

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM223477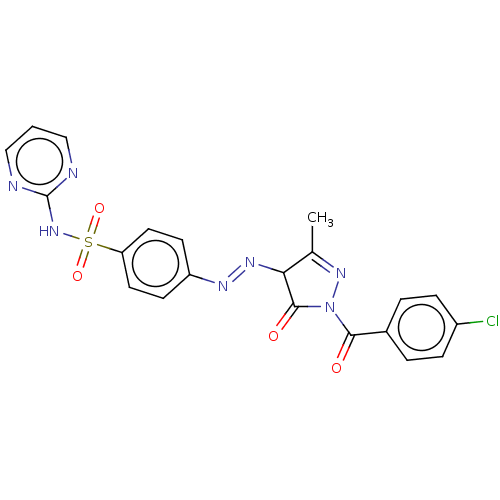 (US9320734, 316) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description KT1 PH domain small molecule inhibitors were identified using the crystal structure of the AKT1 PH domain bound by PtdIns(1,3,4,5)P4 as descried in T... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM223477 (US9320734, 316) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description SPR interaction analyses were performed with a Biacore 2000, using Biacore 2000 Control Software v3.2 and BIAevaluation v4.1 analysis software (Biaco... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM50034417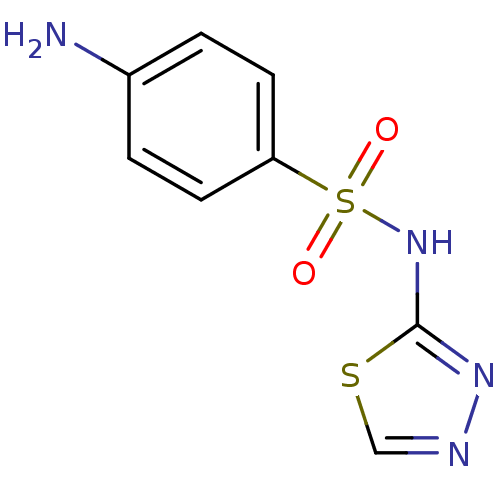 (4-Amino-N-[1,3,4]thiadiazol-2-yl-benzenesulfonamid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description SPR interaction analyses were performed with a Biacore 2000, using Biacore 2000 Control Software v3.2 and BIAevaluation v4.1 analysis software (Biaco... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM223487 (US9320734, 333) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description KT1 PH domain small molecule inhibitors were identified using the crystal structure of the AKT1 PH domain bound by PtdIns(1,3,4,5)P4 as descried in T... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM223490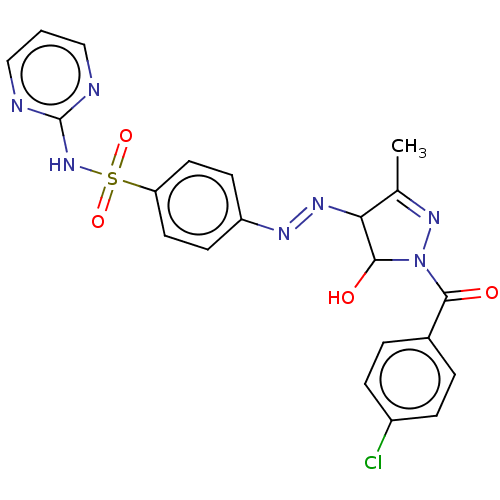 (US9320734, 335) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description KT1 PH domain small molecule inhibitors were identified using the crystal structure of the AKT1 PH domain bound by PtdIns(1,3,4,5)P4 as descried in T... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM223477 (US9320734, 316) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description KT1 PH domain small molecule inhibitors were identified using the crystal structure of the AKT1 PH domain bound by PtdIns(1,3,4,5)P4 as descried in T... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM223488 (US9320734, 332) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description KT1 PH domain small molecule inhibitors were identified using the crystal structure of the AKT1 PH domain bound by PtdIns(1,3,4,5)P4 as descried in T... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM223489 (US9320734, 360) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description KT1 PH domain small molecule inhibitors were identified using the crystal structure of the AKT1 PH domain bound by PtdIns(1,3,4,5)P4 as descried in T... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM223489 (US9320734, 360) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description KT1 PH domain small molecule inhibitors were identified using the crystal structure of the AKT1 PH domain bound by PtdIns(1,3,4,5)P4 as descried in T... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM223488 (US9320734, 332) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 1.37E+3 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description KT1 PH domain small molecule inhibitors were identified using the crystal structure of the AKT1 PH domain bound by PtdIns(1,3,4,5)P4 as descried in T... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM223490 (US9320734, 335) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 1.62E+3 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description KT1 PH domain small molecule inhibitors were identified using the crystal structure of the AKT1 PH domain bound by PtdIns(1,3,4,5)P4 as descried in T... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM223475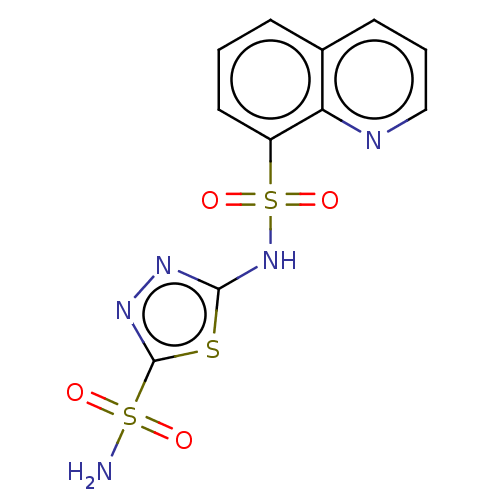 (US9320734, 345) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 1.79E+3 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description SPR interaction analyses were performed with a Biacore 2000, using Biacore 2000 Control Software v3.2 and BIAevaluation v4.1 analysis software (Biaco... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM223486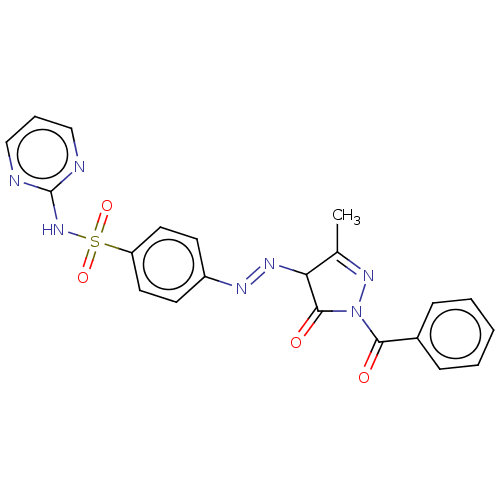 (US9320734, 331) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description KT1 PH domain small molecule inhibitors were identified using the crystal structure of the AKT1 PH domain bound by PtdIns(1,3,4,5)P4 as descried in T... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM223487 (US9320734, 333) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description KT1 PH domain small molecule inhibitors were identified using the crystal structure of the AKT1 PH domain bound by PtdIns(1,3,4,5)P4 as descried in T... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM223480 (US9320734, PtdIns(3,4,5)trisphosphate) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 3.08E+3 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description KT1 PH domain small molecule inhibitors were identified using the crystal structure of the AKT1 PH domain bound by PtdIns(1,3,4,5)P4 as descried in T... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM223486 (US9320734, 331) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 3.66E+3 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description KT1 PH domain small molecule inhibitors were identified using the crystal structure of the AKT1 PH domain bound by PtdIns(1,3,4,5)P4 as descried in T... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM50338954 (CHEMBL1685054 | N-(5-tert-Butyl-1,3,4-thiadiazol-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 4.07E+3 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description SPR interaction analyses were performed with a Biacore 2000, using Biacore 2000 Control Software v3.2 and BIAevaluation v4.1 analysis software (Biaco... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM223476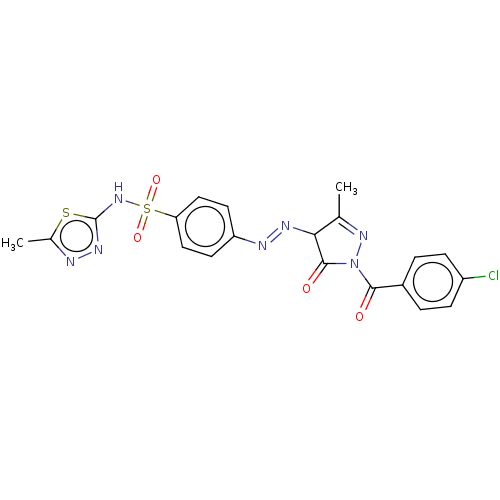 (US9320734, 389) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 4.58E+3 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description SPR interaction analyses were performed with a Biacore 2000, using Biacore 2000 Control Software v3.2 and BIAevaluation v4.1 analysis software (Biaco... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM50338959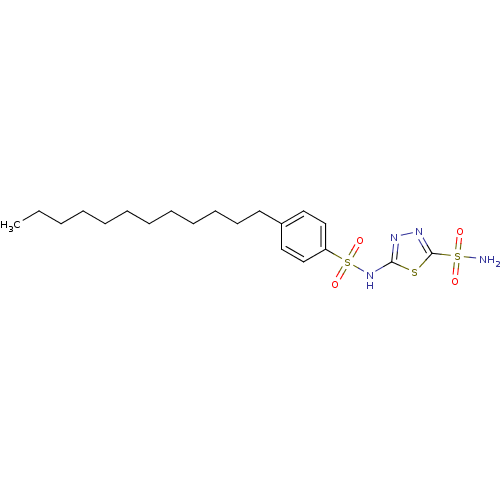 (5-(4-Dodecylphenylsulfonamido)-1,3,4-thiadiazole-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description SPR interaction analyses were performed with a Biacore 2000, using Biacore 2000 Control Software v3.2 and BIAevaluation v4.1 analysis software (Biaco... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM50338939 (CHEMBL1685039 | N-(4-(N-(5-Ethyl-1,3,4-thiadiazol-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | 5.25E+3 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description SPR interaction analyses were performed with a Biacore 2000, using Biacore 2000 Control Software v3.2 and BIAevaluation v4.1 analysis software (Biaco... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM223478 (US9320734, 110) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 6.03E+3 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description SPR interaction analyses were performed with a Biacore 2000, using Biacore 2000 Control Software v3.2 and BIAevaluation v4.1 analysis software (Biaco... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM223474 (US9320734, 415) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 6.27E+3 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description SPR interaction analyses were performed with a Biacore 2000, using Biacore 2000 Control Software v3.2 and BIAevaluation v4.1 analysis software (Biaco... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM50338953 (4-Dodecyl-N-(5-ethyl-1,3,4-thiadiazol-2-yl)benzene...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | 7.59E+3 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description SPR interaction analyses were performed with a Biacore 2000, using Biacore 2000 Control Software v3.2 and BIAevaluation v4.1 analysis software (Biaco... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM223479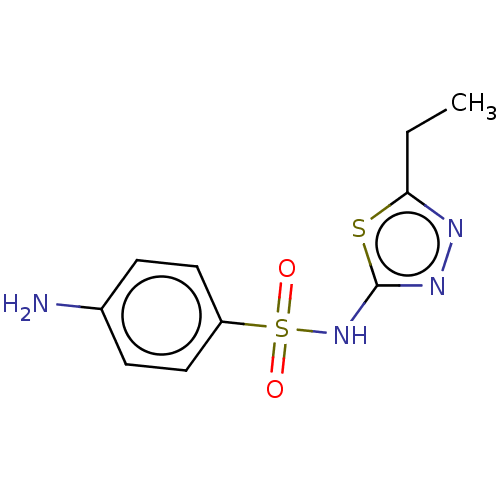 (US9320734, 109) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 7.76E+3 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description SPR interaction analyses were performed with a Biacore 2000, using Biacore 2000 Control Software v3.2 and BIAevaluation v4.1 analysis software (Biaco... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM50338953 (4-Dodecyl-N-(5-ethyl-1,3,4-thiadiazol-2-yl)benzene...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description SPR interaction analyses were performed with a Biacore 2000, using Biacore 2000 Control Software v3.2 and BIAevaluation v4.1 analysis software (Biaco... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM50338952 (4-Dodecyl-N-(5-methyl-1,3,4-thiadiazol-2-yl)benzen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | 8.32E+3 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description SPR interaction analyses were performed with a Biacore 2000, using Biacore 2000 Control Software v3.2 and BIAevaluation v4.1 analysis software (Biaco... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM50304368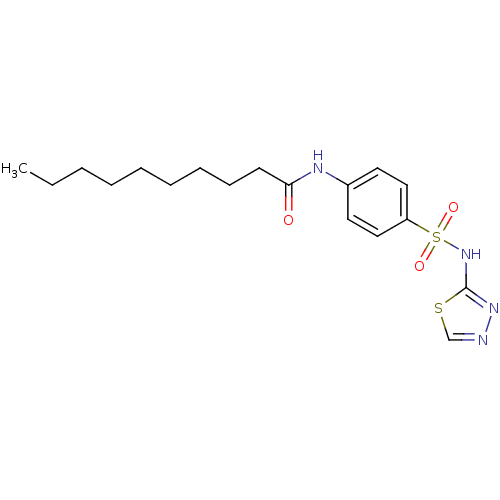 (CHEMBL595582 | N-(4-(N-1,3,4-thiadiazol-2-ylsulfam...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description SPR interaction analyses were performed with a Biacore 2000, using Biacore 2000 Control Software v3.2 and BIAevaluation v4.1 analysis software (Biaco... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM50304370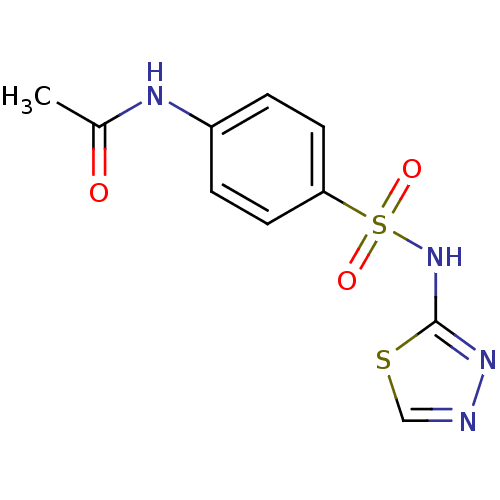 (CHEMBL593946 | N-(4-(N-1,3,4-thiadiazol-2-ylsulfam...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description SPR interaction analyses were performed with a Biacore 2000, using Biacore 2000 Control Software v3.2 and BIAevaluation v4.1 analysis software (Biaco... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM50304369 (4-dodecyl-N-(1,3,4-thiadiazol-2-yl)benzenesulfonam...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description SPR interaction analyses were performed with a Biacore 2000, using Biacore 2000 Control Software v3.2 and BIAevaluation v4.1 analysis software (Biaco... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM50304369 (4-dodecyl-N-(1,3,4-thiadiazol-2-yl)benzenesulfonam...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description SPR interaction analyses were performed with a Biacore 2000, using Biacore 2000 Control Software v3.2 and BIAevaluation v4.1 analysis software (Biaco... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM50034417 (4-Amino-N-[1,3,4]thiadiazol-2-yl-benzenesulfonamid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | 1.51E+4 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description SPR interaction analyses were performed with a Biacore 2000, using Biacore 2000 Control Software v3.2 and BIAevaluation v4.1 analysis software (Biaco... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM50338947 (4-Hexyl-N-(1,3,4-thiadiazol-2-yl)benzenesulfonamid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | 1.91E+4 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description SPR interaction analyses were performed with a Biacore 2000, using Biacore 2000 Control Software v3.2 and BIAevaluation v4.1 analysis software (Biaco... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM50304368 (CHEMBL595582 | N-(4-(N-1,3,4-thiadiazol-2-ylsulfam...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | 1.96E+4 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description SPR interaction analyses were performed with a Biacore 2000, using Biacore 2000 Control Software v3.2 and BIAevaluation v4.1 analysis software (Biaco... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM50304371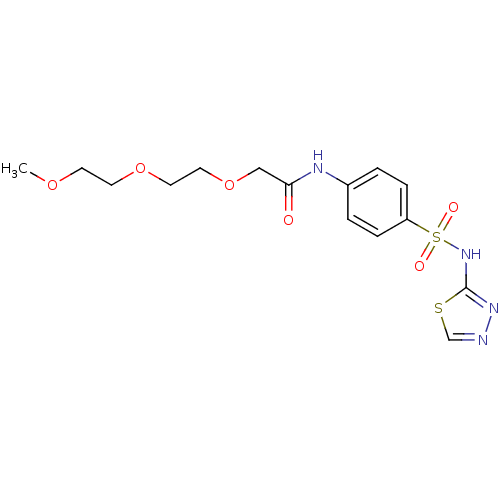 (CHEMBL611216 | N-(4-(N-1,3,4-thiadiazol-2-ylsulfam...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | 2.04E+4 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description SPR interaction analyses were performed with a Biacore 2000, using Biacore 2000 Control Software v3.2 and BIAevaluation v4.1 analysis software (Biaco... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM50338956 (CHEMBL1685055 | Ethyl 2-(5-(4-Dodecylphenylsulfona...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 2.07E+4 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description SPR interaction analyses were performed with a Biacore 2000, using Biacore 2000 Control Software v3.2 and BIAevaluation v4.1 analysis software (Biaco... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM50338946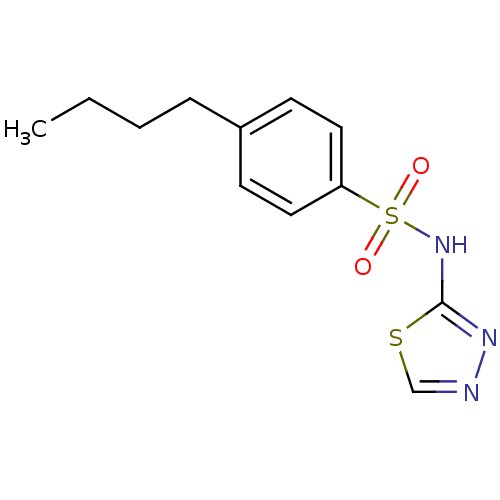 (4-Butyl-N-(1,3,4-thiadiazol-2-yl)benzenesulfonamid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | 2.37E+4 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description SPR interaction analyses were performed with a Biacore 2000, using Biacore 2000 Control Software v3.2 and BIAevaluation v4.1 analysis software (Biaco... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM50338944 (CHEMBL1685044 | N-(4-(N-(5-(hydroxymethyl)-1,3,4-t...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 2.45E+4 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description SPR interaction analyses were performed with a Biacore 2000, using Biacore 2000 Control Software v3.2 and BIAevaluation v4.1 analysis software (Biaco... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM50338948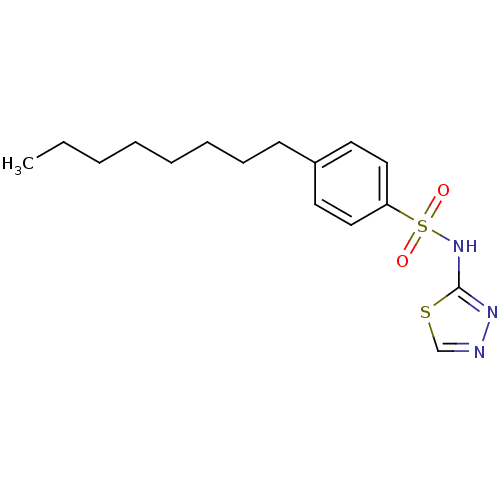 (4-Octyl-N-(1,3,4-thiadiazol-2-yl)benzenesulfonamid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | 2.58E+4 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description SPR interaction analyses were performed with a Biacore 2000, using Biacore 2000 Control Software v3.2 and BIAevaluation v4.1 analysis software (Biaco... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM50338952 (4-Dodecyl-N-(5-methyl-1,3,4-thiadiazol-2-yl)benzen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | 3.63E+4 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description SPR interaction analyses were performed with a Biacore 2000, using Biacore 2000 Control Software v3.2 and BIAevaluation v4.1 analysis software (Biaco... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM50304369 (4-dodecyl-N-(1,3,4-thiadiazol-2-yl)benzenesulfonam...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | 4.08E+4 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description SPR interaction analyses were performed with a Biacore 2000, using Biacore 2000 Control Software v3.2 and BIAevaluation v4.1 analysis software (Biaco... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM50304369 (4-dodecyl-N-(1,3,4-thiadiazol-2-yl)benzenesulfonam...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | 4.08E+4 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description SPR interaction analyses were performed with a Biacore 2000, using Biacore 2000 Control Software v3.2 and BIAevaluation v4.1 analysis software (Biaco... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM50338938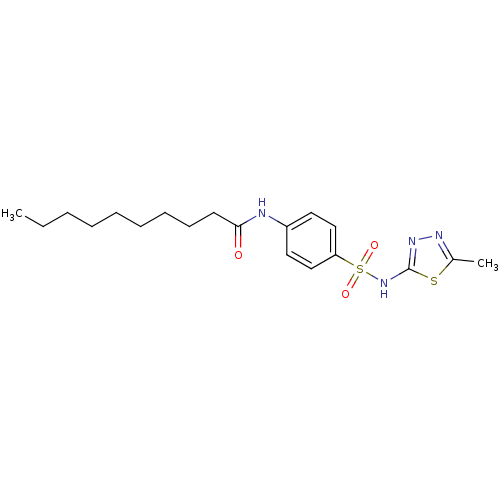 (CHEMBL1685038 | N-(4-(N-(5-Methyl-1,3,4-thiadiazol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | 4.67E+4 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description SPR interaction analyses were performed with a Biacore 2000, using Biacore 2000 Control Software v3.2 and BIAevaluation v4.1 analysis software (Biaco... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM50338952 (4-Dodecyl-N-(5-methyl-1,3,4-thiadiazol-2-yl)benzen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | 4.87E+4 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description SPR interaction analyses were performed with a Biacore 2000, using Biacore 2000 Control Software v3.2 and BIAevaluation v4.1 analysis software (Biaco... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM50338949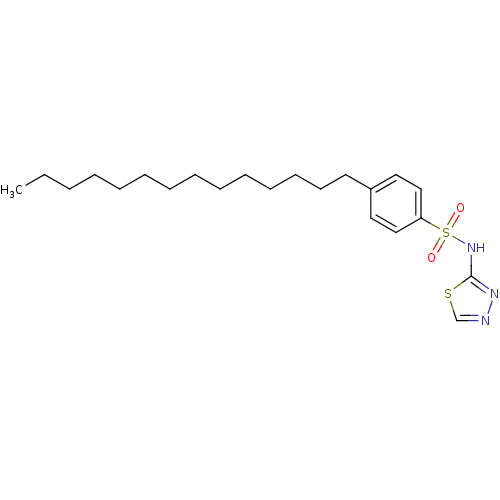 (4-Tetradecyl-N-(1,3,4-thiadiazol-2-yl)benzenesulfo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | 5.89E+4 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description SPR interaction analyses were performed with a Biacore 2000, using Biacore 2000 Control Software v3.2 and BIAevaluation v4.1 analysis software (Biaco... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM50338953 (4-Dodecyl-N-(5-ethyl-1,3,4-thiadiazol-2-yl)benzene...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | 7.30E+4 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description SPR interaction analyses were performed with a Biacore 2000, using Biacore 2000 Control Software v3.2 and BIAevaluation v4.1 analysis software (Biaco... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM50338942 (CHEMBL1685042 | Ethyl 2-(5-(4-Decanamidophenylsulf...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 1.05E+5 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description SPR interaction analyses were performed with a Biacore 2000, using Biacore 2000 Control Software v3.2 and BIAevaluation v4.1 analysis software (Biaco... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM50338943 (CHEMBL1685043 | Ethyl 5-(4-Decanamidophenylsulfona...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 1.09E+5 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description SPR interaction analyses were performed with a Biacore 2000, using Biacore 2000 Control Software v3.2 and BIAevaluation v4.1 analysis software (Biaco... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM50338955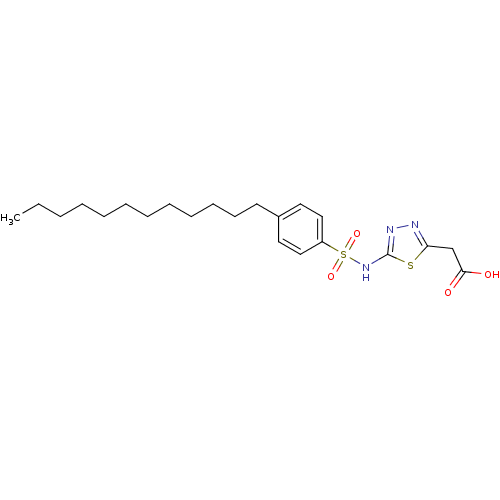 (2-(5-(4-Dodecylphenylsulfonamido)-1,3,4-thiadiazol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description SPR interaction analyses were performed with a Biacore 2000, using Biacore 2000 Control Software v3.2 and BIAevaluation v4.1 analysis software (Biaco... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM50338955 (2-(5-(4-Dodecylphenylsulfonamido)-1,3,4-thiadiazol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 1.14E+5 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description SPR interaction analyses were performed with a Biacore 2000, using Biacore 2000 Control Software v3.2 and BIAevaluation v4.1 analysis software (Biaco... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [1-123] (Homo sapiens (Human)) | BDBM50338939 (CHEMBL1685039 | N-(4-(N-(5-Ethyl-1,3,4-thiadiazol-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | 1.78E+5 | n/a | n/a | n/a | n/a | n/a |
Board of Regents, The University of Texas System; Arizona Board of Regents on behalf of the University of Arizona US Patent | Assay Description SPR interaction analyses were performed with a Biacore 2000, using Biacore 2000 Control Software v3.2 and BIAevaluation v4.1 analysis software (Biaco... | US Patent US9320734 (2016) BindingDB Entry DOI: 10.7270/Q2QF8RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 57 total ) | Next | Last >> |