

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22925 ((8R)-3-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.0330 | -59.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | Bioorg Med Chem Lett 13: 1115-8 (2003) Article DOI: 10.1016/S0960-894X(03)00026-X BindingDB Entry DOI: 10.7270/Q29Z936D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22926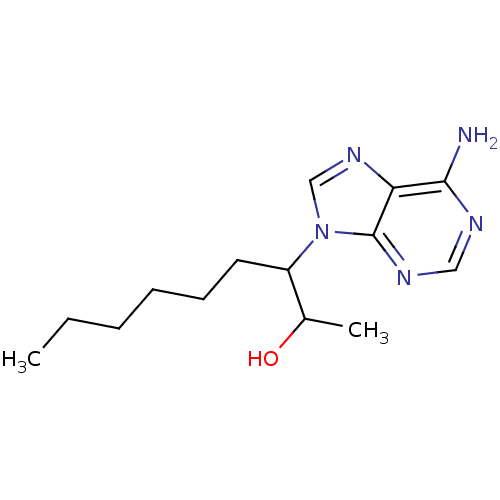 (3-(6-amino-9H-purin-9-yl)nonan-2-ol | EHNA | Eryth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 37 | -42.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | Bioorg Med Chem Lett 13: 1115-8 (2003) Article DOI: 10.1016/S0960-894X(03)00026-X BindingDB Entry DOI: 10.7270/Q29Z936D | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22917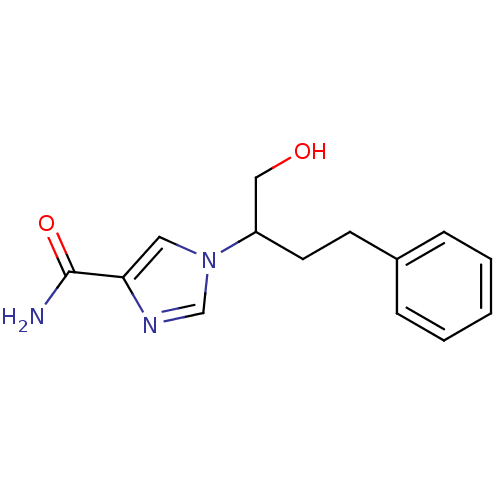 (1-(1-hydroxy-4-phenylbutan-2-yl)-1H-imidazole-4-ca...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 5.90E+3 | -29.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | Bioorg Med Chem Lett 13: 1115-8 (2003) Article DOI: 10.1016/S0960-894X(03)00026-X BindingDB Entry DOI: 10.7270/Q29Z936D | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22921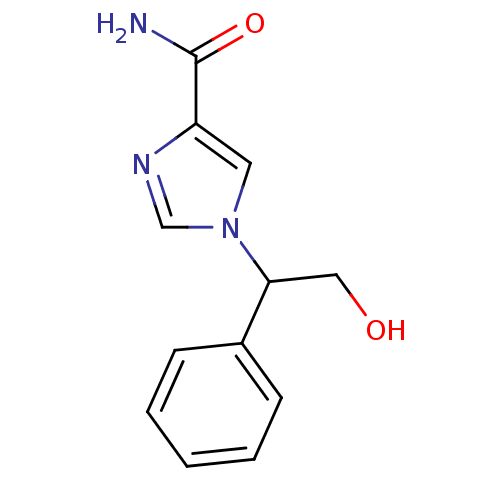 (1-(2-hydroxy-1-phenylethyl)-1H-imidazole-4-carboxa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 5.40E+4 | -24.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | Bioorg Med Chem Lett 13: 1115-8 (2003) Article DOI: 10.1016/S0960-894X(03)00026-X BindingDB Entry DOI: 10.7270/Q29Z936D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22923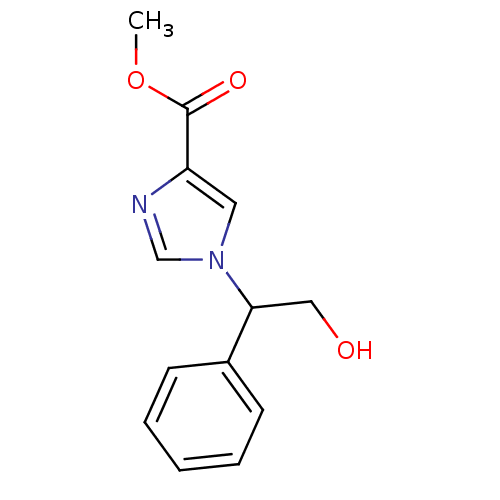 (imidazole-4-carboxylate compound, 5 | methyl 1-(2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-22.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | Bioorg Med Chem Lett 13: 1115-8 (2003) Article DOI: 10.1016/S0960-894X(03)00026-X BindingDB Entry DOI: 10.7270/Q29Z936D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22922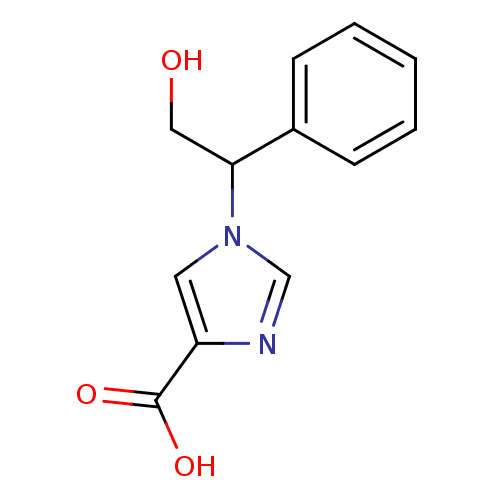 (1-(2-hydroxy-1-phenylethyl)-1H-imidazole-4-carboxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-22.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | Bioorg Med Chem Lett 13: 1115-8 (2003) Article DOI: 10.1016/S0960-894X(03)00026-X BindingDB Entry DOI: 10.7270/Q29Z936D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22924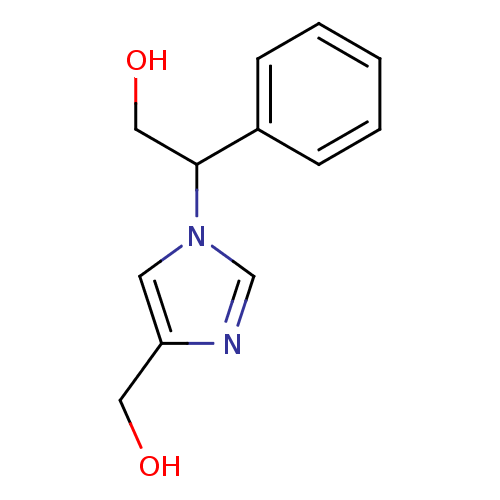 (2-[4-(hydroxymethyl)-1H-imidazol-1-yl]-2-phenyleth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-22.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | Bioorg Med Chem Lett 13: 1115-8 (2003) Article DOI: 10.1016/S0960-894X(03)00026-X BindingDB Entry DOI: 10.7270/Q29Z936D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||