

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50035236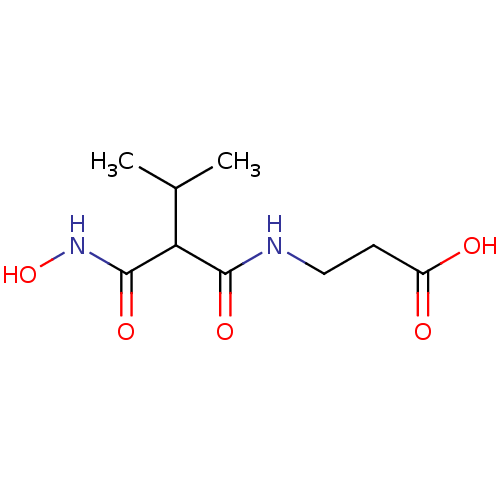 (3-(2-Hydroxycarbamoyl-3-methyl-butyrylamino)-propi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human bronchiolar smooth muscle Endothelin-converting enzyme 1 | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50035247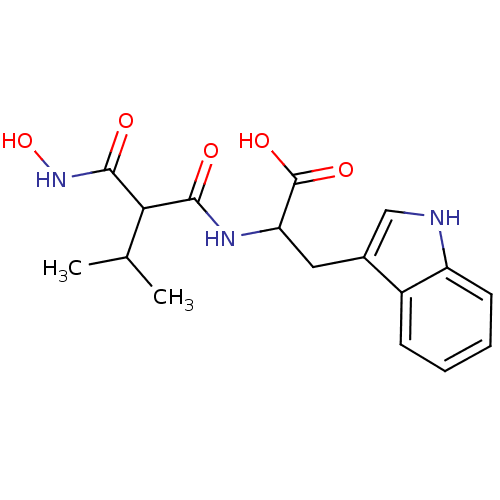 (2-(2-Hydroxycarbamoyl-3-methyl-butyrylamino)-3-(1H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human bronchiolar smooth muscle Endothelin-converting enzyme 1 | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50035246 (2-(2-Hydroxycarbamoyl-4-methyl-pentanoylamino)-3-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human bronchiolar smooth muscle Endothelin-converting enzyme 1 | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50251742 ((3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human bronchiolar smooth muscle Endothelin-converting enzyme 1 | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50035239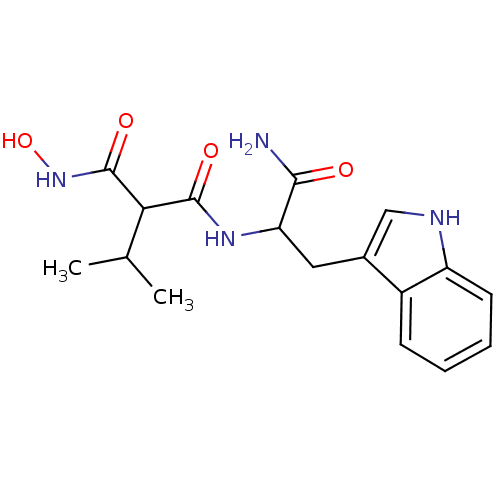 (CHEMBL63317 | N-[1-Carbamoyl-2-(1H-indol-3-yl)-eth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human bronchiolar smooth muscle Endothelin-converting enzyme 1 | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50084905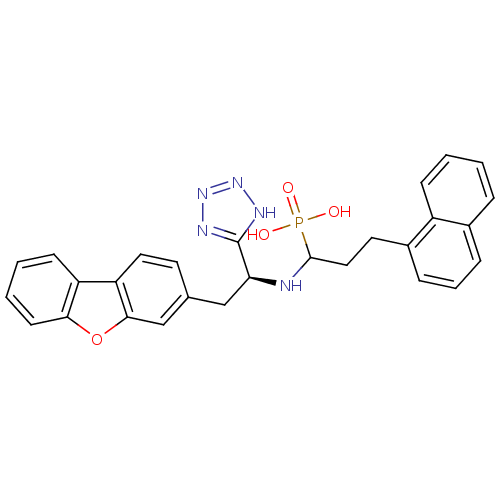 (CHEMBL147964 | {1-[2-Dibenzofuran-3-yl-1-(1H-tetra...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In Vitro inhibition of recombinant human endothelin converting enzyme-1 | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50084905 (CHEMBL147964 | {1-[2-Dibenzofuran-3-yl-1-(1H-tetra...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In Vitro inhibition of recombinant human endothelin converting enzyme-1 | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50035251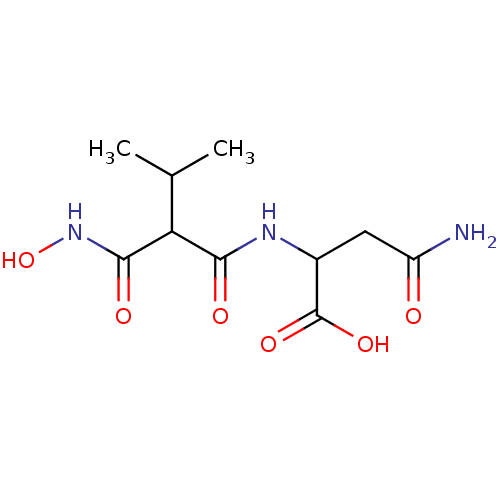 (2-(2-Hydroxycarbamoyl-3-methyl-butyrylamino)-succi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human bronchiolar smooth muscle Endothelin-converting enzyme 1 | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50035249 (CHEMBL303892 | N-[1-Carbamoyl-2-(1H-indol-3-yl)-et...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human bronchiolar smooth muscle Endothelin-converting enzyme 1 | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50084903 (CHEMBL148056 | {[2-Dibenzofuran-3-yl-1-(1H-tetrazo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In Vitro inhibition of recombinant human endothelin converting enzyme-1 | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50084897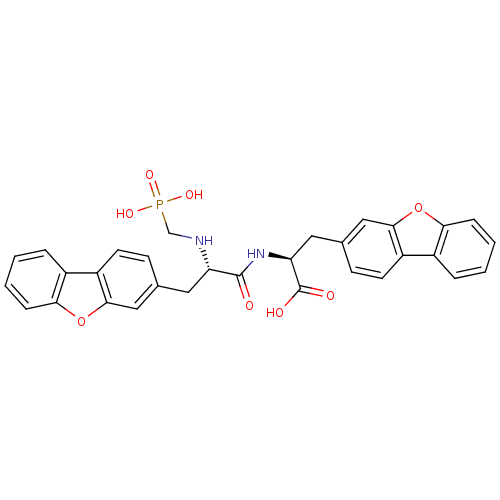 (3-Dibenzofuran-3-yl-2-[3-dibenzofuran-3-yl-2-(phos...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In Vitro inhibition of recombinant human endothelin converting enzyme-1 | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50035240 ((2-Hydroxycarbamoyl-3-methyl-butyrylamino)-acetic ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human bronchiolar smooth muscle Endothelin-converting enzyme 1 | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50159366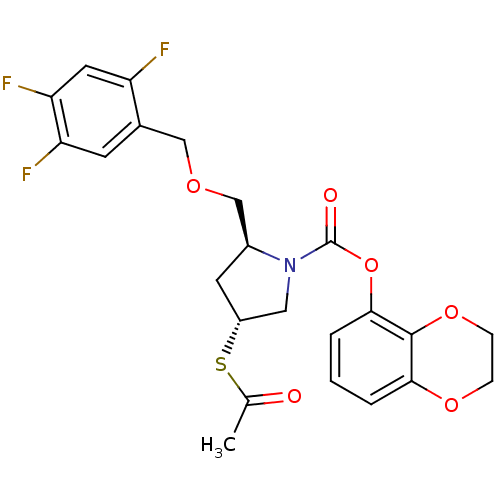 ((2S,4R)-4-Acetylsulfanyl-2-(2,4,5-trifluoro-benzyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pathology Curated by ChEMBL | Assay Description Inhibitory concentration against human ECE-1 by RIA | J Med Chem 48: 483-98 (2005) Article DOI: 10.1021/jm040857x BindingDB Entry DOI: 10.7270/Q2VM4D1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50064110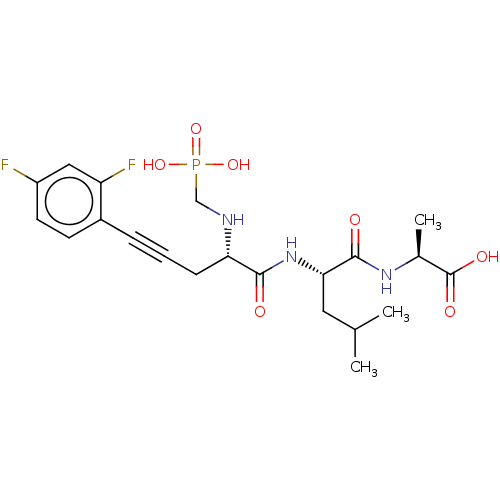 (2-{2-[(S)-5-(2,4-Difluoro-phenyl)-2-(phosphonometh...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In Vitro inhibition of recombinant human endothelin converting enzyme-1 | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM21641 (2-(2-benzyl-3-sulfanylpropanamido)acetic acid | CH...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human bronchiolar smooth muscle Endothelin-converting enzyme 1 | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50105264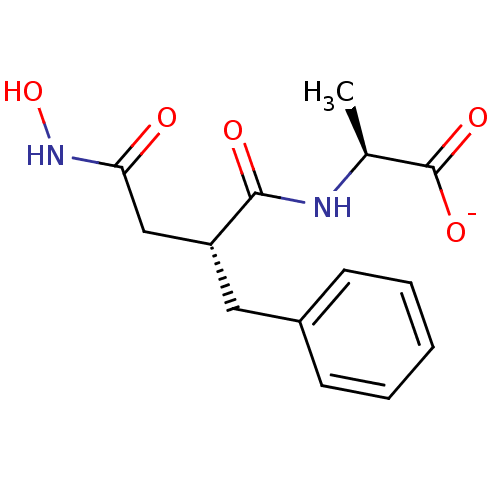 (2-(2-Benzyl-3-hydroxycarbamoyl-propionylamino)-pro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human bronchiolar smooth muscle Endothelin-converting enzyme 1 | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50119751 (2-{[1-(2-Mercapto-4-methyl-pentanoylamino)-cyclope...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibition of Endothelin-converting enzyme 1. | Bioorg Med Chem Lett 12: 3059-62 (2002) BindingDB Entry DOI: 10.7270/Q26D5SCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50119742 (2-{[1-(2-Mercapto-4-methyl-pentanoylamino)-cyclope...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Homo sapiens (human) endothelin converting enzyme-1 | Citation and Details Article DOI: 10.1007/s00044-012-0433-z BindingDB Entry DOI: 10.7270/Q2S185DK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50119742 (2-{[1-(2-Mercapto-4-methyl-pentanoylamino)-cyclope...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibition of Endothelin-converting enzyme 1. | Bioorg Med Chem Lett 12: 3059-62 (2002) BindingDB Entry DOI: 10.7270/Q26D5SCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50159365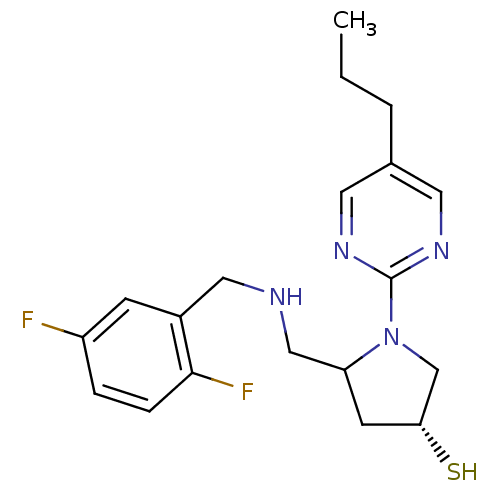 ((3R,5S)-5-{[(2,5-difluorobenzyl)amino]methyl}-1-(5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10.4 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pathology Curated by ChEMBL | Assay Description Inhibitory concentration against human ECE-1 by RIA | J Med Chem 48: 483-98 (2005) Article DOI: 10.1021/jm040857x BindingDB Entry DOI: 10.7270/Q2VM4D1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50488983 (CHEMBL2297552) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Homo sapiens (human) endothelin converting enzyme-1 | Citation and Details Article DOI: 10.1007/s00044-012-0433-z BindingDB Entry DOI: 10.7270/Q2S185DK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50488971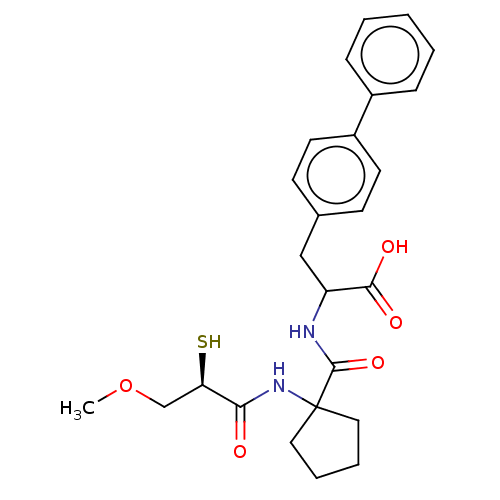 (CHEMBL2297562) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Homo sapiens (human) endothelin converting enzyme-1 | Citation and Details Article DOI: 10.1007/s00044-012-0433-z BindingDB Entry DOI: 10.7270/Q2S185DK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50035235 (CHEMBL2372437 | N-[1-(Carbamoylmethyl-carbamoyl)-e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human bronchiolar smooth muscle Endothelin-converting enzyme 1 | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50091882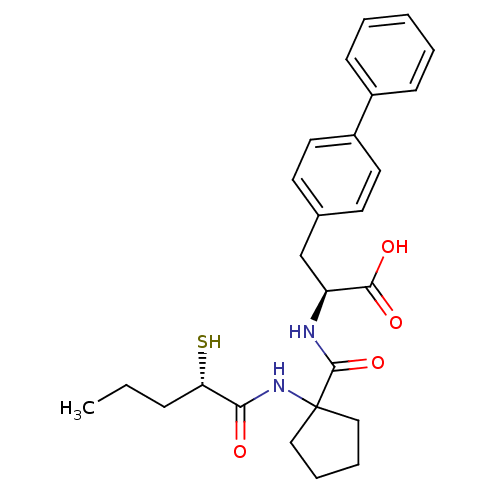 ((S)-3-Biphenyl-4-yl-2-{[1-((S)-2-mercapto-1-oxo-pe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibition of Endothelin-converting enzyme 1. | Bioorg Med Chem Lett 12: 3059-62 (2002) BindingDB Entry DOI: 10.7270/Q26D5SCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50091878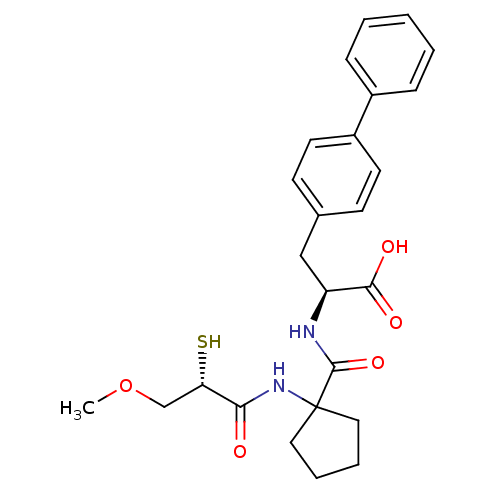 ((S)-3-Biphenyl-4-yl-2-{[1-((S)-2-mercapto-3-methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibition of human Endothelin converting Enzyme-1 activity | Bioorg Med Chem Lett 10: 2037-9 (2001) BindingDB Entry DOI: 10.7270/Q2K35SW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50091882 ((S)-3-Biphenyl-4-yl-2-{[1-((S)-2-mercapto-1-oxo-pe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibition of human Endothelin converting Enzyme-1 activity | Bioorg Med Chem Lett 10: 2037-9 (2001) BindingDB Entry DOI: 10.7270/Q2K35SW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50091882 ((S)-3-Biphenyl-4-yl-2-{[1-((S)-2-mercapto-1-oxo-pe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against Endothelin converting enzyme 1 | Bioorg Med Chem Lett 11: 375-8 (2001) BindingDB Entry DOI: 10.7270/Q2HH6JC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50098118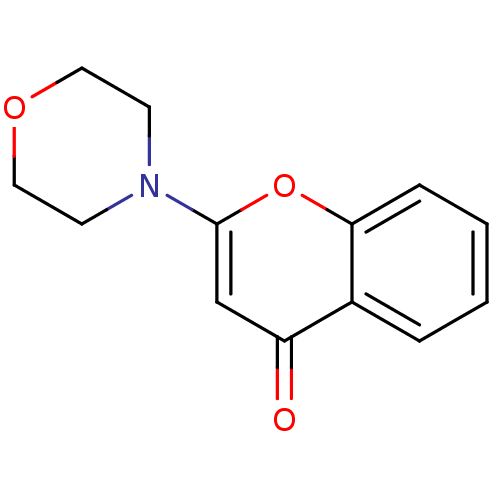 ((2S,4R)-4-Mercapto-2-(2,4,5-trifluoro-benzyloxymet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pathology Curated by ChEMBL | Assay Description Inhibitory concentration against human ECE-1 by RIA | J Med Chem 48: 483-98 (2005) Article DOI: 10.1021/jm040857x BindingDB Entry DOI: 10.7270/Q2VM4D1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50064114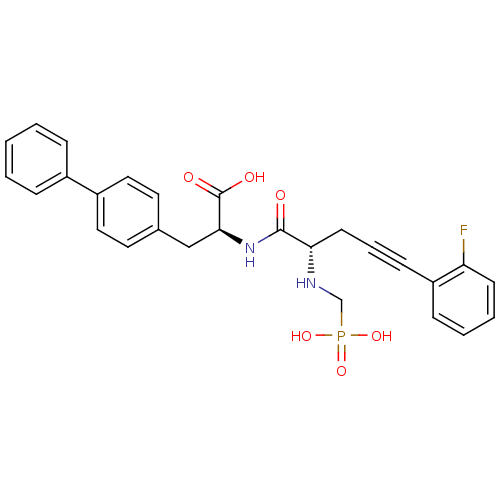 ((S)-3-Biphenyl-4-yl-2-[(S)-5-(2-fluoro-phenyl)-2-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitory activity was assessed on CHO cells expressing recombinant human Endothelin-converting enzyme 1 (ECE-1). | J Med Chem 41: 1513-23 (1998) Article DOI: 10.1021/jm970787c BindingDB Entry DOI: 10.7270/Q2PV6M1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50084898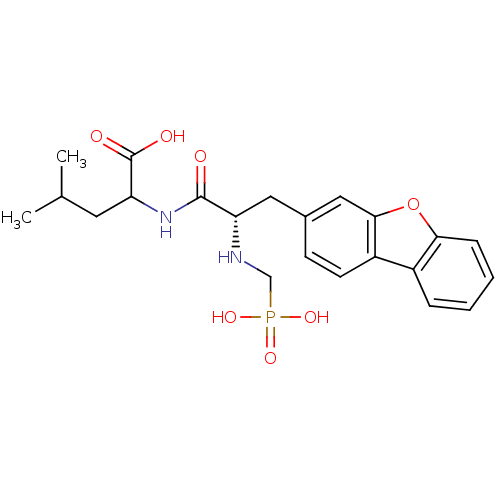 (2-[3-Dibenzofuran-3-yl-2-(phosphonomethyl-amino)-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In Vitro inhibition of recombinant human endothelin converting enzyme-1 | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50064125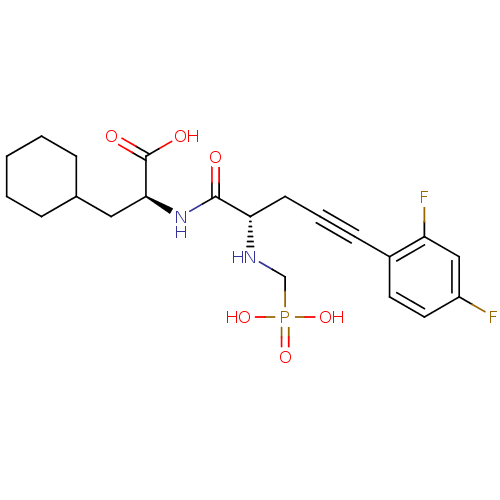 ((S)-3-Cyclohexyl-2-[(S)-5-(2,4-difluoro-phenyl)-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitory activity was assessed on CHO cells expressing recombinant human Endothelin-converting enzyme 1 (ECE-1). | J Med Chem 41: 1513-23 (1998) Article DOI: 10.1021/jm970787c BindingDB Entry DOI: 10.7270/Q2PV6M1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50159364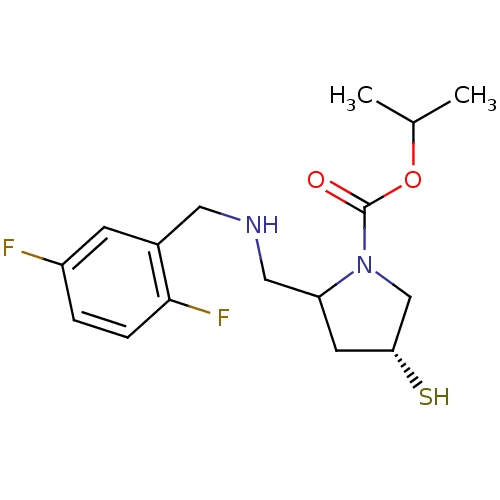 (CHEMBL192441 | isopropyl (2S,4R)-2-{[(2,5-difluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pathology Curated by ChEMBL | Assay Description Inhibitory concentration against human ECE-1 by RIA | J Med Chem 48: 483-98 (2005) Article DOI: 10.1021/jm040857x BindingDB Entry DOI: 10.7270/Q2VM4D1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50064109 (CGS-31447 | CHEMBL285619 | {1-[(S)-2-Biphenyl-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In Vitro inhibition of recombinant human endothelin converting enzyme-1 | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50064109 (CGS-31447 | CHEMBL285619 | {1-[(S)-2-Biphenyl-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitory activity was assessed on CHO cells expressing recombinant human Endothelin-converting enzyme 1 (ECE-1). | J Med Chem 41: 1513-23 (1998) Article DOI: 10.1021/jm970787c BindingDB Entry DOI: 10.7270/Q2PV6M1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50251742 ((3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Chimica Curated by ChEMBL | Assay Description Inhibition of human recombinant ECE1 by fluorimetry | Bioorg Med Chem Lett 19: 4715-9 (2009) Article DOI: 10.1016/j.bmcl.2009.06.064 BindingDB Entry DOI: 10.7270/Q2GH9JWT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50291561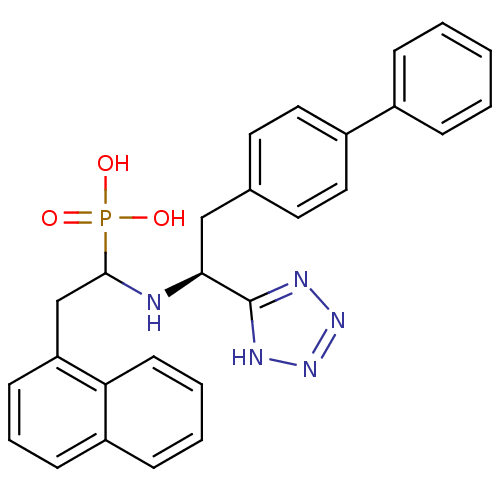 (CHEMBL10251 | {1-[(S)-2-Biphenyl-4-yl-1-(1H-tetraz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition against recombinant human Endothelin converting enzyme 1 activity | Bioorg Med Chem Lett 7: 1059-1064 (1997) Article DOI: 10.1016/S0960-894X(97)00159-5 BindingDB Entry DOI: 10.7270/Q2WH2QGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50035237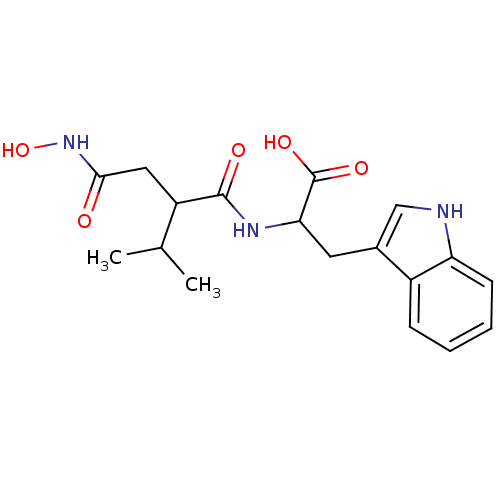 (2-(2-Hydroxycarbamoylmethyl-3-methyl-butyrylamino)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human bronchiolar smooth muscle Endothelin-converting enzyme 1 | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50035237 (2-(2-Hydroxycarbamoylmethyl-3-methyl-butyrylamino)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human bronchiolar smooth muscle Endothelin-converting enzyme 1 | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50488990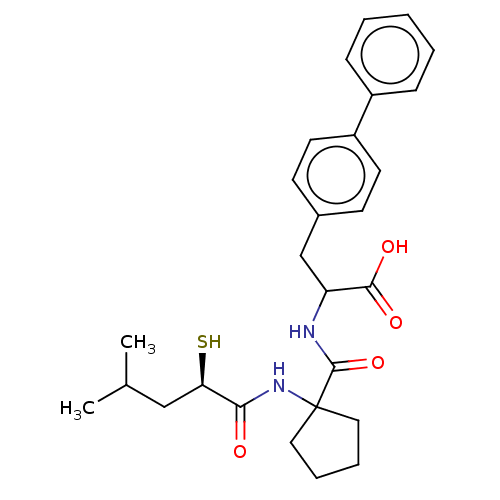 (CHEMBL2297556) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Homo sapiens (human) endothelin converting enzyme-1 | Citation and Details Article DOI: 10.1007/s00044-012-0433-z BindingDB Entry DOI: 10.7270/Q2S185DK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50091888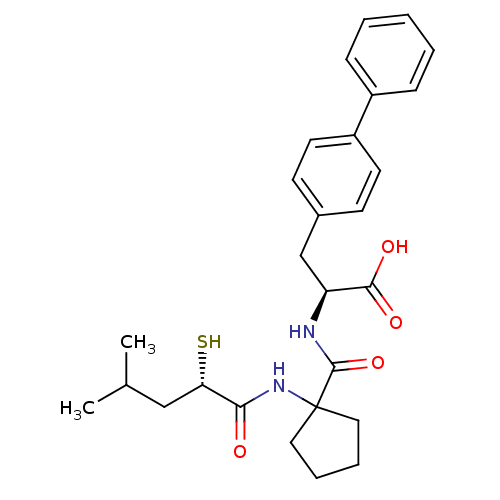 ((S)-3-Biphenyl-4-yl-2-{[1-((S)-2-mercapto-4-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibition of human Endothelin converting Enzyme-1 activity | Bioorg Med Chem Lett 10: 2037-9 (2001) BindingDB Entry DOI: 10.7270/Q2K35SW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50084893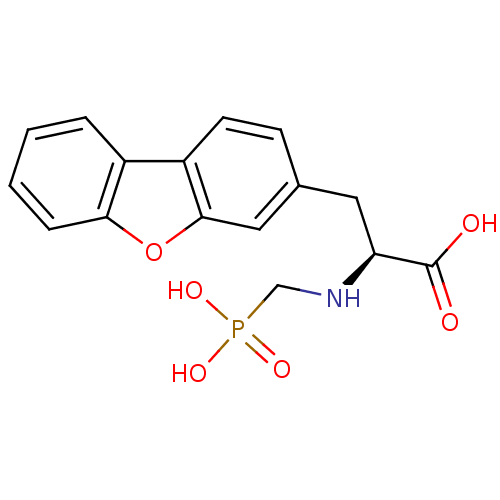 ((S)-3-(dibenzo[b,d]furan-3-yl)-2-(phosphonomethyla...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In Vitro inhibition of recombinant human endothelin converting enzyme-1 | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50084893 ((S)-3-(dibenzo[b,d]furan-3-yl)-2-(phosphonomethyla...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO) Curated by ChEMBL | Assay Description Inhibition of human somatic ECE1 | J Med Chem 53: 208-20 (2010) Article DOI: 10.1021/jm9010803 BindingDB Entry DOI: 10.7270/Q2736R06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50084892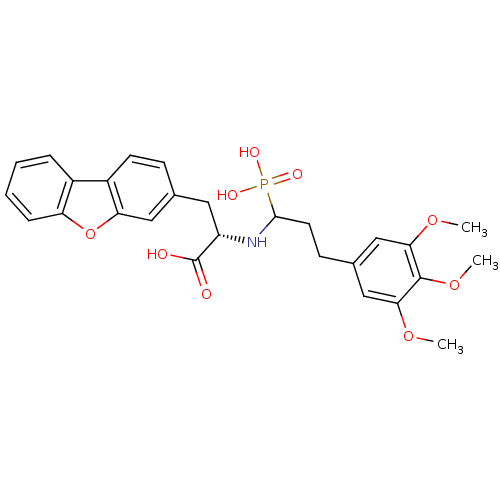 (3-Dibenzofuran-3-yl-2-[1-phosphono-3-(3,4,5-trimet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In Vitro inhibition of recombinant human endothelin converting enzyme-1 | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50119746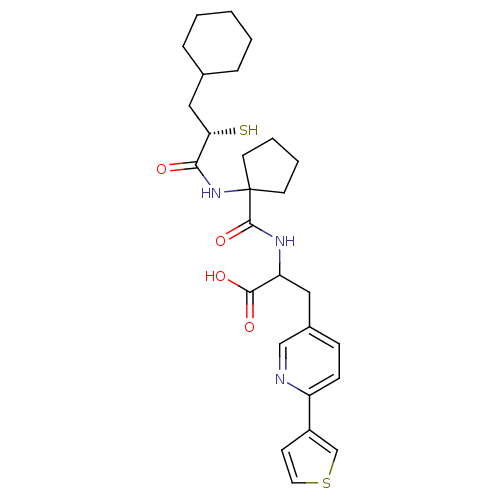 (2-{[1-((S)-3-Cyclohexyl-2-mercapto-propionylamino)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Homo sapiens (human) endothelin converting enzyme-1 | Citation and Details Article DOI: 10.1007/s00044-012-0433-z BindingDB Entry DOI: 10.7270/Q2S185DK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50119746 (2-{[1-((S)-3-Cyclohexyl-2-mercapto-propionylamino)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibition of Endothelin-converting enzyme 1. | Bioorg Med Chem Lett 12: 3059-62 (2002) BindingDB Entry DOI: 10.7270/Q26D5SCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50291557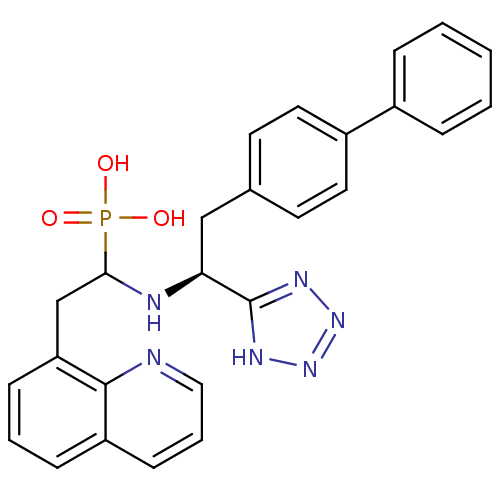 (CHEMBL268761 | {1-[(S)-2-Biphenyl-4-yl-1-(1H-tetra...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition against recombinant human Endothelin converting enzyme 1 activity | Bioorg Med Chem Lett 7: 1059-1064 (1997) Article DOI: 10.1016/S0960-894X(97)00159-5 BindingDB Entry DOI: 10.7270/Q2WH2QGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50084895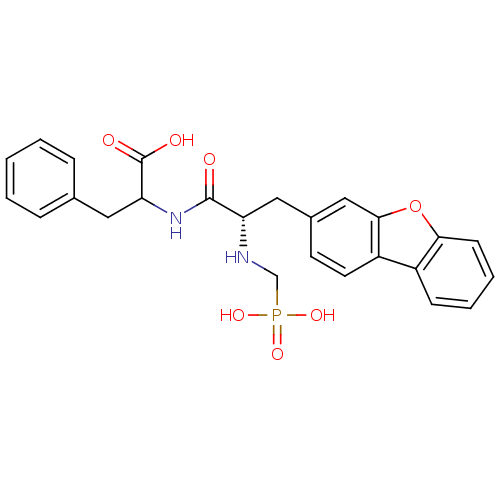 (2-[(S)-3-Dibenzofuran-3-yl-2-(phosphonomethyl-amin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In Vitro inhibition of recombinant human endothelin converting enzyme-1 | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50119757 (2-{[1-((S)-2-Mercapto-hexanoylamino)-cyclopentanec...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibition of Endothelin-converting enzyme 1. | Bioorg Med Chem Lett 12: 3059-62 (2002) BindingDB Entry DOI: 10.7270/Q26D5SCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50119757 (2-{[1-((S)-2-Mercapto-hexanoylamino)-cyclopentanec...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Homo sapiens (human) endothelin converting enzyme-1 | Citation and Details Article DOI: 10.1007/s00044-012-0433-z BindingDB Entry DOI: 10.7270/Q2S185DK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50064124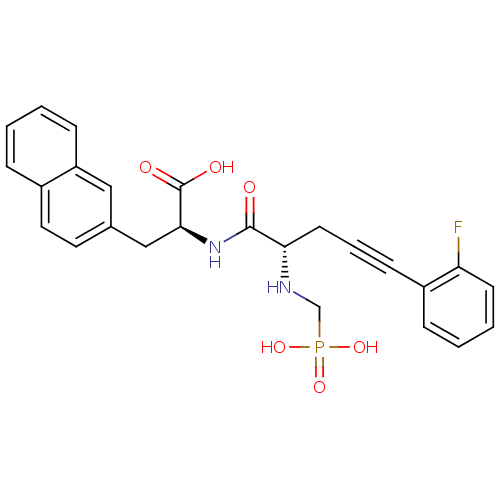 ((S)-2-[(S)-5-(2-Fluoro-phenyl)-2-(phosphonomethyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibitory activity was assessed on CHO cells expressing recombinant human Endothelin-converting enzyme 1 (ECE-1). | J Med Chem 41: 1513-23 (1998) Article DOI: 10.1021/jm970787c BindingDB Entry DOI: 10.7270/Q2PV6M1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 420 total ) | Next | Last >> |