3-13(23)12(4-10)22(25,26)27/h2-6,9,14,18-20H,7-8H2,1H3,(H,28,29,30)/t14-,18-,19+,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
SYMPHONY EVOLUTION, INC.
US Patent
| Assay Description
In AlphaScreen, when the donor and acceptor beads are close in proximity, a series of photochemical events will give rise to a fluorescent signal upo... |
US Patent US9796704 (2017)
BindingDB Entry DOI: 10.7270/Q28K7C6V |
More data for this
Ligand-Target Pair | |
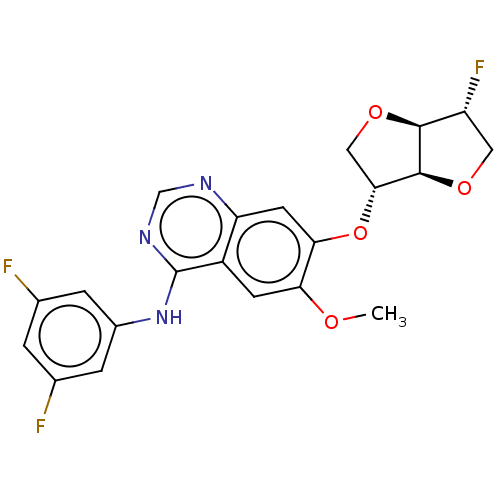
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM351374
(1,4:3,6-dianhydro-2-deoxy-5-O-[4-[(3,5- difluoroph...)Show SMILES COc1cc2c(Nc3cc(F)cc(F)c3)ncnc2cc1O[C@@H]1CO[C@H]2[C@H](F)CO[C@@H]12 |r| Show InChI InChI=1S/C21H18F3N3O4/c1-28-16-5-13-15(6-17(16)31-18-8-30-19-14(24)7-29-20(18)19)25-9-26-21(13)27-12-3-10(22)2-11(23)4-12/h2-6,9,14,18-20H,7-8H2,1H3,(H,25,26,27)/t14-,18-,19+,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
SYMPHONY EVOLUTION, INC.
US Patent
| Assay Description
In AlphaScreen, when the donor and acceptor beads are close in proximity, a series of photochemical events will give rise to a fluorescent signal upo... |
US Patent US9796704 (2017)
BindingDB Entry DOI: 10.7270/Q28K7C6V |
More data for this
Ligand-Target Pair | |
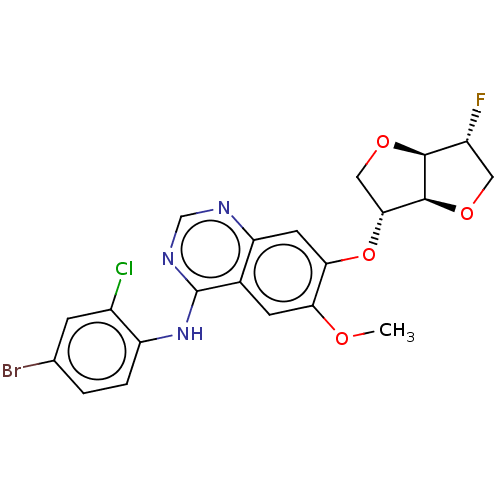
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM351376
(1,4:3,6-dianhydro-5-O-[4-[(4-bromo-2- chlorophenyl...)Show SMILES COc1cc2c(Nc3ccc(Br)cc3Cl)ncnc2cc1O[C@@H]1CO[C@H]2[C@H](F)CO[C@@H]12 |r| Show InChI InChI=1S/C21H18BrClFN3O4/c1-28-16-5-11-15(6-17(16)31-18-8-30-19-13(24)7-29-20(18)19)25-9-26-21(11)27-14-3-2-10(22)4-12(14)23/h2-6,9,13,18-20H,7-8H2,1H3,(H,25,26,27)/t13-,18-,19+,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
SYMPHONY EVOLUTION, INC.
US Patent
| Assay Description
In AlphaScreen, when the donor and acceptor beads are close in proximity, a series of photochemical events will give rise to a fluorescent signal upo... |
US Patent US9796704 (2017)
BindingDB Entry DOI: 10.7270/Q28K7C6V |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data