 Found 46 hits Enz. Inhib. hit(s) with all data for entry = 50011356
Found 46 hits Enz. Inhib. hit(s) with all data for entry = 50011356 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19130
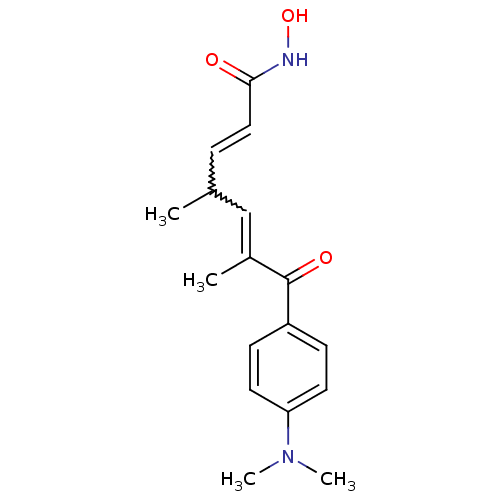
((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of partially purified recombinant human Histone deacetylase 1 |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105678
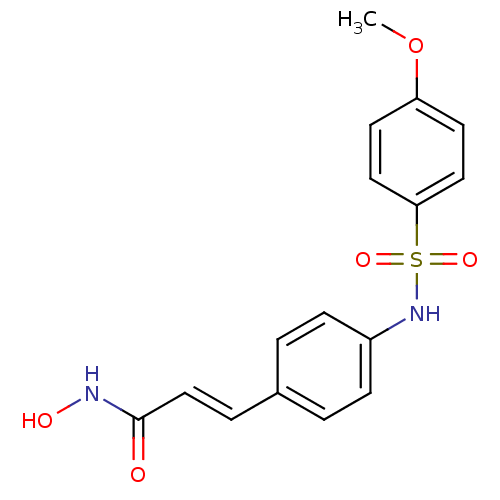
((E)-N-Hydroxy-3-[4-(4-methoxy-benzenesulfonylamino...)Show SMILES COc1ccc(cc1)S(=O)(=O)Nc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C16H16N2O5S/c1-23-14-7-9-15(10-8-14)24(21,22)18-13-5-2-12(3-6-13)4-11-16(19)17-20/h2-11,18,20H,1H3,(H,17,19)/b11-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
Concentration of compound required for acetylation of histone-4 in human T24 cancer cells |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105675
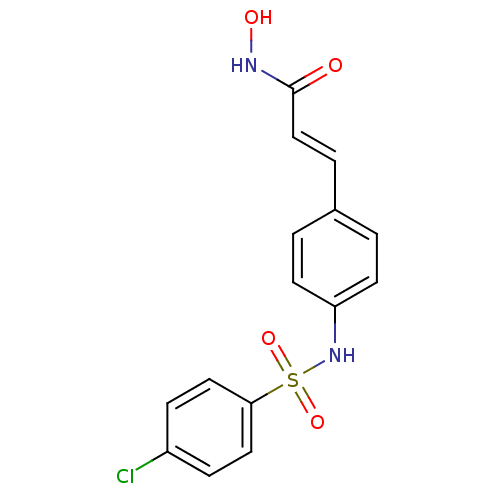
((E)-3-[4-(4-Chloro-benzenesulfonylamino)-phenyl]-N...)Show SMILES ONC(=O)\C=C\c1ccc(NS(=O)(=O)c2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C15H13ClN2O4S/c16-12-4-8-14(9-5-12)23(21,22)18-13-6-1-11(2-7-13)3-10-15(19)17-20/h1-10,18,20H,(H,17,19)/b10-3+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
Concentration of compound required for acetylation of histone-4 in human T24 cancer cells |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105689
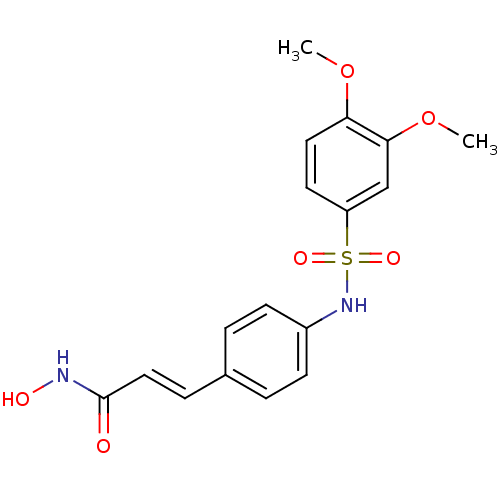
((E)-3-[4-(3,4-Dimethoxy-benzenesulfonylamino)-phen...)Show SMILES COc1ccc(cc1OC)S(=O)(=O)Nc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C17H18N2O6S/c1-24-15-9-8-14(11-16(15)25-2)26(22,23)19-13-6-3-12(4-7-13)5-10-17(20)18-21/h3-11,19,21H,1-2H3,(H,18,20)/b10-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of partially purified recombinant human Histone deacetylase 1 |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105696
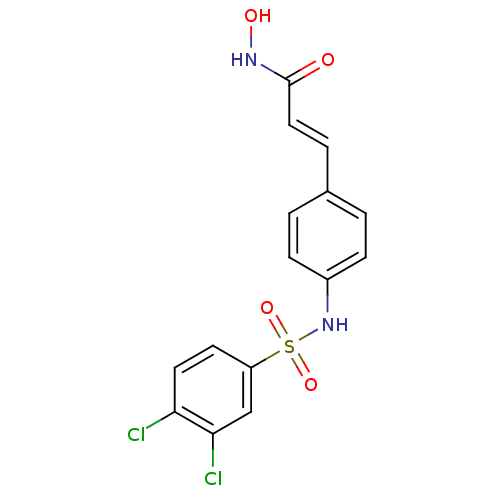
((E)-3-[4-(3,4-Dichloro-benzenesulfonylamino)-pheny...)Show SMILES ONC(=O)\C=C\c1ccc(NS(=O)(=O)c2ccc(Cl)c(Cl)c2)cc1 Show InChI InChI=1S/C15H12Cl2N2O4S/c16-13-7-6-12(9-14(13)17)24(22,23)19-11-4-1-10(2-5-11)3-8-15(20)18-21/h1-9,19,21H,(H,18,20)/b8-3+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
Concentration of compound required for acetylation of histone-4 in human T24 cancer cells |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105688
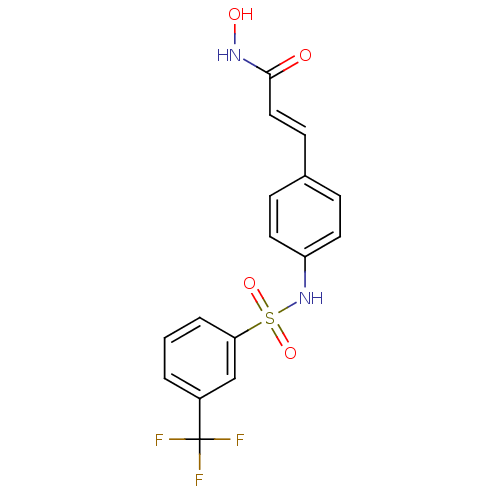
((E)-N-Hydroxy-3-[4-(3-trifluoromethyl-benzenesulfo...)Show SMILES ONC(=O)\C=C\c1ccc(NS(=O)(=O)c2cccc(c2)C(F)(F)F)cc1 Show InChI InChI=1S/C16H13F3N2O4S/c17-16(18,19)12-2-1-3-14(10-12)26(24,25)21-13-7-4-11(5-8-13)6-9-15(22)20-23/h1-10,21,23H,(H,20,22)/b9-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of partially purified recombinant human Histone deacetylase 1 |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105684
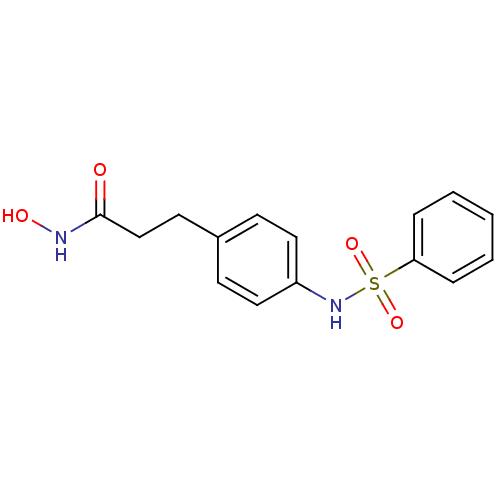
(3-(4-Benzenesulfonylamino-phenyl)-N-hydroxy-propio...)Show InChI InChI=1S/C15H16N2O4S/c18-15(16-19)11-8-12-6-9-13(10-7-12)17-22(20,21)14-4-2-1-3-5-14/h1-7,9-10,17,19H,8,11H2,(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of partially purified recombinant human Histone deacetylase 1 |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105686
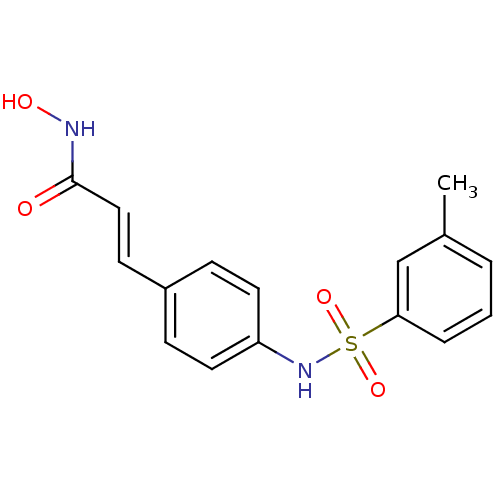
((E)-N-Hydroxy-3-[4-(toluene-3-sulfonylamino)-pheny...)Show SMILES Cc1cccc(c1)S(=O)(=O)Nc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C16H16N2O4S/c1-12-3-2-4-15(11-12)23(21,22)18-14-8-5-13(6-9-14)7-10-16(19)17-20/h2-11,18,20H,1H3,(H,17,19)/b10-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of partially purified recombinant human Histone deacetylase 1 |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105692
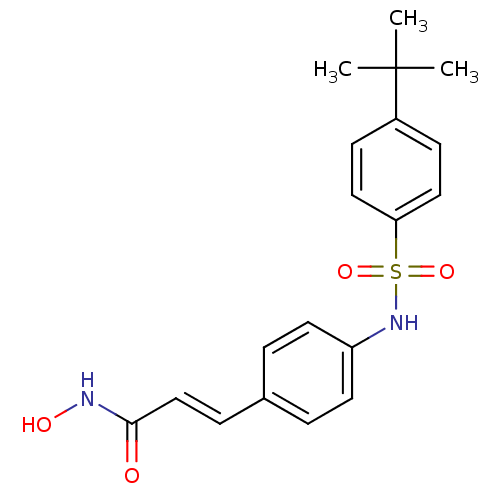
((E)-3-[4-(4-tert-Butyl-benzenesulfonylamino)-pheny...)Show SMILES CC(C)(C)c1ccc(cc1)S(=O)(=O)Nc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C19H22N2O4S/c1-19(2,3)15-7-11-17(12-8-15)26(24,25)21-16-9-4-14(5-10-16)6-13-18(22)20-23/h4-13,21,23H,1-3H3,(H,20,22)/b13-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of partially purified recombinant human Histone deacetylase 1 |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105691
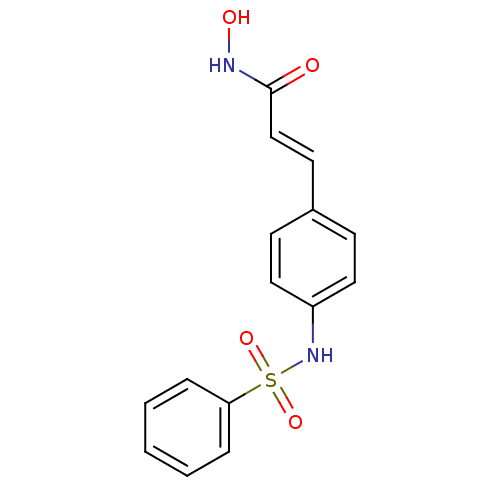
((E)-3-(4-Benzenesulfonylamino-phenyl)-N-hydroxy-ac...)Show InChI InChI=1S/C15H14N2O4S/c18-15(16-19)11-8-12-6-9-13(10-7-12)17-22(20,21)14-4-2-1-3-5-14/h1-11,17,19H,(H,16,18)/b11-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of partially purified recombinant human Histone deacetylase 1 |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105681
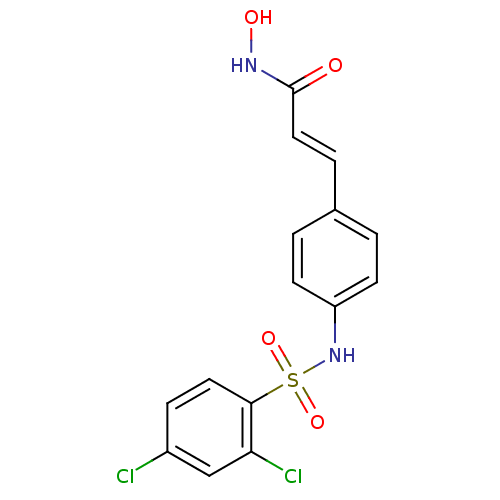
((E)-3-[4-(2,4-Dichloro-benzenesulfonylamino)-pheny...)Show SMILES ONC(=O)\C=C\c1ccc(NS(=O)(=O)c2ccc(Cl)cc2Cl)cc1 Show InChI InChI=1S/C15H12Cl2N2O4S/c16-11-4-7-14(13(17)9-11)24(22,23)19-12-5-1-10(2-6-12)3-8-15(20)18-21/h1-9,19,21H,(H,18,20)/b8-3+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of partially purified recombinant human Histone deacetylase 1 |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105677
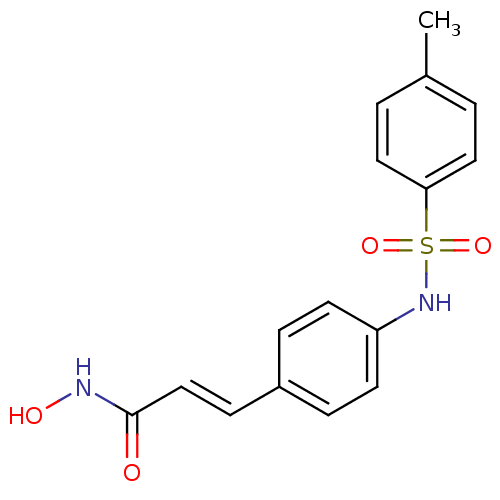
((E)-N-Hydroxy-3-[4-(toluene-4-sulfonylamino)-pheny...)Show SMILES Cc1ccc(cc1)S(=O)(=O)Nc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C16H16N2O4S/c1-12-2-9-15(10-3-12)23(21,22)18-14-7-4-13(5-8-14)6-11-16(19)17-20/h2-11,18,20H,1H3,(H,17,19)/b11-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of partially purified recombinant human Histone deacetylase 1 |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105685
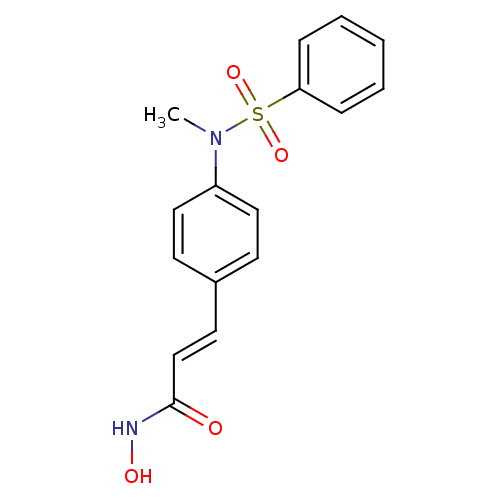
((E)-3-[4-(Benzenesulfonyl-methyl-amino)-phenyl]-N-...)Show SMILES CN(c1ccc(\C=C\C(=O)NO)cc1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C16H16N2O4S/c1-18(23(21,22)15-5-3-2-4-6-15)14-10-7-13(8-11-14)9-12-16(19)17-20/h2-12,20H,1H3,(H,17,19)/b12-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of partially purified recombinant human Histone deacetylase 1 |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105687

((E)-N-Hydroxy-3-[4-(2,4,5-triisopropyl-benzenesulf...)Show SMILES CC(C)c1cc(C(C)C)c(cc1C(C)C)S(=O)(=O)Nc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C24H32N2O4S/c1-15(2)20-13-22(17(5)6)23(14-21(20)16(3)4)31(29,30)26-19-10-7-18(8-11-19)9-12-24(27)25-28/h7-17,26,28H,1-6H3,(H,25,27)/b12-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of partially purified recombinant human Histone deacetylase 1 |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105683
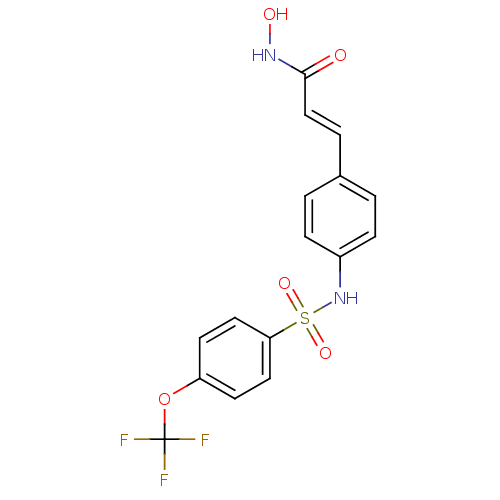
((E)-N-Hydroxy-3-[4-(4-trifluoromethoxy-benzenesulf...)Show SMILES ONC(=O)\C=C\c1ccc(NS(=O)(=O)c2ccc(OC(F)(F)F)cc2)cc1 Show InChI InChI=1S/C16H13F3N2O5S/c17-16(18,19)26-13-6-8-14(9-7-13)27(24,25)21-12-4-1-11(2-5-12)3-10-15(22)20-23/h1-10,21,23H,(H,20,22)/b10-3+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
Concentration of compound required for acetylation of histone-4 in human T24 cancer cells |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105679

((E)-N-Hydroxy-3-[4-(4-nitro-benzenesulfonylamino)-...)Show SMILES ONC(=O)\C=C\c1ccc(NS(=O)(=O)c2ccc(cc2)[N+]([O-])=O)cc1 Show InChI InChI=1S/C15H13N3O6S/c19-15(16-20)10-3-11-1-4-12(5-2-11)17-25(23,24)14-8-6-13(7-9-14)18(21)22/h1-10,17,20H,(H,16,19)/b10-3+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
Concentration of compound required for acetylation of histone-4 in human T24 cancer cells |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105693
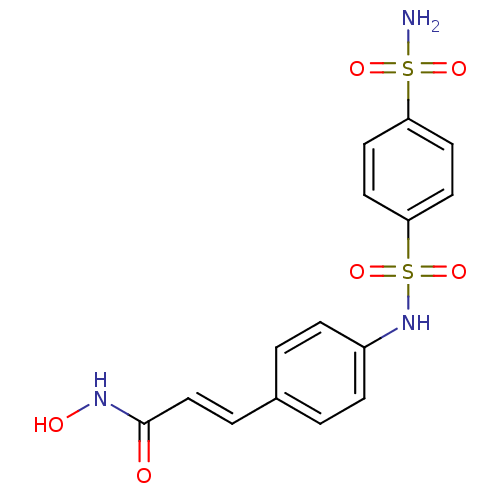
((E)-N-Hydroxy-3-[4-(4-sulfamoyl-benzenesulfonylami...)Show SMILES NS(=O)(=O)c1ccc(cc1)S(=O)(=O)Nc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C15H15N3O6S2/c16-25(21,22)13-6-8-14(9-7-13)26(23,24)18-12-4-1-11(2-5-12)3-10-15(19)17-20/h1-10,18,20H,(H,17,19)(H2,16,21,22)/b10-3+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
Concentration of compound required for acetylation of histone-4 in human T24 cancer cells |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105682
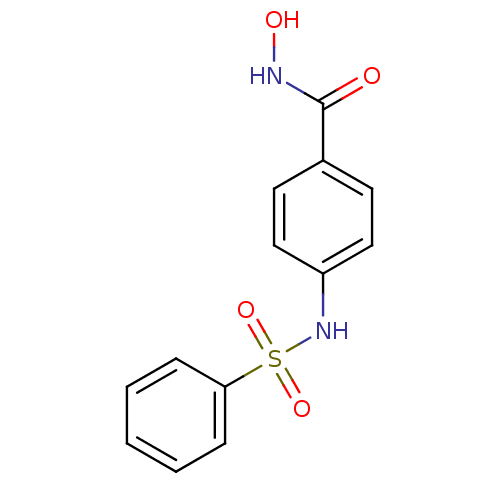
(4-Benzenesulfonylamino-N-hydroxy-benzamide | CHEMB...)Show InChI InChI=1S/C13H12N2O4S/c16-13(14-17)10-6-8-11(9-7-10)15-20(18,19)12-4-2-1-3-5-12/h1-9,15,17H,(H,14,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of partially purified recombinant human Histone deacetylase 1 |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105694
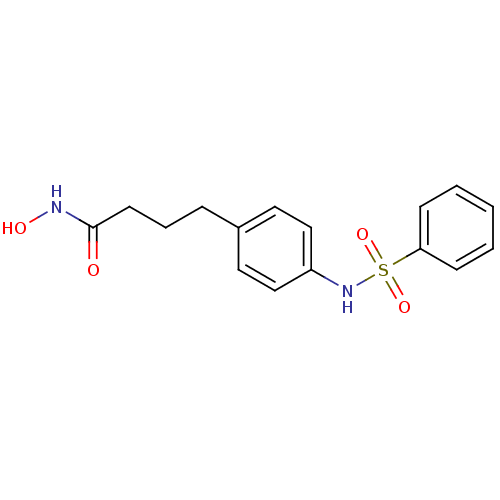
(4-(4-Benzenesulfonylamino-phenyl)-N-hydroxy-butyra...)Show InChI InChI=1S/C16H18N2O4S/c19-16(17-20)8-4-5-13-9-11-14(12-10-13)18-23(21,22)15-6-2-1-3-7-15/h1-3,6-7,9-12,18,20H,4-5,8H2,(H,17,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of partially purified recombinant human Histone deacetylase 1 |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105690
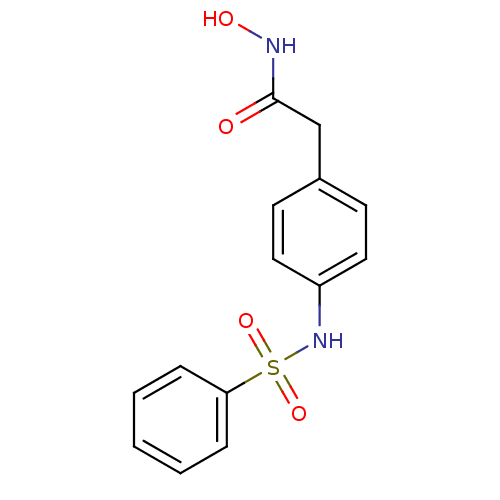
(2-(4-Benzenesulfonylamino-phenyl)-N-hydroxy-acetam...)Show InChI InChI=1S/C14H14N2O4S/c17-14(15-18)10-11-6-8-12(9-7-11)16-21(19,20)13-4-2-1-3-5-13/h1-9,16,18H,10H2,(H,15,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of partially purified recombinant human Histone deacetylase 1 |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105695
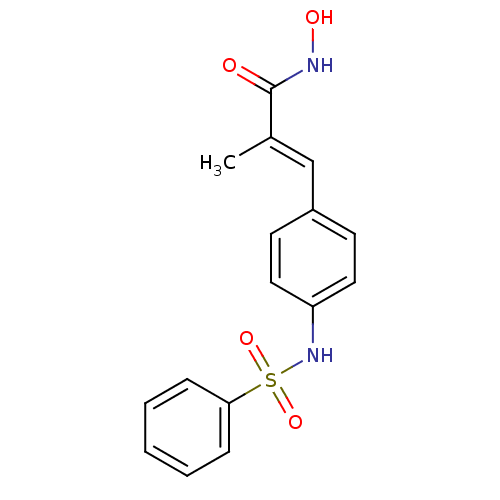
((E)-3-(4-Benzenesulfonylamino-phenyl)-N-hydroxy-2-...)Show SMILES C\C(=C/c1ccc(NS(=O)(=O)c2ccccc2)cc1)C(=O)NO Show InChI InChI=1S/C16H16N2O4S/c1-12(16(19)17-20)11-13-7-9-14(10-8-13)18-23(21,22)15-5-3-2-4-6-15/h2-11,18,20H,1H3,(H,17,19)/b12-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of partially purified recombinant human Histone deacetylase 1 |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105680
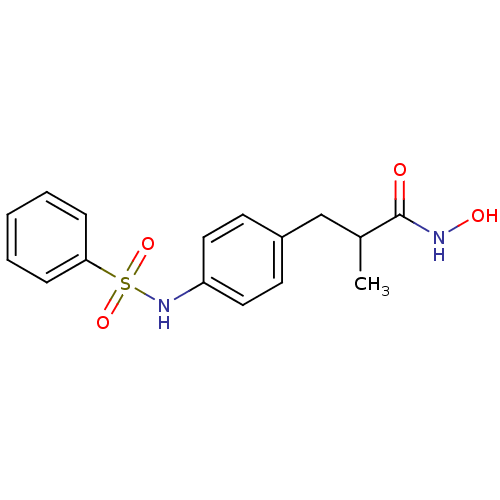
(3-(4-Benzenesulfonylamino-phenyl)-N-hydroxy-2-meth...)Show InChI InChI=1S/C16H18N2O4S/c1-12(16(19)17-20)11-13-7-9-14(10-8-13)18-23(21,22)15-5-3-2-4-6-15/h2-10,12,18,20H,11H2,1H3,(H,17,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of partially purified recombinant human Histone deacetylase 1 |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105676
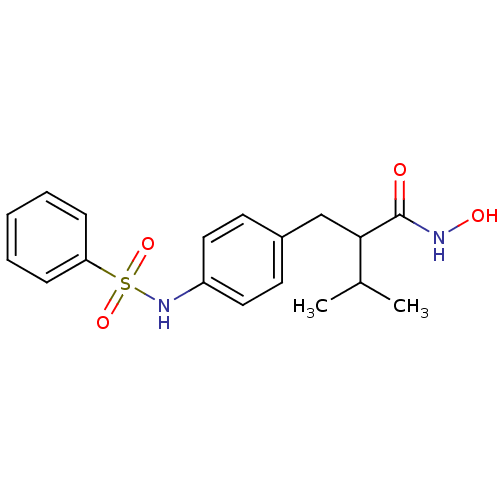
(2-(4-Benzenesulfonylamino-benzyl)-N-hydroxy-3-meth...)Show SMILES CC(C)C(Cc1ccc(NS(=O)(=O)c2ccccc2)cc1)C(=O)NO Show InChI InChI=1S/C18H22N2O4S/c1-13(2)17(18(21)19-22)12-14-8-10-15(11-9-14)20-25(23,24)16-6-4-3-5-7-16/h3-11,13,17,20,22H,12H2,1-2H3,(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of partially purified recombinant human Histone deacetylase 1 |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105681

((E)-3-[4-(2,4-Dichloro-benzenesulfonylamino)-pheny...)Show SMILES ONC(=O)\C=C\c1ccc(NS(=O)(=O)c2ccc(Cl)cc2Cl)cc1 Show InChI InChI=1S/C15H12Cl2N2O4S/c16-11-4-7-14(13(17)9-11)24(22,23)19-12-5-1-10(2-6-12)3-8-15(20)18-21/h1-9,19,21H,(H,18,20)/b8-3+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
Concentration of compound required for acetylation of histone-4 in human T24 cancer cells |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105696

((E)-3-[4-(3,4-Dichloro-benzenesulfonylamino)-pheny...)Show SMILES ONC(=O)\C=C\c1ccc(NS(=O)(=O)c2ccc(Cl)c(Cl)c2)cc1 Show InChI InChI=1S/C15H12Cl2N2O4S/c16-13-7-6-12(9-14(13)17)24(22,23)19-11-4-1-10(2-5-11)3-8-15(20)18-21/h1-9,19,21H,(H,18,20)/b8-3+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
Concentration of compound required for acetylation of histone-4 in human T24 cancer cells |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105688

((E)-N-Hydroxy-3-[4-(3-trifluoromethyl-benzenesulfo...)Show SMILES ONC(=O)\C=C\c1ccc(NS(=O)(=O)c2cccc(c2)C(F)(F)F)cc1 Show InChI InChI=1S/C16H13F3N2O4S/c17-16(18,19)12-2-1-3-14(10-12)26(24,25)21-13-7-4-11(5-8-13)6-9-15(22)20-23/h1-10,21,23H,(H,20,22)/b9-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
Concentration of compound required for acetylation of histone-4 in human T24 cancer cells |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105691

((E)-3-(4-Benzenesulfonylamino-phenyl)-N-hydroxy-ac...)Show InChI InChI=1S/C15H14N2O4S/c18-15(16-19)11-8-12-6-9-13(10-7-12)17-22(20,21)14-4-2-1-3-5-14/h1-11,17,19H,(H,16,18)/b11-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of partially purified recombinant human Histone deacetylase 1 |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105692

((E)-3-[4-(4-tert-Butyl-benzenesulfonylamino)-pheny...)Show SMILES CC(C)(C)c1ccc(cc1)S(=O)(=O)Nc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C19H22N2O4S/c1-19(2,3)15-7-11-17(12-8-15)26(24,25)21-16-9-4-14(5-10-16)6-13-18(22)20-23/h4-13,21,23H,1-3H3,(H,20,22)/b13-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of partially purified recombinant human Histone deacetylase 1 |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105694

(4-(4-Benzenesulfonylamino-phenyl)-N-hydroxy-butyra...)Show InChI InChI=1S/C16H18N2O4S/c19-16(17-20)8-4-5-13-9-11-14(12-10-13)18-23(21,22)15-6-2-1-3-7-15/h1-3,6-7,9-12,18,20H,4-5,8H2,(H,17,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of partially purified recombinant human Histone deacetylase 1 |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105685

((E)-3-[4-(Benzenesulfonyl-methyl-amino)-phenyl]-N-...)Show SMILES CN(c1ccc(\C=C\C(=O)NO)cc1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C16H16N2O4S/c1-18(23(21,22)15-5-3-2-4-6-15)14-10-7-13(8-11-14)9-12-16(19)17-20/h2-12,20H,1H3,(H,17,19)/b12-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
Concentration of compound required for acetylation of histone-4 in human T24 cancer cells |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of partially purified recombinant human Histone deacetylase 1 |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105684

(3-(4-Benzenesulfonylamino-phenyl)-N-hydroxy-propio...)Show InChI InChI=1S/C15H16N2O4S/c18-15(16-19)11-8-12-6-9-13(10-7-12)17-22(20,21)14-4-2-1-3-5-14/h1-7,9-10,17,19H,8,11H2,(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
Concentration of compound required for acetylation of histone-4 in human T24 cancer cells |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105675

((E)-3-[4-(4-Chloro-benzenesulfonylamino)-phenyl]-N...)Show SMILES ONC(=O)\C=C\c1ccc(NS(=O)(=O)c2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C15H13ClN2O4S/c16-12-4-8-14(9-5-12)23(21,22)18-13-6-1-11(2-7-13)3-10-15(19)17-20/h1-10,18,20H,(H,17,19)/b10-3+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
Concentration of compound required for acetylation of histone-4 in human T24 cancer cells |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105687

((E)-N-Hydroxy-3-[4-(2,4,5-triisopropyl-benzenesulf...)Show SMILES CC(C)c1cc(C(C)C)c(cc1C(C)C)S(=O)(=O)Nc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C24H32N2O4S/c1-15(2)20-13-22(17(5)6)23(14-21(20)16(3)4)31(29,30)26-19-10-7-18(8-11-19)9-12-24(27)25-28/h7-17,26,28H,1-6H3,(H,25,27)/b12-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of partially purified recombinant human Histone deacetylase 1 |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105682

(4-Benzenesulfonylamino-N-hydroxy-benzamide | CHEMB...)Show InChI InChI=1S/C13H12N2O4S/c16-13(14-17)10-6-8-11(9-7-10)15-20(18,19)12-4-2-1-3-5-12/h1-9,15,17H,(H,14,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of partially purified recombinant human Histone deacetylase 1 |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105680

(3-(4-Benzenesulfonylamino-phenyl)-N-hydroxy-2-meth...)Show InChI InChI=1S/C16H18N2O4S/c1-12(16(19)17-20)11-13-7-9-14(10-8-13)18-23(21,22)15-5-3-2-4-6-15/h2-10,12,18,20H,11H2,1H3,(H,17,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
Concentration of compound required for acetylation of histone-4 in human T24 cancer cells |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105695

((E)-3-(4-Benzenesulfonylamino-phenyl)-N-hydroxy-2-...)Show SMILES C\C(=C/c1ccc(NS(=O)(=O)c2ccccc2)cc1)C(=O)NO Show InChI InChI=1S/C16H16N2O4S/c1-12(16(19)17-20)11-13-7-9-14(10-8-13)18-23(21,22)15-5-3-2-4-6-15/h2-11,18,20H,1H3,(H,17,19)/b12-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
Concentration of compound required for acetylation of histone-4 in human T24 cancer cells |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105693

((E)-N-Hydroxy-3-[4-(4-sulfamoyl-benzenesulfonylami...)Show SMILES NS(=O)(=O)c1ccc(cc1)S(=O)(=O)Nc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C15H15N3O6S2/c16-25(21,22)13-6-8-14(9-7-13)26(23,24)18-12-4-1-11(2-5-12)3-10-15(19)17-20/h1-10,18,20H,(H,17,19)(H2,16,21,22)/b10-3+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
Concentration of compound required for acetylation of histone-4 in human T24 cancer cells |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105677

((E)-N-Hydroxy-3-[4-(toluene-4-sulfonylamino)-pheny...)Show SMILES Cc1ccc(cc1)S(=O)(=O)Nc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C16H16N2O4S/c1-12-2-9-15(10-3-12)23(21,22)18-14-7-4-13(5-8-14)6-11-16(19)17-20/h2-11,18,20H,1H3,(H,17,19)/b11-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
Concentration of compound required for acetylation of histone-4 in human T24 cancer cells |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105679

((E)-N-Hydroxy-3-[4-(4-nitro-benzenesulfonylamino)-...)Show SMILES ONC(=O)\C=C\c1ccc(NS(=O)(=O)c2ccc(cc2)[N+]([O-])=O)cc1 Show InChI InChI=1S/C15H13N3O6S/c19-15(16-20)10-3-11-1-4-12(5-2-11)17-25(23,24)14-8-6-13(7-9-14)18(21)22/h1-10,17,20H,(H,16,19)/b10-3+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
Concentration of compound required for acetylation of histone-4 in human T24 cancer cells |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105690

(2-(4-Benzenesulfonylamino-phenyl)-N-hydroxy-acetam...)Show InChI InChI=1S/C14H14N2O4S/c17-14(15-18)10-11-6-8-12(9-7-11)16-21(19,20)13-4-2-1-3-5-13/h1-9,16,18H,10H2,(H,15,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
Concentration of compound required for acetylation of histone-4 in human T24 cancer cells |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105683

((E)-N-Hydroxy-3-[4-(4-trifluoromethoxy-benzenesulf...)Show SMILES ONC(=O)\C=C\c1ccc(NS(=O)(=O)c2ccc(OC(F)(F)F)cc2)cc1 Show InChI InChI=1S/C16H13F3N2O5S/c17-16(18,19)26-13-6-8-14(9-7-13)27(24,25)21-12-4-1-11(2-5-12)3-10-15(22)20-23/h1-10,21,23H,(H,20,22)/b10-3+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
Concentration of compound required for acetylation of histone-4 in human T24 cancer cells |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105686

((E)-N-Hydroxy-3-[4-(toluene-3-sulfonylamino)-pheny...)Show SMILES Cc1cccc(c1)S(=O)(=O)Nc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C16H16N2O4S/c1-12-3-2-4-15(11-12)23(21,22)18-14-8-5-13(6-9-14)7-10-16(19)17-20/h2-11,18,20H,1H3,(H,17,19)/b10-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
Concentration of compound required for acetylation of histone-4 in human T24 cancer cells |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105689

((E)-3-[4-(3,4-Dimethoxy-benzenesulfonylamino)-phen...)Show SMILES COc1ccc(cc1OC)S(=O)(=O)Nc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C17H18N2O6S/c1-24-15-9-8-14(11-16(15)25-2)26(22,23)19-13-6-3-12(4-7-13)5-10-17(20)18-21/h3-11,19,21H,1-2H3,(H,18,20)/b10-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
Concentration of compound required for acetylation of histone-4 in human T24 cancer cells |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105678

((E)-N-Hydroxy-3-[4-(4-methoxy-benzenesulfonylamino...)Show SMILES COc1ccc(cc1)S(=O)(=O)Nc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C16H16N2O5S/c1-23-14-7-9-15(10-8-14)24(21,22)18-13-5-2-12(3-6-13)4-11-16(19)17-20/h2-11,18,20H,1H3,(H,17,19)/b11-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
Concentration of compound required for acetylation of histone-4 in human T24 cancer cells |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105676

(2-(4-Benzenesulfonylamino-benzyl)-N-hydroxy-3-meth...)Show SMILES CC(C)C(Cc1ccc(NS(=O)(=O)c2ccccc2)cc1)C(=O)NO Show InChI InChI=1S/C18H22N2O4S/c1-13(2)17(18(21)19-22)12-14-8-10-15(11-9-14)20-25(23,24)16-6-4-3-5-7-16/h3-11,13,17,20,22H,12H2,1-2H3,(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
Concentration of compound required for acetylation of histone-4 in human T24 cancer cells |
Bioorg Med Chem Lett 11: 2847-50 (2001)
BindingDB Entry DOI: 10.7270/Q2SF2VF5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data