 Found 38 hits of ic50 for UniProtKB: P29320
Found 38 hits of ic50 for UniProtKB: P29320 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM50345579

(5-((5-(4-((4-(2-hydroxyethyl)piperazin-1-yl)methyl...)Show SMILES CNC(=O)c1ncc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)n1C Show InChI InChI=1S/C30H33F3N6O3/c1-20-4-5-22(16-21(20)7-9-25-18-35-27(37(25)3)29(42)34-2)28(41)36-24-8-6-23(26(17-24)30(31,32)33)19-39-12-10-38(11-13-39)14-15-40/h4-6,8,16-18,40H,10-15,19H2,1-3H3,(H,34,42)(H,36,41) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human EPHA3 using poly[Glu:Tyr] peptide substrate by Hotspot assay |
Bioorg Med Chem Lett 21: 3743-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.060
BindingDB Entry DOI: 10.7270/Q21G0MM8 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM50430875

(CHEMBL2336005 | US9062066, LCB 03-0110)Show SMILES Oc1cccc(Nc2ccnc3cc(sc23)-c2cccc(CN3CCOCC3)c2)c1 Show InChI InChI=1S/C24H23N3O2S/c28-20-6-2-5-19(14-20)26-21-7-8-25-22-15-23(30-24(21)22)18-4-1-3-17(13-18)16-27-9-11-29-12-10-27/h1-8,13-15,28H,9-12,16H2,(H,25,26) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | 8.0 | n/a |
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY
US Patent
| Assay Description
The inhibitory activity measurement against the kinases mentioned above was measured in the kinase inhibition reaction mixture containing 2 ul purifi... |
US Patent US9062066 (2015)
BindingDB Entry DOI: 10.7270/Q21G0K17 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM93207
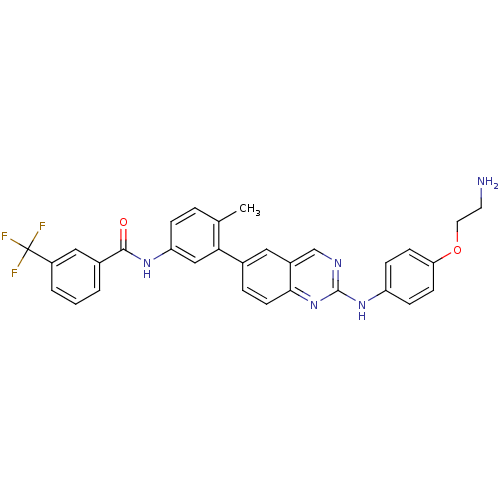
(Kinase inhibitor, 5)Show SMILES Cc1ccc(NC(=O)c2cccc(c2)C(F)(F)F)cc1-c1ccc2nc(Nc3ccc(OCCN)cc3)ncc2c1 Show InChI InChI=1S/C31H26F3N5O2/c1-19-5-7-25(37-29(40)21-3-2-4-23(16-21)31(32,33)34)17-27(19)20-6-12-28-22(15-20)18-36-30(39-28)38-24-8-10-26(11-9-24)41-14-13-35/h2-12,15-18H,13-14,35H2,1H3,(H,37,40)(H,36,38,39) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Washington
| Assay Description
Fluorescence assay used for determination of catch and release efficiency. |
ACS Chem Biol 8: 691-9 (2013)
Article DOI: 10.1021/cb300623a
BindingDB Entry DOI: 10.7270/Q20R9N1X |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of EPHA3 (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM50536679
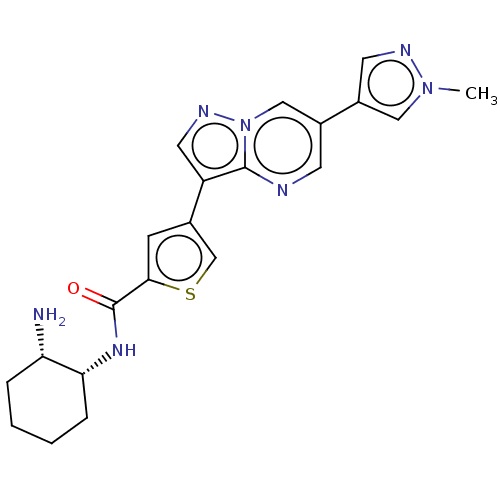
(CHEMBL4568087)Show SMILES Cn1cc(cn1)-c1cnc2c(cnn2c1)-c1csc(c1)C(=O)N[C@@H]1CCCC[C@@H]1N |r| Show InChI InChI=1S/C21H23N7OS/c1-27-10-15(8-24-27)14-7-23-20-16(9-25-28(20)11-14)13-6-19(30-12-13)21(29)26-18-5-3-2-4-17(18)22/h6-12,17-18H,2-5,22H2,1H3,(H,26,29)/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged cytoplasmic EPHA3 expressed in baculovirus expression system by LanthaScreen assay |
Bioorg Med Chem Lett 26: 4362-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.003
BindingDB Entry DOI: 10.7270/Q2NP27X5 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 31.9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human EPHA3 using poly[Glu:Tyr] (4:1) as substrate by [gamma-33P]-ATP assay |
Eur J Med Chem 161: 456-467 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.052
BindingDB Entry DOI: 10.7270/Q2W380MT |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human EPHA3 using poly[Glu:Tyr] (4:1) as substrate by [gamma-33P]-ATP assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2018.02.022
BindingDB Entry DOI: 10.7270/Q2DJ5KB8 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM50299218

(8-(2-Methoxyphenyl)-1-methyl-7-(2'-methyl-5'-hydro...)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(O)ccc1C |(16.89,-42.99,;16.89,-41.45,;18.23,-40.68,;19.55,-41.45,;20.88,-40.68,;20.88,-39.14,;19.55,-38.38,;18.23,-39.15,;16.9,-38.38,;16.91,-36.84,;15.44,-36.35,;14.53,-37.6,;15.43,-38.85,;14.53,-40.09,;13.06,-39.61,;11.74,-40.37,;11.73,-41.91,;10.41,-39.61,;9.07,-40.38,;10.41,-38.07,;11.74,-37.29,;11.74,-35.75,;13.06,-38.07,;18.24,-36.06,;19.58,-36.83,;20.9,-36.05,;22.24,-36.81,;20.9,-34.51,;19.55,-33.75,;18.23,-34.53,;16.89,-33.77,)| Show InChI InChI=1S/C22H19N5O4/c1-12-8-9-13(28)10-14(12)16-11-26-18-19(25(2)22(30)24-20(18)29)23-21(26)27(16)15-6-4-5-7-17(15)31-3/h4-11,28H,1-3H3,(H,24,29,30) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphA3 by [gamma33-P]ATP based assay |
J Med Chem 52: 6433-46 (2009)
Article DOI: 10.1021/jm9009444
BindingDB Entry DOI: 10.7270/Q2BZ663G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of EPHA3 |
J Med Chem 52: 3191-204 (2009)
Article DOI: 10.1021/jm800861c
BindingDB Entry DOI: 10.7270/Q23J3DWT |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM50536678
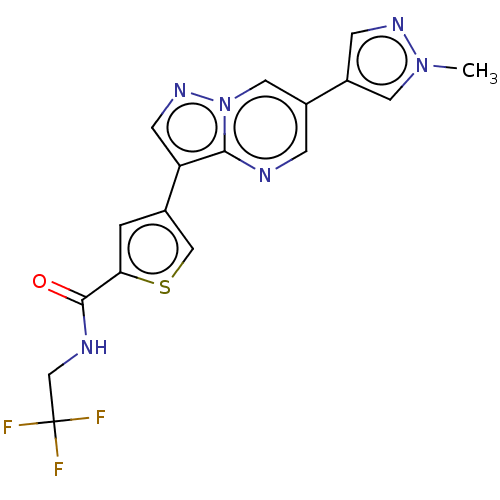
(CHEMBL4550702)Show SMILES Cn1cc(cn1)-c1cnc2c(cnn2c1)-c1csc(c1)C(=O)NCC(F)(F)F Show InChI InChI=1S/C17H13F3N6OS/c1-25-6-12(4-23-25)11-3-21-15-13(5-24-26(15)7-11)10-2-14(28-8-10)16(27)22-9-17(18,19)20/h2-8H,9H2,1H3,(H,22,27) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged cytoplasmic EPHA3 expressed in baculovirus expression system by LanthaScreen assay |
Bioorg Med Chem Lett 26: 4362-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.003
BindingDB Entry DOI: 10.7270/Q2NP27X5 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM50536675
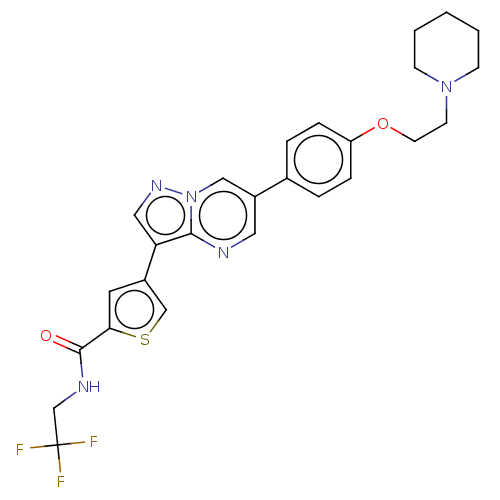
(CHEMBL4569508)Show SMILES FC(F)(F)CNC(=O)c1cc(cs1)-c1cnn2cc(cnc12)-c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C26H26F3N5O2S/c27-26(28,29)17-31-25(35)23-12-19(16-37-23)22-14-32-34-15-20(13-30-24(22)34)18-4-6-21(7-5-18)36-11-10-33-8-2-1-3-9-33/h4-7,12-16H,1-3,8-11,17H2,(H,31,35) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged cytoplasmic EPHA3 expressed in baculovirus expression system by LanthaScreen assay |
Bioorg Med Chem Lett 26: 4362-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.003
BindingDB Entry DOI: 10.7270/Q2NP27X5 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM50563891
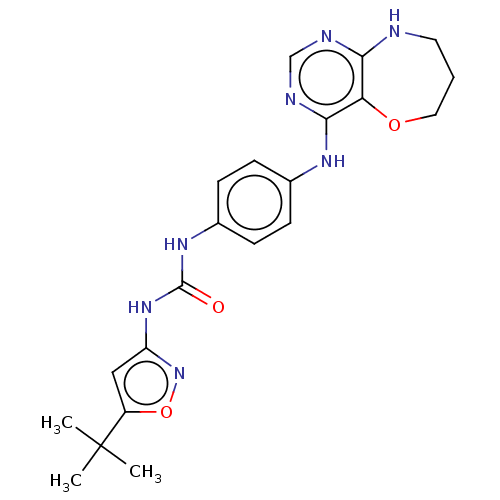
(CHEMBL4793380)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2ccc(Nc3ncnc4NCCCOc34)cc2)no1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 552 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human EphA3 (578 to end residues) using poly(Glu, Tyr) 4:1 as substrate incubated for 40 mins in presence of [gamma-33ATP] ... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2017.09.018
BindingDB Entry DOI: 10.7270/Q270854P |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the phosphorylation of a 14-residue fragment of phospholipase C gamma 1 by Epidermal growth factor receptor from A4... |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of EPHA3 |
Bioorg Med Chem Lett 21: 7155-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.078
BindingDB Entry DOI: 10.7270/Q2NC61NH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM50345739
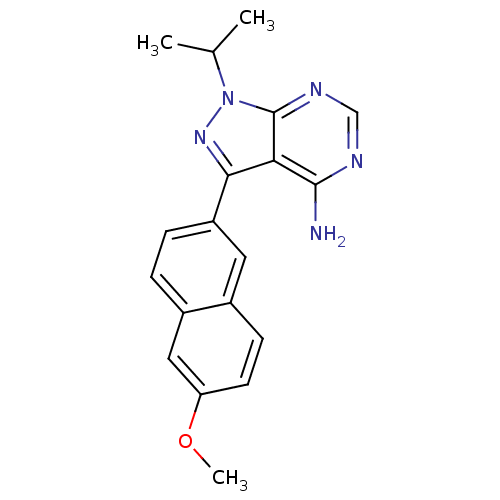
(1-isopropyl-3-(6-methoxynaphthalen-2-yl)-1H-pyrazo...)Show InChI InChI=1S/C19H19N5O/c1-11(2)24-19-16(18(20)21-10-22-19)17(23-24)14-5-4-13-9-15(25-3)7-6-12(13)8-14/h4-11H,1-3H3,(H2,20,21,22) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human EPHA3 using myelin basic protein as substrate after 120 mins |
J Med Chem 55: 2416-26 (2012)
Article DOI: 10.1021/jm201713h
BindingDB Entry DOI: 10.7270/Q2P2706V |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM50208911
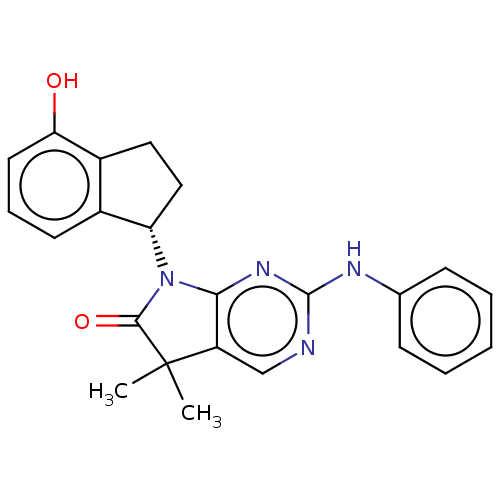
(CHEMBL3884319)Show SMILES CC1(C)C(=O)N([C@H]2CCc3c2cccc3O)c2nc(Nc3ccccc3)ncc12 |r| Show InChI InChI=1S/C23H22N4O2/c1-23(2)17-13-24-22(25-14-7-4-3-5-8-14)26-20(17)27(21(23)29)18-12-11-16-15(18)9-6-10-19(16)28/h3-10,13,18,28H,11-12H2,1-2H3,(H,24,25,26)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged EPHA3 cytoplasmic domain (569 to 976 residues) expressed in baculovirus expression system |
Bioorg Med Chem Lett 27: 114-120 (2017)
Article DOI: 10.1016/j.bmcl.2016.08.068
BindingDB Entry DOI: 10.7270/Q2N018JC |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM50428059
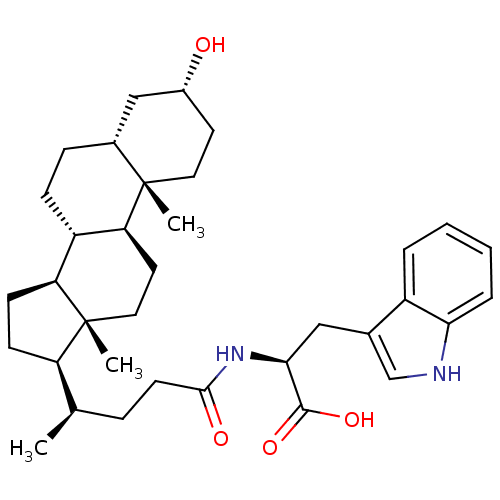
(CHEMBL2322989)Show SMILES C[C@H](CCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3CC[C@]12C |r| Show InChI InChI=1S/C35H50N2O4/c1-21(8-13-32(39)37-31(33(40)41)18-22-20-36-30-7-5-4-6-25(22)30)27-11-12-28-26-10-9-23-19-24(38)14-16-34(23,2)29(26)15-17-35(27,28)3/h4-7,20-21,23-24,26-29,31,36,38H,8-19H2,1-3H3,(H,37,39)(H,40,41)/t21-,23-,24-,26+,27-,28+,29+,31+,34+,35-/m1/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Parma
Curated by ChEMBL
| Assay Description
Displacement of ephrin-A1-Fc from EphA3 receptor Fc ectodomain (unknown origin) after 1 hr by ELISA |
J Med Chem 56: 2936-47 (2013)
Article DOI: 10.1021/jm301890k
BindingDB Entry DOI: 10.7270/Q2JW8G7H |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM50277623

(CHEMBL451523 | N-(5-tert-butyl-2-methoxy-3-(3-(4-(...)Show SMILES COc1c(NC(=O)Nc2ccc(-c3ccc(CN4CCOCC4)nc3)c3ccccc23)cc(cc1NS(C)(=O)=O)C(C)(C)C Show InChI InChI=1S/C33H39N5O5S/c1-33(2,3)23-18-29(31(42-4)30(19-23)37-44(5,40)41)36-32(39)35-28-13-12-25(26-8-6-7-9-27(26)28)22-10-11-24(34-20-22)21-38-14-16-43-17-15-38/h6-13,18-20,37H,14-17,21H2,1-5H3,(H2,35,36,39) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical
Curated by ChEMBL
| Assay Description
Inhibition of HEK (unknown origin) |
Bioorg Med Chem Lett 19: 2386-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.104
BindingDB Entry DOI: 10.7270/Q22N525R |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM50345740

(3-(6-ethoxynaphthalen-2-yl)-1-isopropyl-1H-pyrazol...)Show SMILES CCOc1ccc2cc(ccc2c1)-c1nn(C(C)C)c2ncnc(N)c12 Show InChI InChI=1S/C20H21N5O/c1-4-26-16-8-7-13-9-15(6-5-14(13)10-16)18-17-19(21)22-11-23-20(17)25(24-18)12(2)3/h5-12H,4H2,1-3H3,(H2,21,22,23) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human EPHA3 using myelin basic protein as substrate after 120 mins |
J Med Chem 55: 2416-26 (2012)
Article DOI: 10.1021/jm201713h
BindingDB Entry DOI: 10.7270/Q2P2706V |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM50302166

(3-amino-6-(3-hydroxypiperidin-1-yl)-4-propylthieno...)Show InChI InChI=1S/C16H22N4O2S/c1-2-4-9-7-11(20-6-3-5-10(21)8-20)19-16-12(9)13(17)14(23-16)15(18)22/h7,10,21H,2-6,8,17H2,1H3,(H2,18,22) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Hek |
Bioorg Med Chem Lett 19: 5547-51 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.054
BindingDB Entry DOI: 10.7270/Q22Z16G1 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM50302163

(3-amino-6-(4-aminopiperidin-1-yl)-4-propylthieno[2...)Show InChI InChI=1S/C16H23N5OS/c1-2-3-9-8-11(21-6-4-10(17)5-7-21)20-16-12(9)13(18)14(23-16)15(19)22/h8,10H,2-7,17-18H2,1H3,(H2,19,22) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Hek |
Bioorg Med Chem Lett 19: 5547-51 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.054
BindingDB Entry DOI: 10.7270/Q22Z16G1 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM50302164
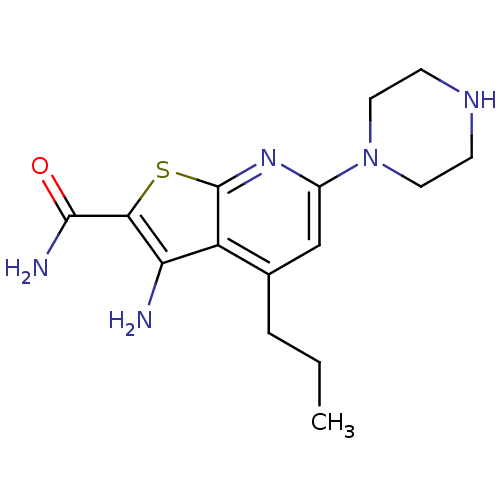
(3-amino-6-(piperazin-1-yl)-4-propylthieno[2,3-b]py...)Show InChI InChI=1S/C15H21N5OS/c1-2-3-9-8-10(20-6-4-18-5-7-20)19-15-11(9)12(16)13(22-15)14(17)21/h8,18H,2-7,16H2,1H3,(H2,17,21) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Hek |
Bioorg Med Chem Lett 19: 5547-51 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.054
BindingDB Entry DOI: 10.7270/Q22Z16G1 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM50302165
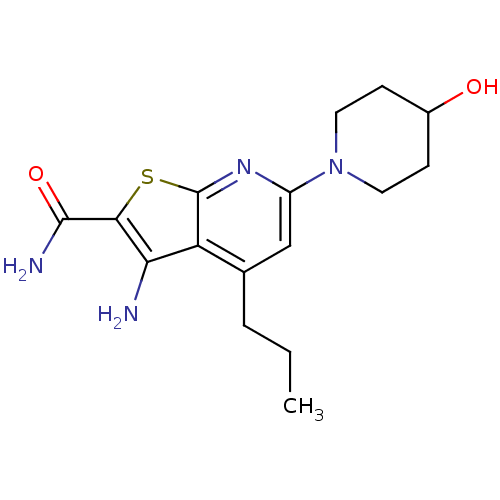
(3-amino-6-(4-hydroxypiperidin-1-yl)-4-propylthieno...)Show InChI InChI=1S/C16H22N4O2S/c1-2-3-9-8-11(20-6-4-10(21)5-7-20)19-16-12(9)13(17)14(23-16)15(18)22/h8,10,21H,2-7,17H2,1H3,(H2,18,22) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Hek |
Bioorg Med Chem Lett 19: 5547-51 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.054
BindingDB Entry DOI: 10.7270/Q22Z16G1 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM50389704

(CHEMBL2070081 | US10544104, Compound 150 | US95180...)Show SMILES CCOc1ccc2cc(ccc2c1)-c1nn(CC2CCN(C)CC2)c2ncnc(N)c12 Show InChI InChI=1S/C24H28N6O/c1-3-31-20-7-6-17-12-19(5-4-18(17)13-20)22-21-23(25)26-15-27-24(21)30(28-22)14-16-8-10-29(2)11-9-16/h4-7,12-13,15-16H,3,8-11,14H2,1-2H3,(H2,25,26,27) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human EPHA3 using myelin basic protein as substrate after 120 mins |
J Med Chem 55: 2416-26 (2012)
Article DOI: 10.1021/jm201713h
BindingDB Entry DOI: 10.7270/Q2P2706V |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM50389703

(CHEMBL2070075 | US10544104, Compound 148 | US97650...)Show SMILES CCOc1ccc2cc(ccc2c1)-c1nn(C2CCN(C)CC2)c2ncnc(N)c12 Show InChI InChI=1S/C23H26N6O/c1-3-30-19-7-6-15-12-17(5-4-16(15)13-19)21-20-22(24)25-14-26-23(20)29(27-21)18-8-10-28(2)11-9-18/h4-7,12-14,18H,3,8-11H2,1-2H3,(H2,24,25,26) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human EPHA3 using myelin basic protein as substrate after 120 mins |
J Med Chem 55: 2416-26 (2012)
Article DOI: 10.1021/jm201713h
BindingDB Entry DOI: 10.7270/Q2P2706V |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM50389684
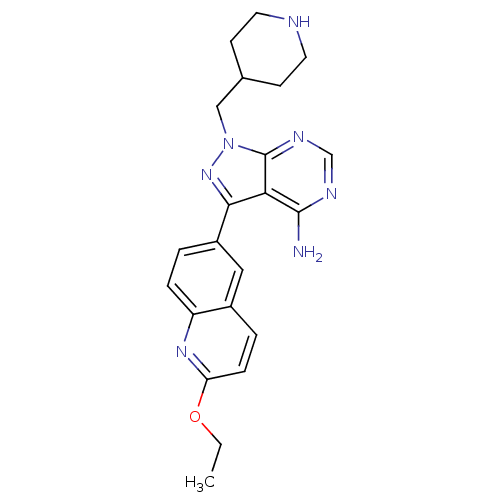
(CHEMBL2070051 | US10307425, Example 119 | US106321...)Show SMILES CCOc1ccc2cc(ccc2n1)-c1nn(CC2CCNCC2)c2ncnc(N)c12 Show InChI InChI=1S/C22H25N7O/c1-2-30-18-6-4-15-11-16(3-5-17(15)27-18)20-19-21(23)25-13-26-22(19)29(28-20)12-14-7-9-24-10-8-14/h3-6,11,13-14,24H,2,7-10,12H2,1H3,(H2,23,25,26) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human EPHA3 using myelin basic protein as substrate after 120 mins |
J Med Chem 55: 2416-26 (2012)
Article DOI: 10.1021/jm201713h
BindingDB Entry DOI: 10.7270/Q2P2706V |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM50383380
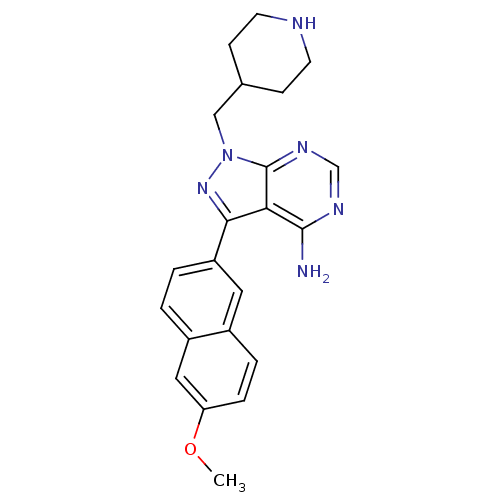
(CHEMBL2030553 | CHEMBL2070047 | US10544104, Compou...)Show SMILES COc1ccc2cc(ccc2c1)-c1nn(CC2CCNCC2)c2ncnc(N)c12 Show InChI InChI=1S/C22H24N6O/c1-29-18-5-4-15-10-17(3-2-16(15)11-18)20-19-21(23)25-13-26-22(19)28(27-20)12-14-6-8-24-9-7-14/h2-5,10-11,13-14,24H,6-9,12H2,1H3,(H2,23,25,26) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human EPHA3 using myelin basic protein as substrate after 120 mins |
J Med Chem 55: 2416-26 (2012)
Article DOI: 10.1021/jm201713h
BindingDB Entry DOI: 10.7270/Q2P2706V |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM50401152
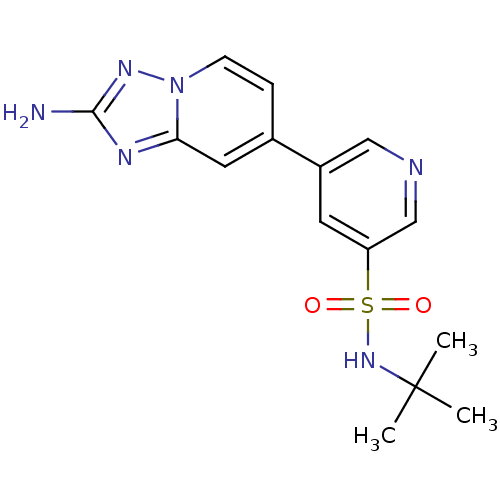
(CHEMBL2205766)Show SMILES CC(C)(C)NS(=O)(=O)c1cncc(c1)-c1ccn2nc(N)nc2c1 Show InChI InChI=1S/C15H18N6O2S/c1-15(2,3)20-24(22,23)12-6-11(8-17-9-12)10-4-5-21-13(7-10)18-14(16)19-21/h4-9,20H,1-3H3,(H2,16,19) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cellzome Ltd
Curated by ChEMBL
| Assay Description
Inhibition of EPHA3 |
Bioorg Med Chem Lett 22: 4613-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.090
BindingDB Entry DOI: 10.7270/Q2HQ412B |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM50135286

(CHEMBL3745885)Show SMILES Cn1c2nc(Nc3ccc4[nH]ccc4c3)ncc2cc(c1=O)S(=O)(=O)c1ccc(F)cc1F Show InChI InChI=1S/C22H15F2N5O3S/c1-29-20-13(9-19(21(29)30)33(31,32)18-5-2-14(23)10-16(18)24)11-26-22(28-20)27-15-3-4-17-12(8-15)6-7-25-17/h2-11,25H,1H3,(H,26,27,28) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
| Assay Description
Inhibition of human EPHA3 using poly[Glu:Tyr] (4:1) as substrate |
Bioorg Med Chem 24: 521-44 (2016)
Article DOI: 10.1016/j.bmc.2015.11.045
BindingDB Entry DOI: 10.7270/Q24Q7WT8 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM50383381
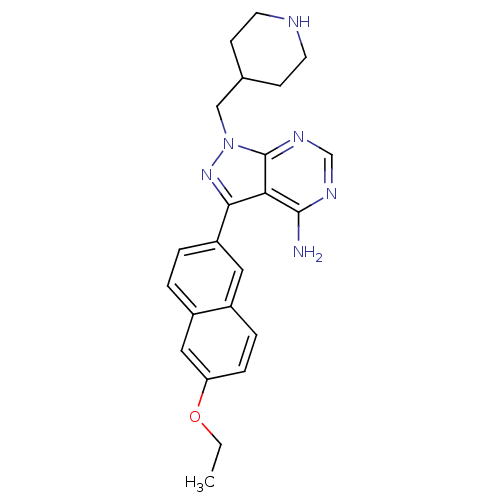
(CHEMBL2030554 | CHEMBL2070050 | US10544104, Compou...)Show SMILES CCOc1ccc2cc(ccc2c1)-c1nn(CC2CCNCC2)c2ncnc(N)c12 Show InChI InChI=1S/C23H26N6O/c1-2-30-19-6-5-16-11-18(4-3-17(16)12-19)21-20-22(24)26-14-27-23(20)29(28-21)13-15-7-9-25-10-8-15/h3-6,11-12,14-15,25H,2,7-10,13H2,1H3,(H2,24,26,27) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human EPHA3 using myelin basic protein as substrate after 120 mins |
J Med Chem 55: 2416-26 (2012)
Article DOI: 10.1021/jm201713h
BindingDB Entry DOI: 10.7270/Q2P2706V |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM50519662
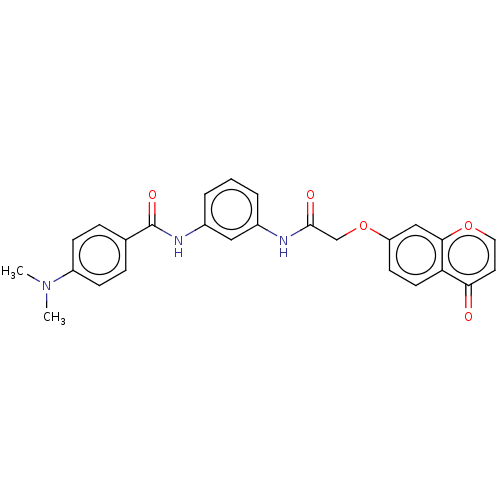
(CHEMBL4438748)Show SMILES CN(C)c1ccc(cc1)C(=O)Nc1cccc(NC(=O)COc2ccc3c(c2)occc3=O)c1 Show InChI InChI=1S/C26H23N3O5/c1-29(2)20-8-6-17(7-9-20)26(32)28-19-5-3-4-18(14-19)27-25(31)16-34-21-10-11-22-23(30)12-13-33-24(22)15-21/h3-15H,16H2,1-2H3,(H,27,31)(H,28,32) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human EphA3 (578 to end residues) using poly(Glu, Tyr) 4:1 as substrate measured after 40 mins in presence of [gamma33ATP] ... |
J Med Chem 62: 10691-10710 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01143
BindingDB Entry DOI: 10.7270/Q2MC93FG |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM50212270
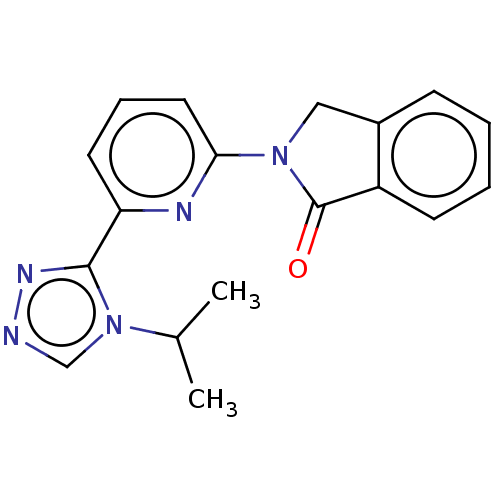
(CHEMBL3952214)Show InChI InChI=1S/C18H17N5O/c1-12(2)23-11-19-21-17(23)15-8-5-9-16(20-15)22-10-13-6-3-4-7-14(13)18(22)24/h3-9,11-12H,10H2,1-2H3 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EPHA3 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127405
BindingDB Entry DOI: 10.7270/Q2JQ14Q8 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM50334268

(CHEMBL1642655 | CHEMBL2205637 | N-(6-(1H-imidazol-...)Show SMILES CC(C)(C)c1ccc(cc1)C(=O)Nc1cn2cc(ccc2n1)-n1ccnc1 Show InChI InChI=1S/C21H21N5O/c1-21(2,3)16-6-4-15(5-7-16)20(27)24-18-13-26-12-17(8-9-19(26)23-18)25-11-10-22-14-25/h4-14H,1-3H3,(H,24,27) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged EPHA3 cytoplasmic domain (569 to 976 residues) expressed in baculovirus expression system |
Bioorg Med Chem Lett 27: 1709-1713 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.079
BindingDB Entry DOI: 10.7270/Q2J38VV6 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM50560385

(CHEMBL4759397) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | <2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EPHA3 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127405
BindingDB Entry DOI: 10.7270/Q2JQ14Q8 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM50237288
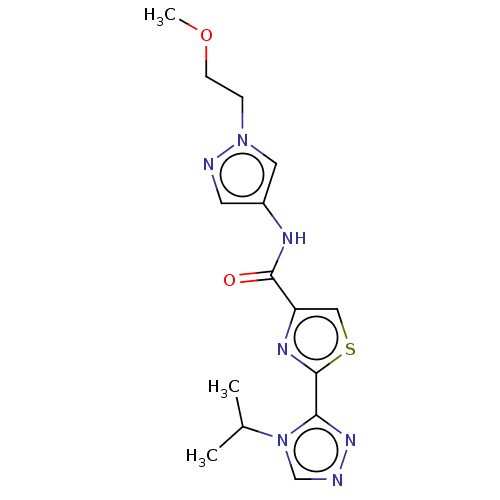
(CHEMBL4073198)Show InChI InChI=1S/C15H19N7O2S/c1-10(2)22-9-16-20-13(22)15-19-12(8-25-15)14(23)18-11-6-17-21(7-11)4-5-24-3/h6-10H,4-5H2,1-3H3,(H,18,23) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged EPHA3 cytoplasmic domain (569 to 976 residues) expressed in baculovirus expression system |
Bioorg Med Chem Lett 27: 1709-1713 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.079
BindingDB Entry DOI: 10.7270/Q2J38VV6 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM50359359

(CHEMBL1929238)Show SMILES CN(C)CCN1CCN(CCC1=O)C(=O)c1cc(sc1NC(=O)Nc1cccc(Cl)c1Cl)C(C)(C)C Show InChI InChI=1S/C25H33Cl2N5O3S/c1-25(2,3)19-15-16(22(36-19)29-24(35)28-18-8-6-7-17(26)21(18)27)23(34)32-10-9-20(33)31(13-14-32)12-11-30(4)5/h6-8,15H,9-14H2,1-5H3,(H2,28,29,35) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of EPHA3 |
Bioorg Med Chem Lett 21: 7155-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.078
BindingDB Entry DOI: 10.7270/Q2NC61NH |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM50537742
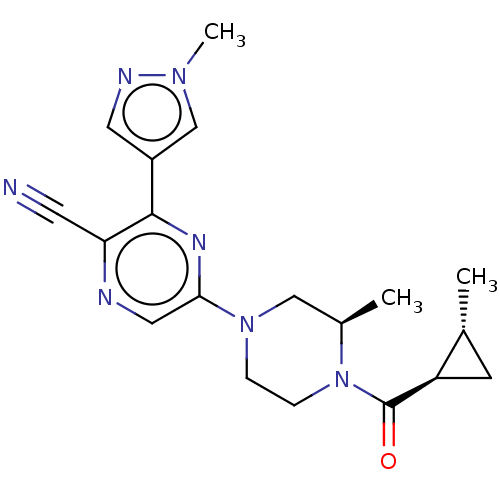
(CHEMBL4634634 | US11179389, Compound 1-14)Show SMILES C[C@@H]1C[C@H]1C(=O)N1CCN(C[C@H]1C)c1cnc(C#N)c(n1)-c1cnn(C)c1 |r| Show InChI InChI=1S/C19H23N7O/c1-12-6-15(12)19(27)26-5-4-25(10-13(26)2)17-9-21-16(7-20)18(23-17)14-8-22-24(3)11-14/h8-9,11-13,15H,4-6,10H2,1-3H3/t12-,13-,15-/m1/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of tracer 236 binding to recombinant human His-tagged EPHA3 (569 to 976 residues) expressed in baculovirus expression system measured afte... |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126715
BindingDB Entry DOI: 10.7270/Q2HM5CZG |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM50536681
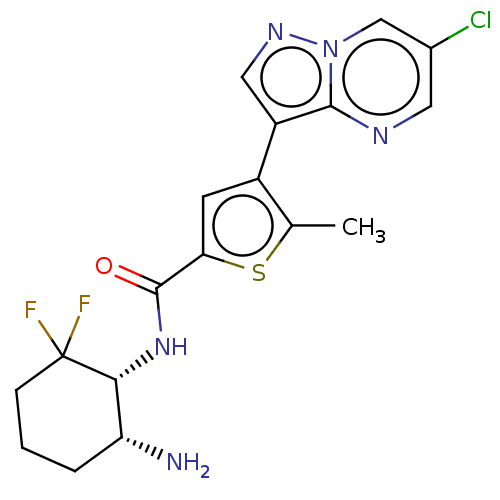
(CHEMBL4552628)Show SMILES Cc1sc(cc1-c1cnn2cc(Cl)cnc12)C(=O)N[C@@H]1[C@H](N)CCCC1(F)F |r| Show InChI InChI=1S/C18H18ClF2N5OS/c1-9-11(12-7-24-26-8-10(19)6-23-16(12)26)5-14(28-9)17(27)25-15-13(22)3-2-4-18(15,20)21/h5-8,13,15H,2-4,22H2,1H3,(H,25,27)/t13-,15-/m1/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged cytoplasmic EPHA3 expressed in baculovirus expression system by LanthaScreen assay |
Bioorg Med Chem Lett 26: 4362-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.003
BindingDB Entry DOI: 10.7270/Q2NP27X5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data