

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type-1 angiotensin II receptor (RABBIT) | BDBM50240609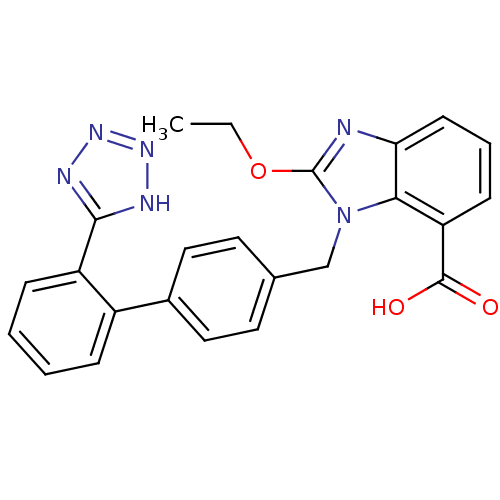 (2-Ethoxy-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmet...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin II receptor, type 1 in rat adrenal membrane | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50241491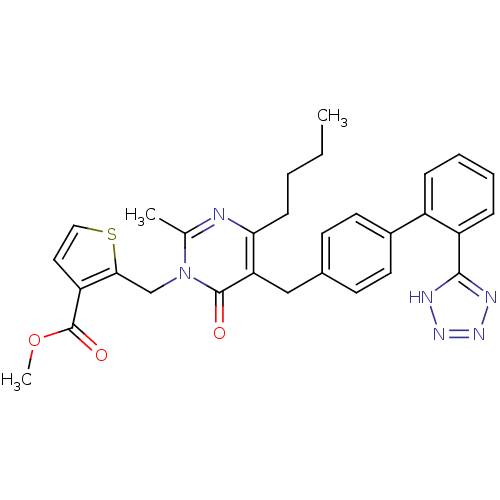 (2-{4-Butyl-2-methyl-6-oxo-5-[2'-(1H-tetrazol-5-yl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Binding affinity was evaluated towards Angiotensin II receptor, type 1 in rabbit aorta | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50049210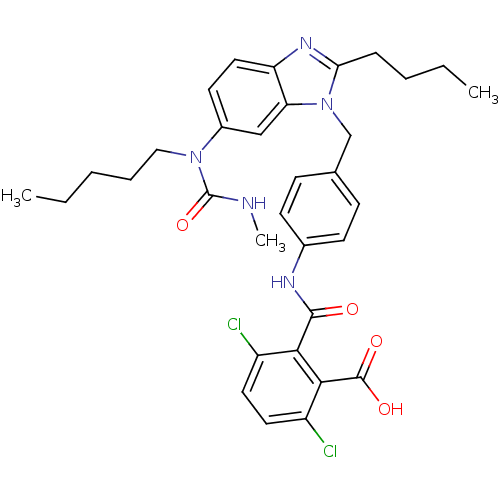 (CHEMBL348126 | N-{4-[2-Butyl-6-(3-methyl-1-pentyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Ki value was evaluated against Angiotensin II receptor, type 1 | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50035429 (4'-[2-Butyl-6-(3-cyclohexyl-ureido)-benzoimidazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Ki value was evaluated against Angiotensin II receptor, type 1 | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50035429 (4'-[2-Butyl-6-(3-cyclohexyl-ureido)-benzoimidazol-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Ki value was evaluated against Angiotensin II receptor, type 2 | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50049210 (CHEMBL348126 | N-{4-[2-Butyl-6-(3-methyl-1-pentyl-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Ki value was evaluated against Angiotensin II receptor, type 2 | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50049199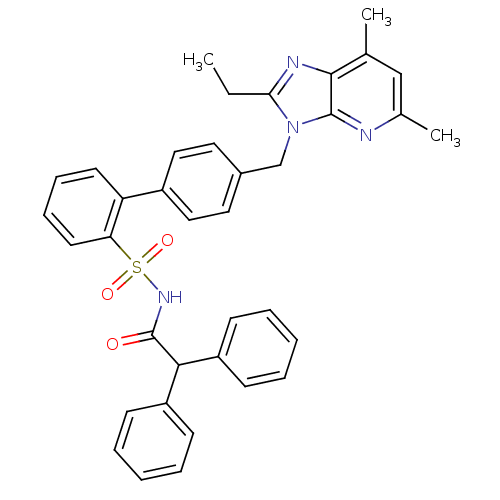 (4'-(2-Ethyl-5,7-dimethyl-imidazo[4,5-b]pyridin-3-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of Angiotensin II receptor, type 1 | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50041969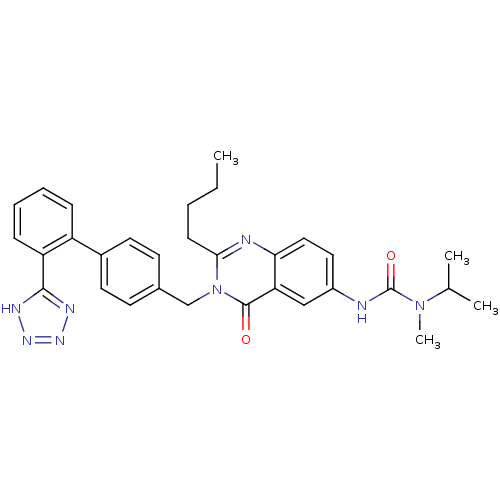 (3-{2-Butyl-4-oxo-3-[2'-(1H-tetrazol-5-yl)-biphenyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity was evaluated against Angiotensin II receptor, type 1 in rabbit aorta | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50030711 (CHEMBL338027 | L-163958 | Pentanoic acid {4-bromo-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of Angiotensin II receptor, type 2 | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50030711 (CHEMBL338027 | L-163958 | Pentanoic acid {4-bromo-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of Angiotensin II receptor, type 1 | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50038189 (4'-(2-Ethyl-5,7-dimethyl-imidazo[4,5-b]pyridin-3-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity was evaluated against Angiotensin II receptor, type 1 in rabbit aorta | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50049181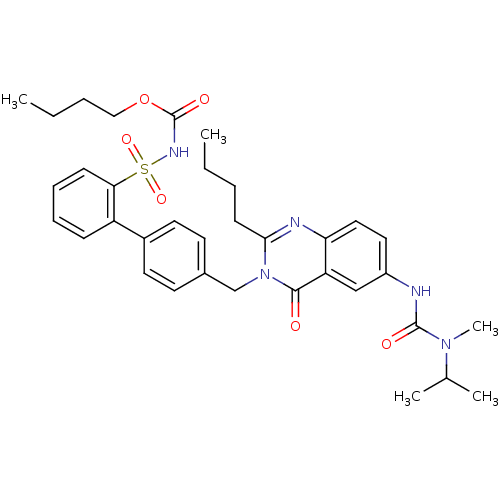 (2-Butyl-3-(2'-(butyloxycarbonylaminosulphonyl)-bip...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of Angiotensin II receptor, type 1 | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM50032353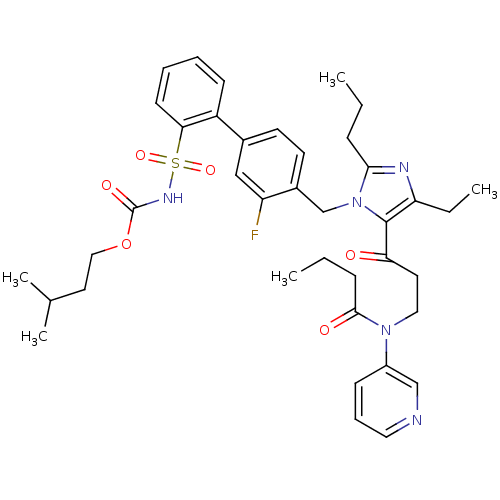 (CHEMBL100119 | N-{3-[5-Ethyl-3-(3-fluoro-2'-isopen...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity at Angiotensin II type 2 receptor. | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM50049203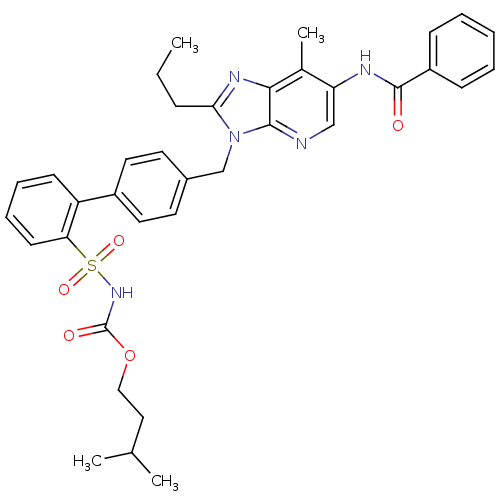 (CHEMBL160494 | L-163017 | N-[7-Methyl-2-propyl-3-(...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity at Angiotensin II type 2 receptor. | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50009718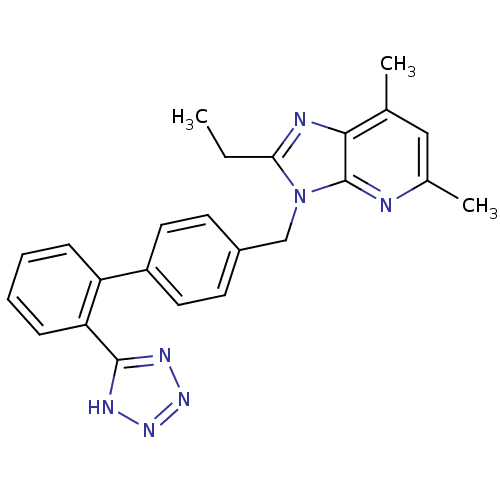 (2-Ethyl-5,7-dimethyl-3-[2'-(1H-tetrazol-5-yl)-biph...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity was evaluated against Angiotensin II receptor, type 1 in rabbit aorta | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50031491 (2-propyl-4-(methythio)-1-[[[2'-[(propylamino)carbo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of Angiotensin II receptor, type 1 | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50049214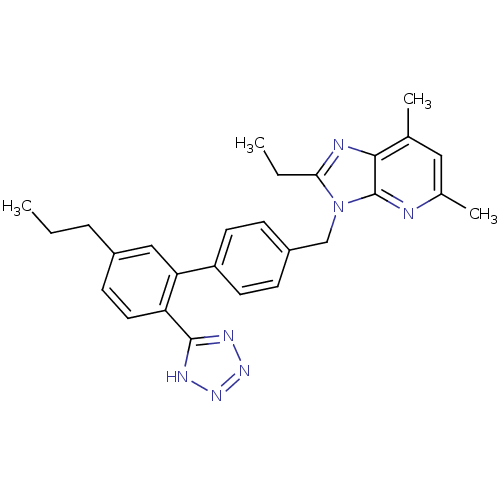 (2-Ethyl-5,7-dimethyl-3-[5'-propyl-2'-(1H-tetrazol-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of Angiotensin II receptor, type 1 | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50049185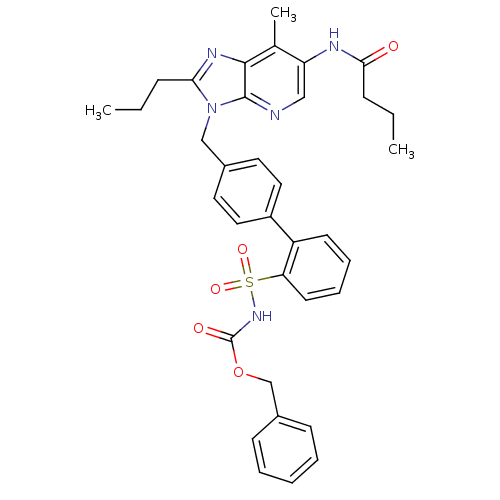 (4'-[2-Butyl-6-(3-isopropyl-3-methyl-ureido)-4-oxo-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of Angiotensin II receptor, type 1 | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM50049204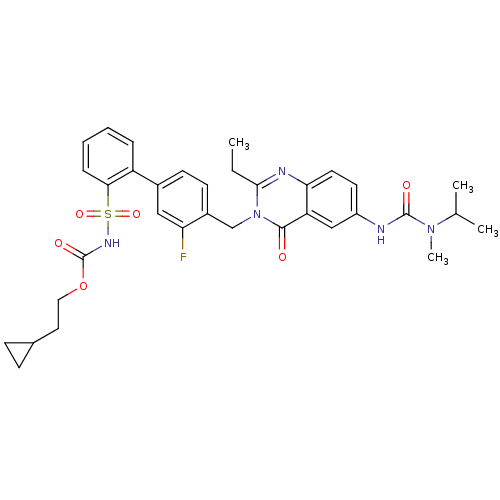 (2-ethyl-3-(3-fluoro-2'-(2-cyclopropylethyloxycarbo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity at Angiotensin II type 2 receptor. | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50035451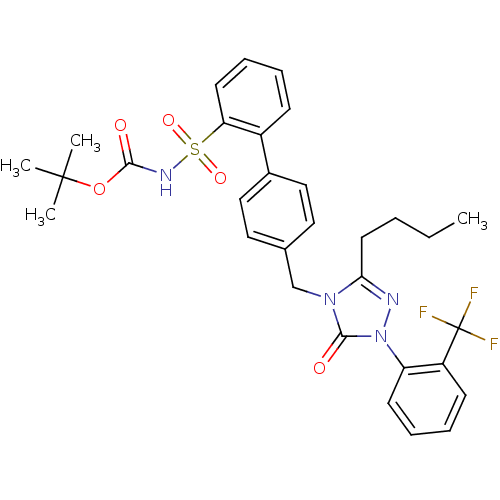 (2-{4-[3-butyl-5-oxo-1-(2-trifluoromethylphenyl)-4,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of Angiotensin II receptor, type 1 | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50049204 (2-ethyl-3-(3-fluoro-2'-(2-cyclopropylethyloxycarbo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of Angiotensin II receptor, type 1 | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50049198 (3-{2-Butyl-7-methyl-3-[2'-(1H-tetrazol-5-yl)-biphe...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of Angiotensin II receptor, type 1 | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50049182 ((S)-5-Benzyloxy-2-diphenylacetyl-6-methoxy-1,2,3,4...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity was evaluated against Angiotensin II receptor, type 2 | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50049206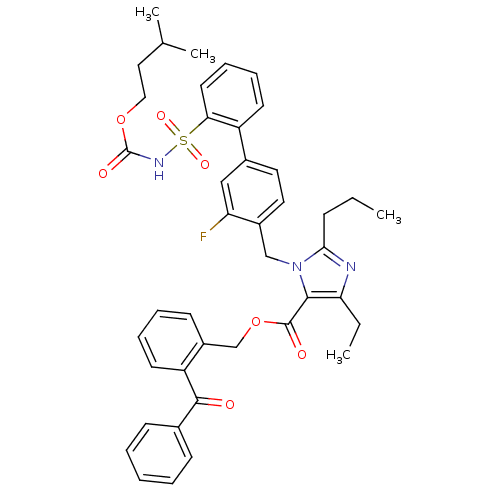 (5-Ethyl-3-(3-fluoro-2'-isopentyloxycarbaronylsulfa...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of Angiotensin II receptor, type 2 | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM82433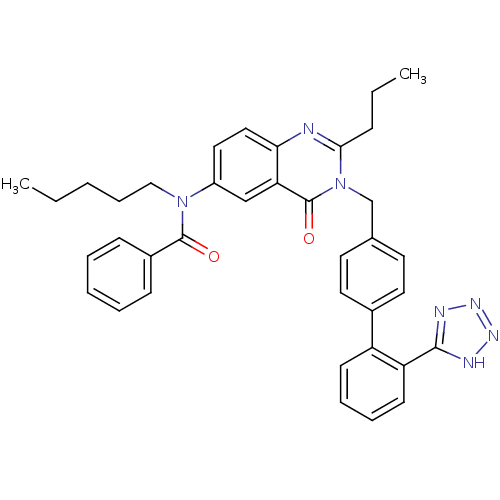 (CHEMBL302102 | L-159,689 | L-159689 | N-Pentyl-N-[...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity at Angiotensin II type 2 receptor. | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM50049185 (4'-[2-Butyl-6-(3-isopropyl-3-methyl-ureido)-4-oxo-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity at Angiotensin II type 2 receptor. | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50032362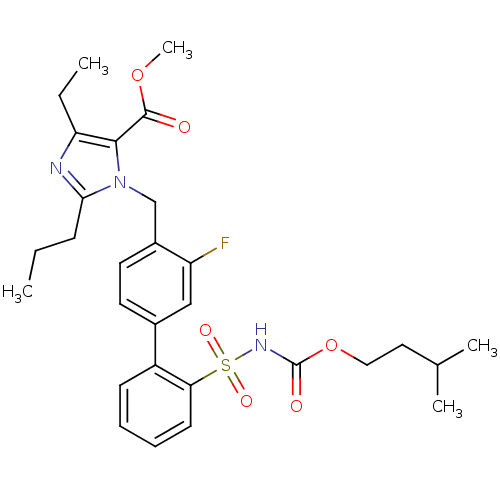 (3-(2'-Isopentyloxycarbonylsulfamoyl-3-fluoro-biphe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of Angiotensin II receptor, type 2 | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50032362 (3-(2'-Isopentyloxycarbonylsulfamoyl-3-fluoro-biphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of Angiotensin II receptor, type 1 | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50049189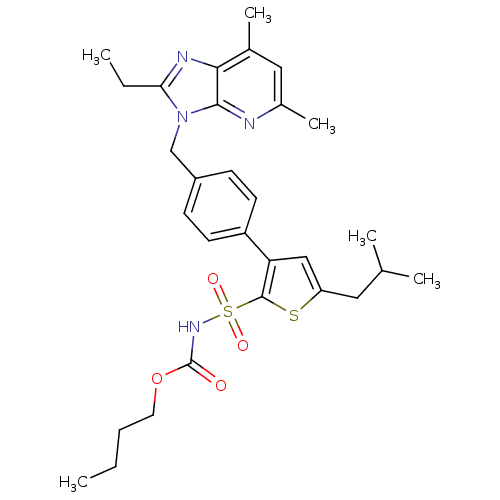 (3-[4-(2-Ethyl-57-dimethyl-imidazo[45-b]pyridin-3-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of Angiotensin II receptor, type 1 | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50369183 (CHEMBL290214 | L-163491) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of Angiotensin II receptor, type 1 | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50049195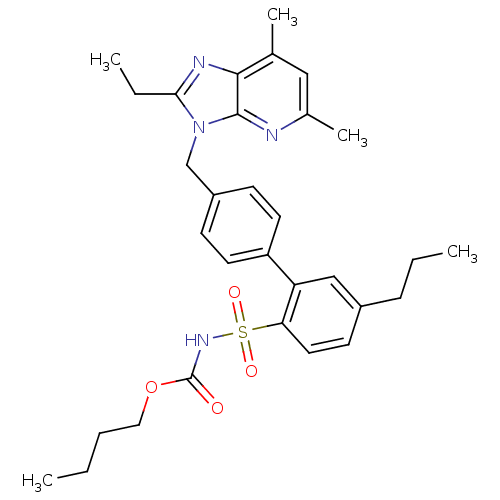 (3-[(2'-(butylsulfonylcarbamate)-3'-propyl-1,1'-bip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of Angiotensin II receptor, type 1 | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM82433 (CHEMBL302102 | L-159,689 | L-159689 | N-Pentyl-N-[...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of Angiotensin II receptor, type 1 | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50049183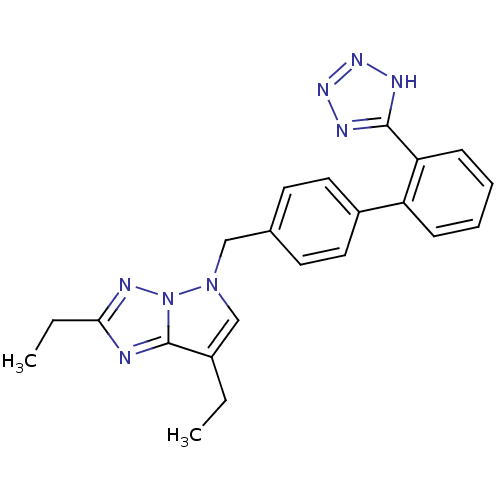 (2,7-Diethyl-5-[2'-(1H-tetrazol-5-yl)-biphenyl-4-yl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity was evaluated against angiotensin II type 1 rabbit aorta | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50049179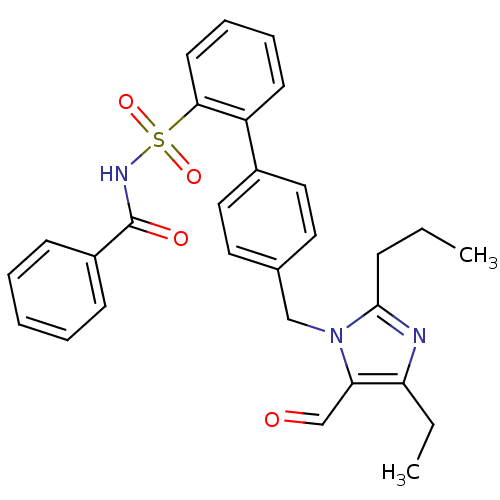 (4'-(4-Ethyl-5-formyl-2-propyl-imidazol-1-ylmethyl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of Angiotensin II receptor, type 1 | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50049189 (3-[4-(2-Ethyl-57-dimethyl-imidazo[45-b]pyridin-3-y...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of Angiotensin II receptor, type 2 | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM50049214 (2-Ethyl-5,7-dimethyl-3-[5'-propyl-2'-(1H-tetrazol-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity at Angiotensin II type 2 receptor. | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM50049181 (2-Butyl-3-(2'-(butyloxycarbonylaminosulphonyl)-bip...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity at Angiotensin II type 2 receptor. | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50041963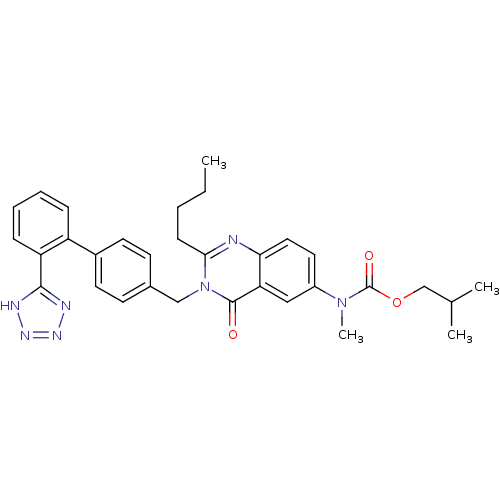 (CHEMBL24861 | {2-Butyl-4-oxo-3-[2'-(1H-tetrazol-5-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of Angiotensin II receptor, type 1 | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM50032364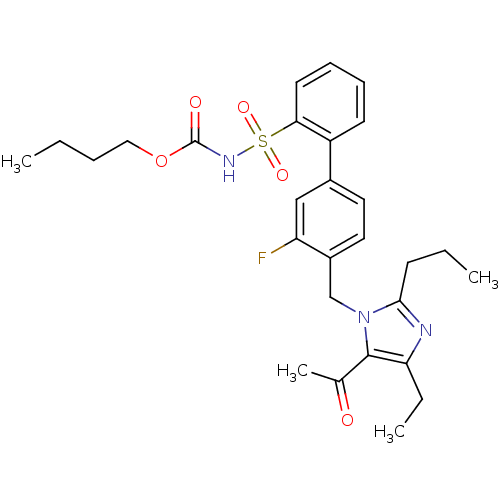 (4'-(5-Acetyl-4-ethyl-2-propyl-imidazol-1-ylmethyl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity at Angiotensin II type 2 receptor. | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50049187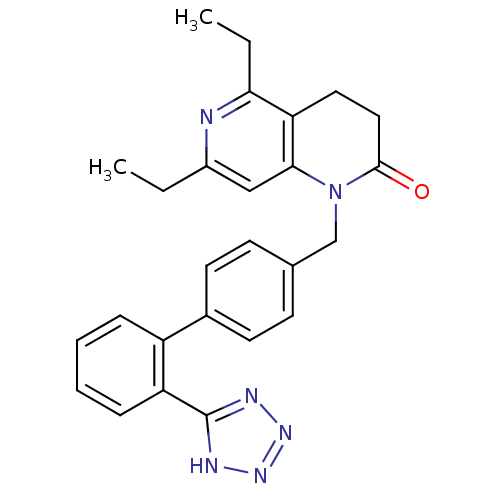 (5,7-Diethyl-1-[2'-(1H-tetrazol-5-yl)-biphenyl-4-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description In vitro antagonistic potency against Angiotensin II receptor, type 1 in guinea pig adrenal membrane | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50049193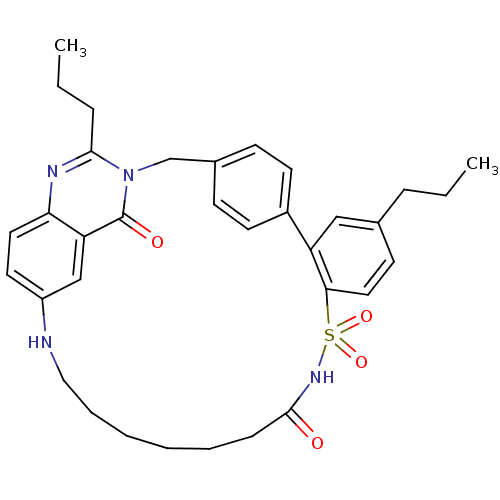 (9,28-dipropyl-13-sulfurdioxo-1,14,22,27-tetraazape...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of Angiotensin II receptor, type 1 | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50049178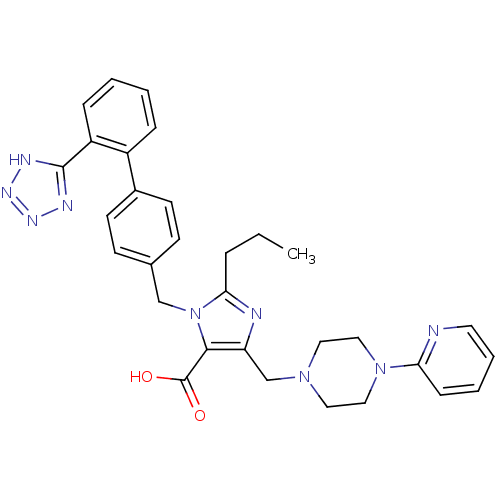 (2-Propyl-5-(4-pyridin-2-yl-piperazin-1-ylmethyl)-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of Angiotensin II receptor, type 1 | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50049197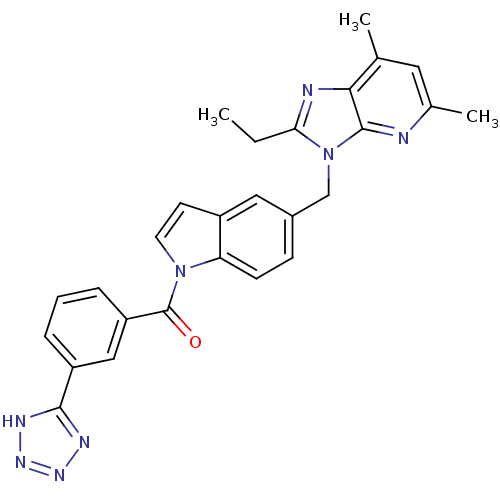 (CHEMBL159004 | [5-(2-Ethyl-5,7-dimethyl-imidazo[4,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of Angiotensin II receptor, type 1 | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50047126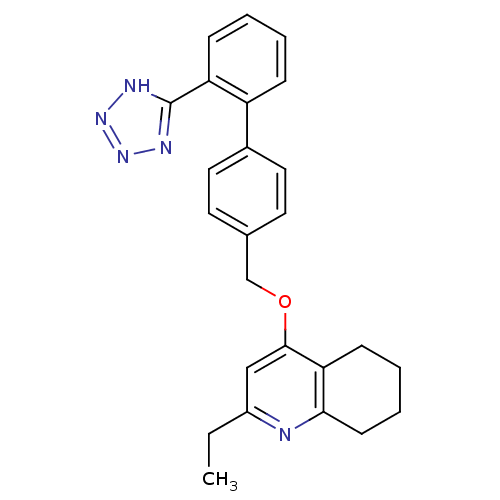 (2-Ethyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description In vitro antagonistic potency against Angiotensin II receptor, type 1 in guinea pig adrenal membrane | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50043469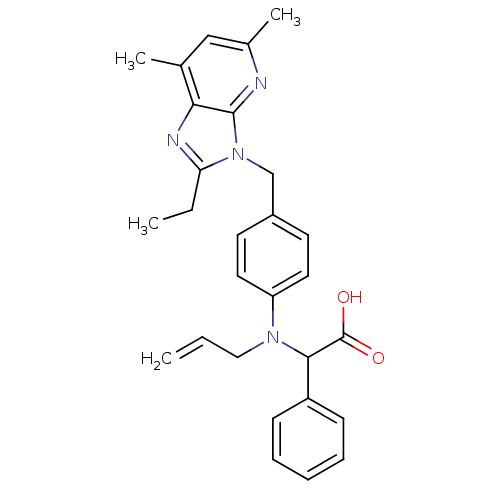 (CHEMBL135089 | {Allyl-[4-(2-ethyl-5,7-dimethyl-imi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of Angiotensin II receptor, type 1 | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50049195 (3-[(2'-(butylsulfonylcarbamate)-3'-propyl-1,1'-bip...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of Angiotensin II receptor, type 2 | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049200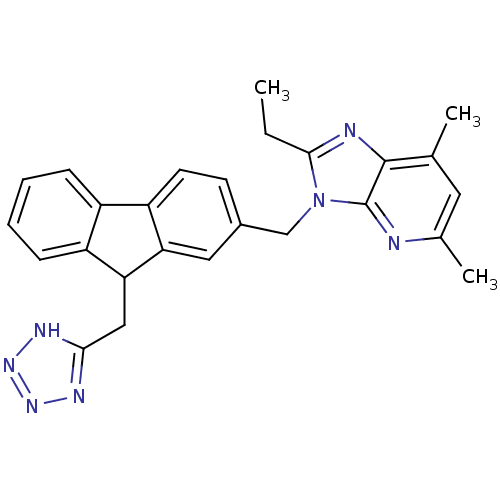 (2-Ethyl-5,7-dimethyl-3-[9-(1H-tetrazol-5-ylmethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description In vitro antagonistic potency against Angiotensin II receptor, type 1 in rat adrenal cortex | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50049202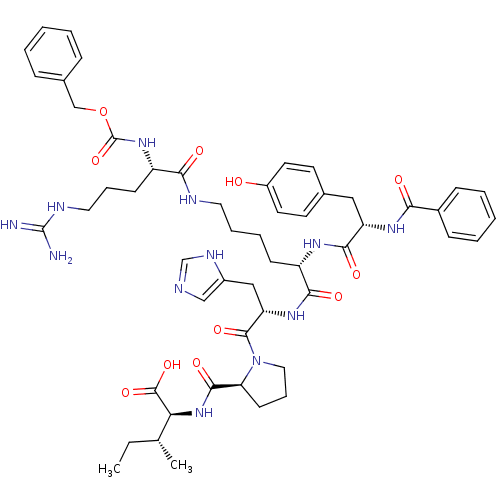 ((2S,3R)-2-({(S)-1-[(S)-2-[(S)-2-[(S)-2-Benzoylamin...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of Angiotensin II receptor, type 2 | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50049180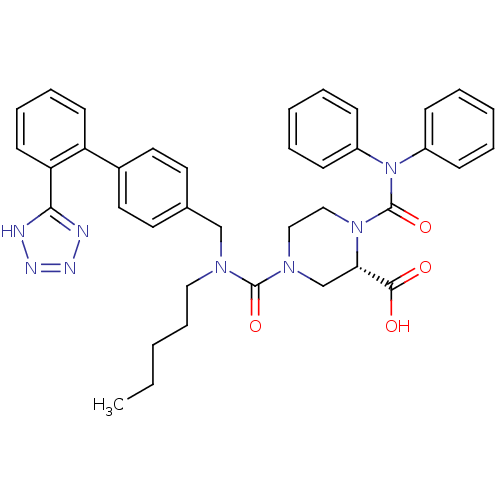 ((S)-1-Diphenylcarbamoyl-4-{pentyl-[2'-(1H-tetrazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of Angiotensin II receptor, type 1 | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM50035451 (2-{4-[3-butyl-5-oxo-1-(2-trifluoromethylphenyl)-4,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity at Angiotensin II type 2 receptor. | J Med Chem 39: 625-56 (1996) Article DOI: 10.1021/jm9504722 BindingDB Entry DOI: 10.7270/Q29P3299 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 78 total ) | Next | Last >> |