 Found 28 hits Enz. Inhib. hit(s) with all data for entry = 50001054
Found 28 hits Enz. Inhib. hit(s) with all data for entry = 50001054 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Thymidylate synthase
(Escherichia coli) | BDBM50008294

(2-(4-(((2-amino-4-oxo-1,4-dihydroquinazolin-6-yl)m...)Show SMILES Nc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 Show InChI InChI=1S/C24H23N5O6/c1-2-11-29(13-14-3-8-18-17(12-14)22(33)28-24(25)27-18)16-6-4-15(5-7-16)21(32)26-19(23(34)35)9-10-20(30)31/h1,3-8,12,19H,9-11,13H2,(H,26,32)(H,30,31)(H,34,35)(H3,25,27,28,33) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Thymidylate synthase of E. coli (Ki) |
J Med Chem 34: 1925-34 (1991)
BindingDB Entry DOI: 10.7270/Q25Q4V1Q |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Escherichia coli) | BDBM50012244
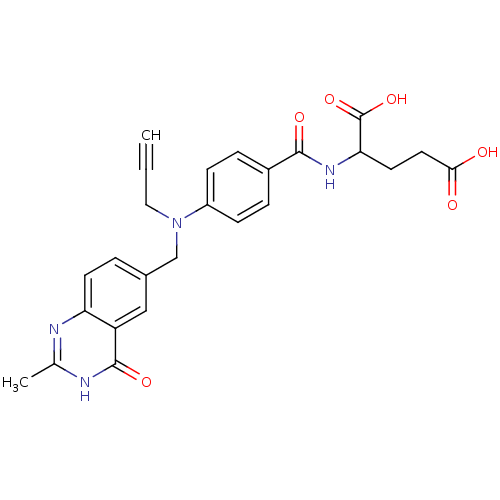
(2-{4-[(2-Methyl-4-oxo-3,4-dihydro-quinazolin-6-ylm...)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 Show InChI InChI=1S/C25H24N4O6/c1-3-12-29(14-16-4-9-20-19(13-16)24(33)27-15(2)26-20)18-7-5-17(6-8-18)23(32)28-21(25(34)35)10-11-22(30)31/h1,4-9,13,21H,10-12,14H2,2H3,(H,28,32)(H,30,31)(H,34,35)(H,26,27,33) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Thymidylate synthase of E. coli (Ki) |
J Med Chem 34: 1925-34 (1991)
BindingDB Entry DOI: 10.7270/Q25Q4V1Q |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50012244

(2-{4-[(2-Methyl-4-oxo-3,4-dihydro-quinazolin-6-ylm...)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 Show InChI InChI=1S/C25H24N4O6/c1-3-12-29(14-16-4-9-20-19(13-16)24(33)27-15(2)26-20)18-7-5-17(6-8-18)23(32)28-21(25(34)35)10-11-22(30)31/h1,4-9,13,21H,10-12,14H2,2H3,(H,28,32)(H,30,31)(H,34,35)(H,26,27,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Thymidylate synthase (Ki) |
J Med Chem 34: 1925-34 (1991)
BindingDB Entry DOI: 10.7270/Q25Q4V1Q |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50008294

(2-(4-(((2-amino-4-oxo-1,4-dihydroquinazolin-6-yl)m...)Show SMILES Nc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 Show InChI InChI=1S/C24H23N5O6/c1-2-11-29(13-14-3-8-18-17(12-14)22(33)28-24(25)27-18)16-6-4-15(5-7-16)21(32)26-19(23(34)35)9-10-20(30)31/h1,3-8,12,19H,9-11,13H2,(H,26,32)(H,30,31)(H,34,35)(H3,25,27,28,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Thymidylate synthase (Ki) |
J Med Chem 34: 1925-34 (1991)
BindingDB Entry DOI: 10.7270/Q25Q4V1Q |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50011244

(6-{[(4-Benzenesulfonyl-phenyl)-prop-2-ynyl-amino]-...)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(cc3)S(=O)(=O)c3ccccc3)cc2c(=O)[nH]1 Show InChI InChI=1S/C25H21N3O3S/c1-3-15-28(17-19-9-14-24-23(16-19)25(29)27-18(2)26-24)20-10-12-22(13-11-20)32(30,31)21-7-5-4-6-8-21/h1,4-14,16H,15,17H2,2H3,(H,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Thymidylate synthase (Ki) |
J Med Chem 34: 1925-34 (1991)
BindingDB Entry DOI: 10.7270/Q25Q4V1Q |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Escherichia coli) | BDBM50011244

(6-{[(4-Benzenesulfonyl-phenyl)-prop-2-ynyl-amino]-...)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(cc3)S(=O)(=O)c3ccccc3)cc2c(=O)[nH]1 Show InChI InChI=1S/C25H21N3O3S/c1-3-15-28(17-19-9-14-24-23(16-19)25(29)27-18(2)26-24)20-10-12-22(13-11-20)32(30,31)21-7-5-4-6-8-21/h1,4-14,16H,15,17H2,2H3,(H,26,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Thymidylate synthase of E. coli (Ki) |
J Med Chem 34: 1925-34 (1991)
BindingDB Entry DOI: 10.7270/Q25Q4V1Q |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50005203
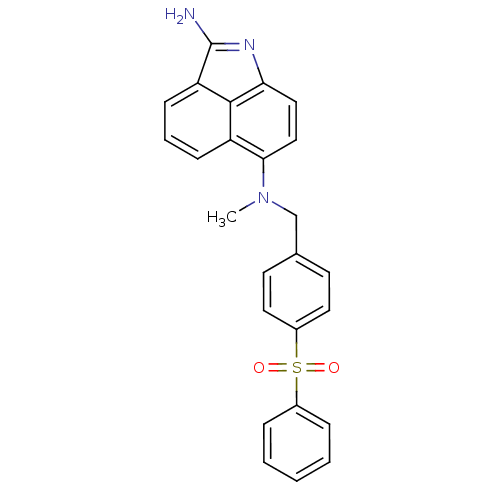
((4-Benzenesulfonyl-benzyl)-(2-imino-1-methyl-1,2-d...)Show SMILES CN(Cc1ccc(cc1)S(=O)(=O)c1ccccc1)c1ccc2N=C(N)c3cccc1c23 |t:24| Show InChI InChI=1S/C25H21N3O2S/c1-28(23-15-14-22-24-20(23)8-5-9-21(24)25(26)27-22)16-17-10-12-19(13-11-17)31(29,30)18-6-3-2-4-7-18/h2-15H,16H2,1H3,(H2,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Thymidylate synthase (Ki) |
J Med Chem 34: 1925-34 (1991)
BindingDB Entry DOI: 10.7270/Q25Q4V1Q |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50005330
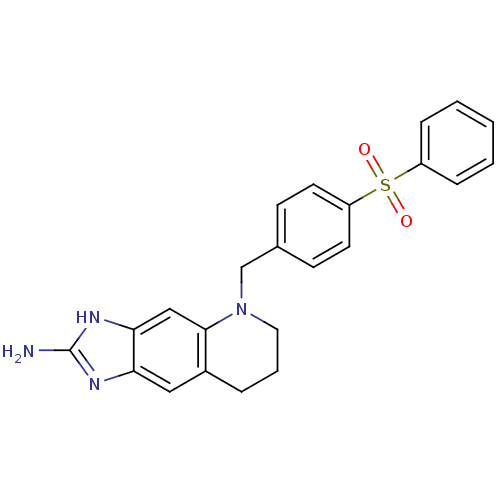
(5-(4-Benzenesulfonyl-benzyl)-5,6,7,8-tetrahydro-1H...)Show SMILES Nc1nc2cc3CCCN(Cc4ccc(cc4)S(=O)(=O)c4ccccc4)c3cc2[nH]1 Show InChI InChI=1S/C23H22N4O2S/c24-23-25-20-13-17-5-4-12-27(22(17)14-21(20)26-23)15-16-8-10-19(11-9-16)30(28,29)18-6-2-1-3-7-18/h1-3,6-11,13-14H,4-5,12,15H2,(H3,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Thymidylate synthase (Ki) |
J Med Chem 34: 1925-34 (1991)
BindingDB Entry DOI: 10.7270/Q25Q4V1Q |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Escherichia coli) | BDBM50012245

(6-{[(4-Benzenesulfonyl-3-trifluoromethyl-phenyl)-p...)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(c(c3)C(F)(F)F)S(=O)(=O)c3ccccc3)cc2c(=O)[nH]1 Show InChI InChI=1S/C26H20F3N3O3S/c1-3-13-32(16-18-9-11-23-21(14-18)25(33)31-17(2)30-23)19-10-12-24(22(15-19)26(27,28)29)36(34,35)20-7-5-4-6-8-20/h1,4-12,14-15H,13,16H2,2H3,(H,30,31,33) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Thymidylate synthase of E. coli (Ki) |
J Med Chem 34: 1925-34 (1991)
BindingDB Entry DOI: 10.7270/Q25Q4V1Q |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Escherichia coli) | BDBM50005330

(5-(4-Benzenesulfonyl-benzyl)-5,6,7,8-tetrahydro-1H...)Show SMILES Nc1nc2cc3CCCN(Cc4ccc(cc4)S(=O)(=O)c4ccccc4)c3cc2[nH]1 Show InChI InChI=1S/C23H22N4O2S/c24-23-25-20-13-17-5-4-12-27(22(17)14-21(20)26-23)15-16-8-10-19(11-9-16)30(28,29)18-6-2-1-3-7-18/h1-3,6-11,13-14H,4-5,12,15H2,(H3,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Thymidylate synthase of E. coli (Ki) |
J Med Chem 34: 1925-34 (1991)
BindingDB Entry DOI: 10.7270/Q25Q4V1Q |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50012245

(6-{[(4-Benzenesulfonyl-3-trifluoromethyl-phenyl)-p...)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(c(c3)C(F)(F)F)S(=O)(=O)c3ccccc3)cc2c(=O)[nH]1 Show InChI InChI=1S/C26H20F3N3O3S/c1-3-13-32(16-18-9-11-23-21(14-18)25(33)31-17(2)30-23)19-10-12-24(22(15-19)26(27,28)29)36(34,35)20-7-5-4-6-8-20/h1,4-12,14-15H,13,16H2,2H3,(H,30,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Thymidylate synthase (Ki) |
J Med Chem 34: 1925-34 (1991)
BindingDB Entry DOI: 10.7270/Q25Q4V1Q |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50005323

(5-[4-(Piperazine-1-sulfonyl)-benzyl]-5,6,7,8-tetra...)Show SMILES Nc1nc2cc3CCCN(Cc4ccc(cc4)S(=O)(=O)N4CCNCC4)c3cc2[nH]1 Show InChI InChI=1S/C21H26N6O2S/c22-21-24-18-12-16-2-1-9-26(20(16)13-19(18)25-21)14-15-3-5-17(6-4-15)30(28,29)27-10-7-23-8-11-27/h3-6,12-13,23H,1-2,7-11,14H2,(H3,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Thymidylate synthase (Ki) |
J Med Chem 34: 1925-34 (1991)
BindingDB Entry DOI: 10.7270/Q25Q4V1Q |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50012240
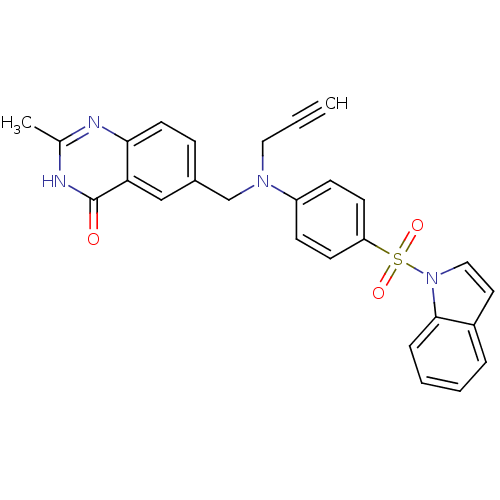
(6-({[4-(Indole-1-sulfonyl)-phenyl]-prop-2-ynyl-ami...)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(cc3)S(=O)(=O)n3ccc4ccccc34)cc2c(=O)[nH]1 Show InChI InChI=1S/C27H22N4O3S/c1-3-15-30(18-20-8-13-25-24(17-20)27(32)29-19(2)28-25)22-9-11-23(12-10-22)35(33,34)31-16-14-21-6-4-5-7-26(21)31/h1,4-14,16-17H,15,18H2,2H3,(H,28,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Thymidylate synthase (Ki) |
J Med Chem 34: 1925-34 (1991)
BindingDB Entry DOI: 10.7270/Q25Q4V1Q |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Escherichia coli) | BDBM50005323

(5-[4-(Piperazine-1-sulfonyl)-benzyl]-5,6,7,8-tetra...)Show SMILES Nc1nc2cc3CCCN(Cc4ccc(cc4)S(=O)(=O)N4CCNCC4)c3cc2[nH]1 Show InChI InChI=1S/C21H26N6O2S/c22-21-24-18-12-16-2-1-9-26(20(16)13-19(18)25-21)14-15-3-5-17(6-4-15)30(28,29)27-10-7-23-8-11-27/h3-6,12-13,23H,1-2,7-11,14H2,(H3,22,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Thymidylate synthase of E. coli (Ki) |
J Med Chem 34: 1925-34 (1991)
BindingDB Entry DOI: 10.7270/Q25Q4V1Q |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Escherichia coli) | BDBM50012240

(6-({[4-(Indole-1-sulfonyl)-phenyl]-prop-2-ynyl-ami...)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(cc3)S(=O)(=O)n3ccc4ccccc34)cc2c(=O)[nH]1 Show InChI InChI=1S/C27H22N4O3S/c1-3-15-30(18-20-8-13-25-24(17-20)27(32)29-19(2)28-25)22-9-11-23(12-10-22)35(33,34)31-16-14-21-6-4-5-7-26(21)31/h1,4-14,16-17H,15,18H2,2H3,(H,28,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Thymidylate synthase of E. coli (Ki) |
J Med Chem 34: 1925-34 (1991)
BindingDB Entry DOI: 10.7270/Q25Q4V1Q |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50012243
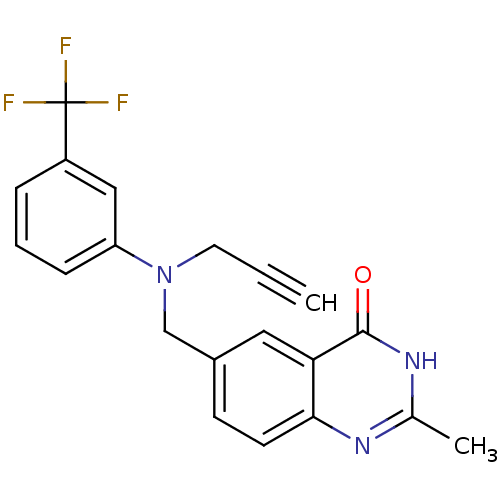
(2-Methyl-6-{[prop-2-ynyl-(3-trifluoromethyl-phenyl...)Show SMILES Cc1nc2ccc(CN(CC#C)c3cccc(c3)C(F)(F)F)cc2c(=O)[nH]1 Show InChI InChI=1S/C20H16F3N3O/c1-3-9-26(16-6-4-5-15(11-16)20(21,22)23)12-14-7-8-18-17(10-14)19(27)25-13(2)24-18/h1,4-8,10-11H,9,12H2,2H3,(H,24,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Thymidylate synthase (Ki) |
J Med Chem 34: 1925-34 (1991)
BindingDB Entry DOI: 10.7270/Q25Q4V1Q |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Escherichia coli) | BDBM50012243

(2-Methyl-6-{[prop-2-ynyl-(3-trifluoromethyl-phenyl...)Show SMILES Cc1nc2ccc(CN(CC#C)c3cccc(c3)C(F)(F)F)cc2c(=O)[nH]1 Show InChI InChI=1S/C20H16F3N3O/c1-3-9-26(16-6-4-5-15(11-16)20(21,22)23)12-14-7-8-18-17(10-14)19(27)25-13(2)24-18/h1,4-8,10-11H,9,12H2,2H3,(H,24,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Thymidylate synthase of E. coli (Ki) |
J Med Chem 34: 1925-34 (1991)
BindingDB Entry DOI: 10.7270/Q25Q4V1Q |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50005202
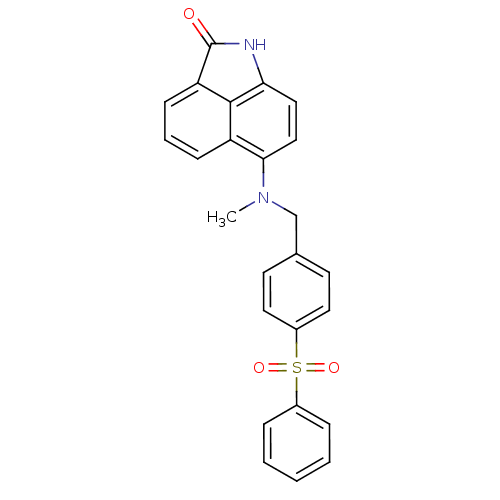
(6-[(4-Benzenesulfonyl-benzyl)-methyl-amino]-1-meth...)Show SMILES CN(Cc1ccc(cc1)S(=O)(=O)c1ccccc1)c1ccc2NC(=O)c3cccc1c23 Show InChI InChI=1S/C25H20N2O3S/c1-27(23-15-14-22-24-20(23)8-5-9-21(24)25(28)26-22)16-17-10-12-19(13-11-17)31(29,30)18-6-3-2-4-7-18/h2-15H,16H2,1H3,(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Thymidylate synthase (Ki) |
J Med Chem 34: 1925-34 (1991)
BindingDB Entry DOI: 10.7270/Q25Q4V1Q |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50005183

(1-Methyl-6-{methyl-[4-(piperazine-1-sulfonyl)-benz...)Show SMILES CN(Cc1ccc(cc1)S(=O)(=O)N1CCNCC1)c1ccc2NC(=O)c3cccc1c23 Show InChI InChI=1S/C23H24N4O3S/c1-26(21-10-9-20-22-18(21)3-2-4-19(22)23(28)25-20)15-16-5-7-17(8-6-16)31(29,30)27-13-11-24-12-14-27/h2-10,24H,11-15H2,1H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Thymidylate synthase (Ki) |
J Med Chem 34: 1925-34 (1991)
BindingDB Entry DOI: 10.7270/Q25Q4V1Q |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Escherichia coli) | BDBM50005202

(6-[(4-Benzenesulfonyl-benzyl)-methyl-amino]-1-meth...)Show SMILES CN(Cc1ccc(cc1)S(=O)(=O)c1ccccc1)c1ccc2NC(=O)c3cccc1c23 Show InChI InChI=1S/C25H20N2O3S/c1-27(23-15-14-22-24-20(23)8-5-9-21(24)25(28)26-22)16-17-10-12-19(13-11-17)31(29,30)18-6-3-2-4-7-18/h2-15H,16H2,1H3,(H,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Thymidylate synthase of E. coli (Ki) |
J Med Chem 34: 1925-34 (1991)
BindingDB Entry DOI: 10.7270/Q25Q4V1Q |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Escherichia coli) | BDBM50005203

((4-Benzenesulfonyl-benzyl)-(2-imino-1-methyl-1,2-d...)Show SMILES CN(Cc1ccc(cc1)S(=O)(=O)c1ccccc1)c1ccc2N=C(N)c3cccc1c23 |t:24| Show InChI InChI=1S/C25H21N3O2S/c1-28(23-15-14-22-24-20(23)8-5-9-21(24)25(26)27-22)16-17-10-12-19(13-11-17)31(29,30)18-6-3-2-4-7-18/h2-15H,16H2,1H3,(H2,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Thymidylate synthase of E. coli (Ki) |
J Med Chem 34: 1925-34 (1991)
BindingDB Entry DOI: 10.7270/Q25Q4V1Q |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50012242
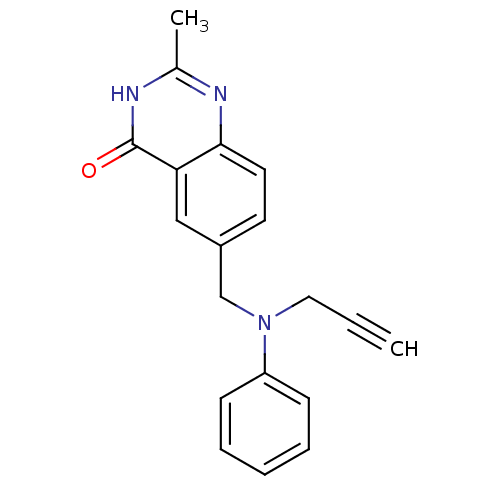
(2-Methyl-6-[(phenyl-prop-2-ynyl-amino)-methyl]-3H-...)Show InChI InChI=1S/C19H17N3O/c1-3-11-22(16-7-5-4-6-8-16)13-15-9-10-18-17(12-15)19(23)21-14(2)20-18/h1,4-10,12H,11,13H2,2H3,(H,20,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Thymidylate synthase (Ki) |
J Med Chem 34: 1925-34 (1991)
BindingDB Entry DOI: 10.7270/Q25Q4V1Q |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50012241
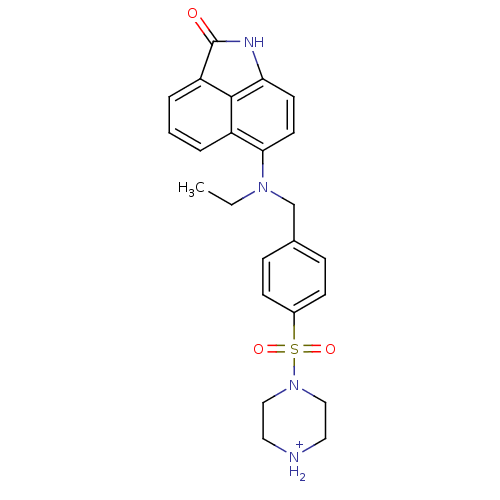
(4-(4-{[Ethyl-(2-oxo-1,2-dihydro-benzo[cd]indol-6-y...)Show SMILES CCN(Cc1ccc(cc1)S(=O)(=O)N1CC[NH2+]CC1)c1ccc2NC(=O)c3cccc1c23 Show InChI InChI=1S/C24H26N4O3S/c1-2-27(22-11-10-21-23-19(22)4-3-5-20(23)24(29)26-21)16-17-6-8-18(9-7-17)32(30,31)28-14-12-25-13-15-28/h3-11,25H,2,12-16H2,1H3,(H,26,29)/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Thymidylate synthase (Ki) |
J Med Chem 34: 1925-34 (1991)
BindingDB Entry DOI: 10.7270/Q25Q4V1Q |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Escherichia coli) | BDBM50012242

(2-Methyl-6-[(phenyl-prop-2-ynyl-amino)-methyl]-3H-...)Show InChI InChI=1S/C19H17N3O/c1-3-11-22(16-7-5-4-6-8-16)13-15-9-10-18-17(12-15)19(23)21-14(2)20-18/h1,4-10,12H,11,13H2,2H3,(H,20,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Thymidylate synthase of E. coli (Ki) |
J Med Chem 34: 1925-34 (1991)
BindingDB Entry DOI: 10.7270/Q25Q4V1Q |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Escherichia coli) | BDBM50005328
(5-[4-(Piperazine-1-sulfonyl)-benzyl]-5,6,7,8-tetra...)Show SMILES O=S(=O)(N1CCNCC1)c1ccc(CN2CCCc3cc4nc[nH]c4cc23)cc1 Show InChI InChI=1S/C21H25N5O2S/c27-29(28,26-10-7-22-8-11-26)18-5-3-16(4-6-18)14-25-9-1-2-17-12-19-20(13-21(17)25)24-15-23-19/h3-6,12-13,15,22H,1-2,7-11,14H2,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Thymidylate synthase of E. coli (Ki) |
J Med Chem 34: 1925-34 (1991)
BindingDB Entry DOI: 10.7270/Q25Q4V1Q |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50005328

(5-[4-(Piperazine-1-sulfonyl)-benzyl]-5,6,7,8-tetra...)Show SMILES O=S(=O)(N1CCNCC1)c1ccc(CN2CCCc3cc4nc[nH]c4cc23)cc1 Show InChI InChI=1S/C21H25N5O2S/c27-29(28,26-10-7-22-8-11-26)18-5-3-16(4-6-18)14-25-9-1-2-17-12-19-20(13-21(17)25)24-15-23-19/h3-6,12-13,15,22H,1-2,7-11,14H2,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Thymidylate synthase (Ki) |
J Med Chem 34: 1925-34 (1991)
BindingDB Entry DOI: 10.7270/Q25Q4V1Q |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Escherichia coli) | BDBM50005183

(1-Methyl-6-{methyl-[4-(piperazine-1-sulfonyl)-benz...)Show SMILES CN(Cc1ccc(cc1)S(=O)(=O)N1CCNCC1)c1ccc2NC(=O)c3cccc1c23 Show InChI InChI=1S/C23H24N4O3S/c1-26(21-10-9-20-22-18(21)3-2-4-19(22)23(28)25-20)15-16-5-7-17(8-6-16)31(29,30)27-13-11-24-12-14-27/h2-10,24H,11-15H2,1H3,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Thymidylate synthase of E. coli (Ki) |
J Med Chem 34: 1925-34 (1991)
BindingDB Entry DOI: 10.7270/Q25Q4V1Q |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Escherichia coli) | BDBM50012241

(4-(4-{[Ethyl-(2-oxo-1,2-dihydro-benzo[cd]indol-6-y...)Show SMILES CCN(Cc1ccc(cc1)S(=O)(=O)N1CC[NH2+]CC1)c1ccc2NC(=O)c3cccc1c23 Show InChI InChI=1S/C24H26N4O3S/c1-2-27(22-11-10-21-23-19(22)4-3-5-20(23)24(29)26-21)16-17-6-8-18(9-7-17)32(30,31)28-14-12-25-13-15-28/h3-11,25H,2,12-16H2,1H3,(H,26,29)/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Thymidylate synthase of E. coli (Ki) |
J Med Chem 34: 1925-34 (1991)
BindingDB Entry DOI: 10.7270/Q25Q4V1Q |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data