

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50130172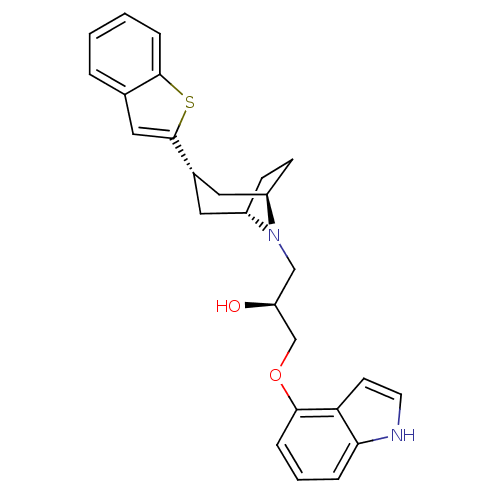 (1-(3-Benzo[b]thiophen-2-yl-8-aza-bicyclo[3.2.1]oct...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50130170 ((S)-1-(1H-Indol-4-yloxy)-3-[(1R,3S,5S)-3-(4-methox...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity at 5-hydroxytryptamine 1A receptor by [3H]-8-OH-DPAT displacement. | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50130170 ((S)-1-(1H-Indol-4-yloxy)-3-[(1R,3S,5S)-3-(4-methox...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50130163 ((S)-1-(1H-Indol-4-yloxy)-3-[(4S,6R)-4-(4-methoxy-b...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50130152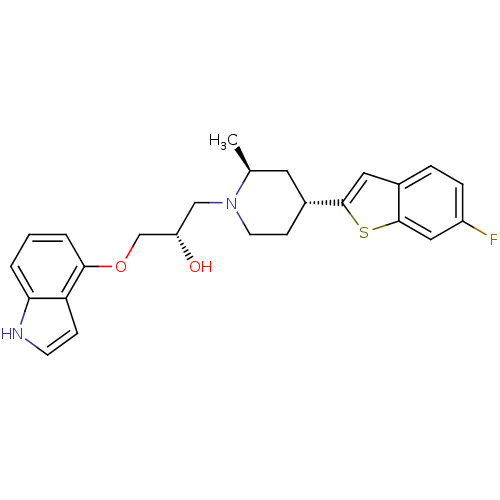 ((S)-1-(1H-indol-4-yloxy)-3-((2S,4R)-4-(6-fluoroben...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50130173 ((S)-1-[(4S,6R)-4-(5-Fluoro-benzo[b]thiophen-2-yl)-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50130168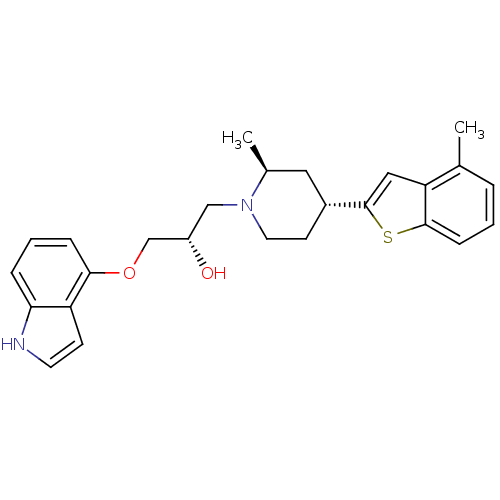 ((S)-1-(1H-Indol-4-yloxy)-3-[(4S,6R)-2-methyl-4-(4-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50130157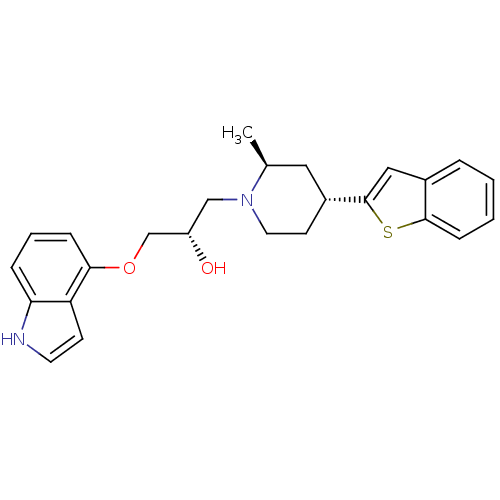 ((S)-1-((4S,6R)-4-Benzo[b]thiophen-2-yl-2-methyl-pi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50130169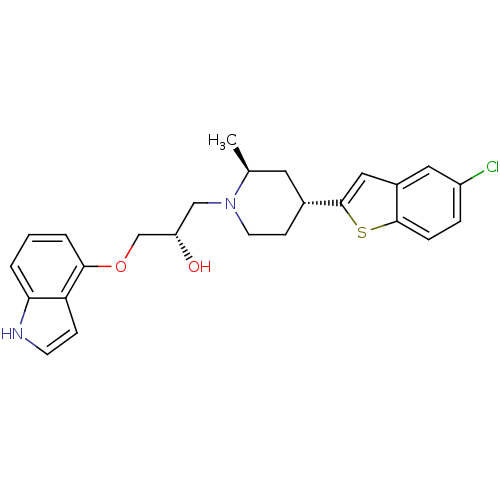 ((S)-1-(1H-indol-4-yloxy)-3-((2S,4R)-4-(5-chloroben...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50130162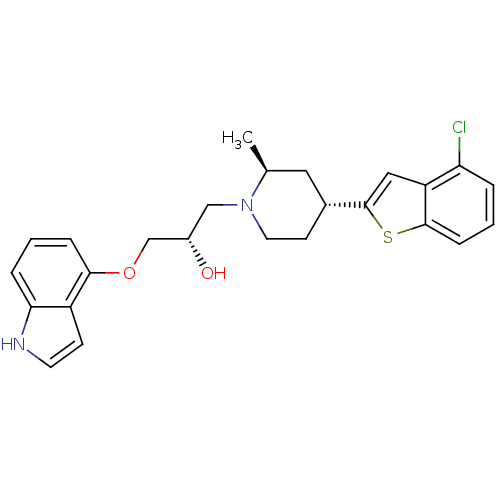 ((S)-1-[(4S,6R)-4-(4-Chloro-benzo[b]thiophen-2-yl)-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50130164 ((S)-1-[(4S,6R)-4-(4,5-Dimethoxy-benzo[b]thiophen-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of 5-HT induced [35S]-GTP-gammaS, binding at human cloned 5-HT 1A receptors. | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50130159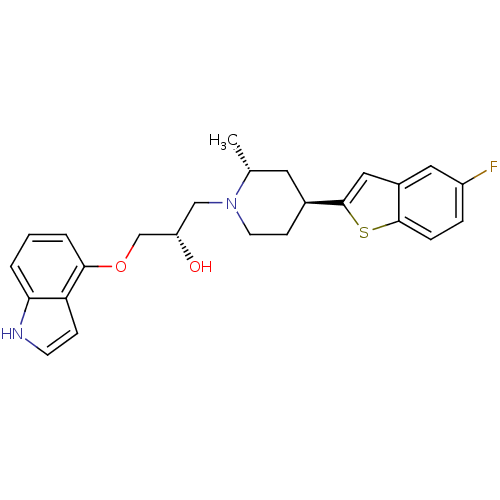 ((S)-1-[(4R,6S)-4-(5-Fluoro-benzo[b]thiophen-2-yl)-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50130154 ((S)-1-[(4S,6R)-4-(6-Chloro-benzo[b]thiophen-2-yl)-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50130167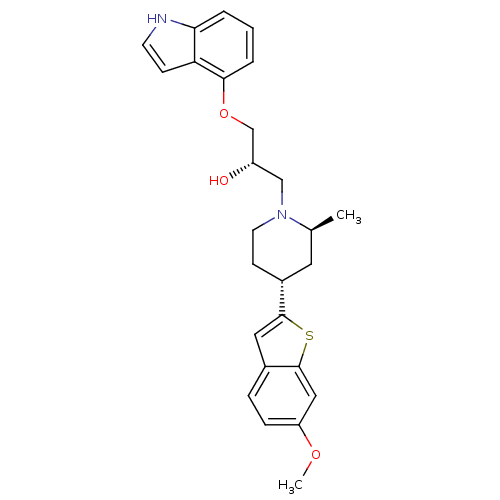 ((S)-1-(1H-Indol-4-yloxy)-3-[(4S,6R)-4-(6-methoxy-b...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50130165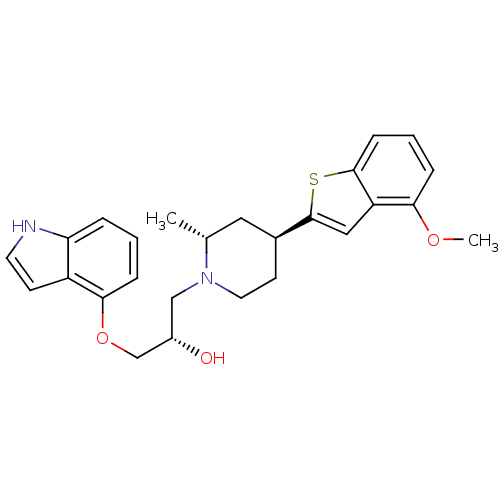 ((S)-1-(1H-Indol-4-yloxy)-3-[(4R,6S)-4-(4-methoxy-b...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50130171 ((S)-1-[(4S,6R)-4-(4-Fluoro-benzo[b]thiophen-2-yl)-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50130164 ((S)-1-[(4S,6R)-4-(4,5-Dimethoxy-benzo[b]thiophen-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity at 5-hydroxytryptamine 1A receptor by [3H]-8-OH-DPAT displacement. | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50130160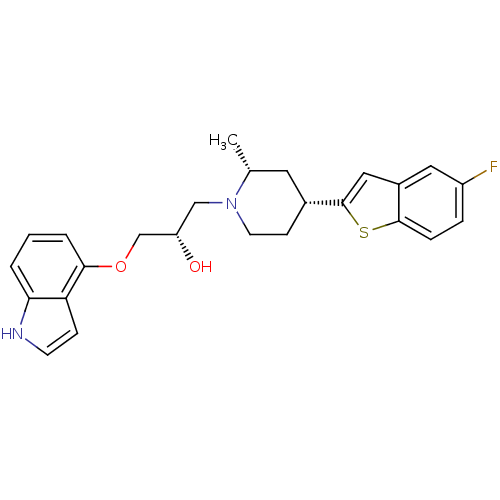 ((S)-1-[(4R,6R)-4-(5-Fluoro-benzo[b]thiophen-2-yl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of 5-HT induced [35S]-GTP-gammaS, binding at human cloned 5-HT 1A receptors. | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50130161 ((S)-1-(1H-Indol-4-yloxy)-3-[(4R,6R)-4-(4-methoxy-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50130153 ((S)-1-[(4S,6R)-4-(4,6-Dimethyl-benzo[b]thiophen-2-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50130156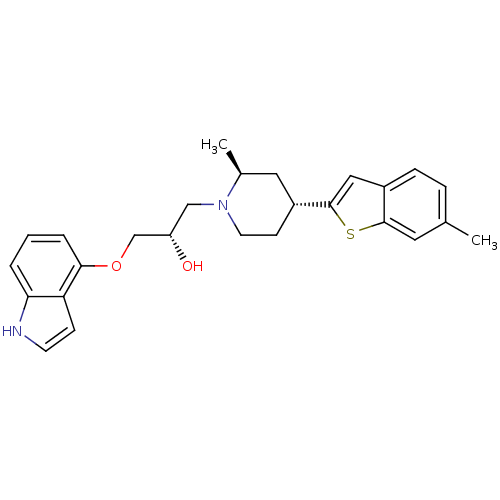 ((S)-1-(1H-Indol-4-yloxy)-3-[(4S,6R)-2-methyl-4-(6-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50130163 ((S)-1-(1H-Indol-4-yloxy)-3-[(4S,6R)-4-(4-methoxy-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of 5-HT induced [35S]-GTP-gammaS, binding at human cloned 5-HT 1A receptors. | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50130161 ((S)-1-(1H-Indol-4-yloxy)-3-[(4R,6R)-4-(4-methoxy-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity at 5-hydroxytryptamine 1A receptor by [3H]-8-OH-DPAT displacement. | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50130167 ((S)-1-(1H-Indol-4-yloxy)-3-[(4S,6R)-4-(6-methoxy-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity at 5-hydroxytryptamine 1A receptor by [3H]-8-OH-DPAT displacement. | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50130157 ((S)-1-((4S,6R)-4-Benzo[b]thiophen-2-yl-2-methyl-pi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of 5-HT induced [35S]-GTP-gammaS, binding at human cloned 5-HT 1A receptors. | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50130157 ((S)-1-((4S,6R)-4-Benzo[b]thiophen-2-yl-2-methyl-pi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of 5-HT induced [35S]-GTP-gammaS, binding at human cloned 5-HT 1A receptors. | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50130155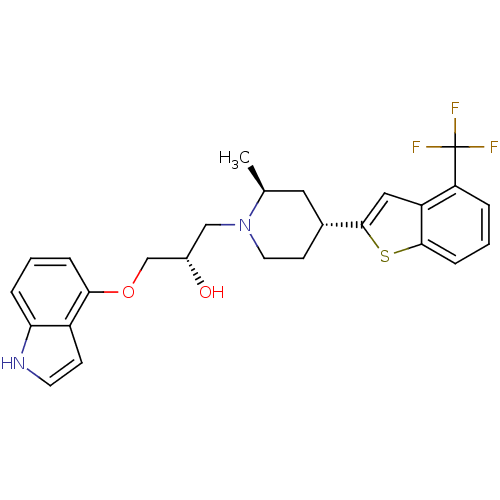 ((S)-1-(1H-Indol-4-yloxy)-3-[(4S,6R)-2-methyl-4-(4-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50130160 ((S)-1-[(4R,6R)-4-(5-Fluoro-benzo[b]thiophen-2-yl)-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50130166 ((S)-1-[(4S,6R)-4-(7-Fluoro-benzo[b]thiophen-2-yl)-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50130163 ((S)-1-(1H-Indol-4-yloxy)-3-[(4S,6R)-4-(4-methoxy-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity at 5-hydroxytryptamine 1A receptor by [3H]-8-OH-DPAT displacement. | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50130166 ((S)-1-[(4S,6R)-4-(7-Fluoro-benzo[b]thiophen-2-yl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity at 5-hydroxytryptamine 1A receptor by [3H]-8-OH-DPAT displacement. | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50130151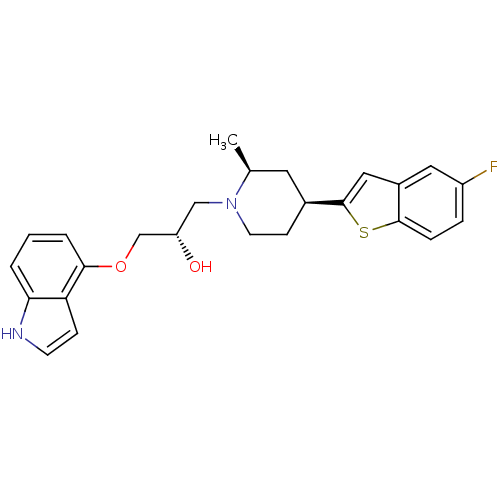 ((S)-1-[(4S,6S)-4-(5-Fluoro-benzo[b]thiophen-2-yl)-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50130171 ((S)-1-[(4S,6R)-4-(4-Fluoro-benzo[b]thiophen-2-yl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of 5-HT induced [35S]-GTP-gammaS, binding at human cloned 5-HT 1A receptors. | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50130169 ((S)-1-(1H-indol-4-yloxy)-3-((2S,4R)-4-(5-chloroben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of 5-HT induced [35S]-GTP-gammaS, binding at human cloned 5-HT 1A receptors. | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50130152 ((S)-1-(1H-indol-4-yloxy)-3-((2S,4R)-4-(6-fluoroben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity at 5-hydroxytryptamine 1A receptor by [3H]-8-OH-DPAT displacement. | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50130173 ((S)-1-[(4S,6R)-4-(5-Fluoro-benzo[b]thiophen-2-yl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity at 5-hydroxytryptamine 1A receptor by [3H]-8-OH-DPAT displacement. | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50130173 ((S)-1-[(4S,6R)-4-(5-Fluoro-benzo[b]thiophen-2-yl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity at 5-hydroxytryptamine 1A receptor by [3H]-8-OH-DPAT displacement. | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50130168 ((S)-1-(1H-Indol-4-yloxy)-3-[(4S,6R)-2-methyl-4-(4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of 5-HT induced [35S]-GTP-gammaS, binding at human cloned 5-HT 1A receptors. | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50130152 ((S)-1-(1H-indol-4-yloxy)-3-((2S,4R)-4-(6-fluoroben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of 5-HT induced [35S]-GTP-gammaS, binding at human cloned 5-HT 1A receptors. | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50130169 ((S)-1-(1H-indol-4-yloxy)-3-((2S,4R)-4-(5-chloroben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of 5-HT induced [35S]-GTP-gammaS, binding at human cloned 5-HT 1A receptors. | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50130165 ((S)-1-(1H-Indol-4-yloxy)-3-[(4R,6S)-4-(4-methoxy-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity at 5-hydroxytryptamine 1A receptor by [3H]-8-OH-DPAT displacement. | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50130165 ((S)-1-(1H-Indol-4-yloxy)-3-[(4R,6S)-4-(4-methoxy-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity at 5-hydroxytryptamine 1A receptor by [3H]-8-OH-DPAT displacement. | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50130168 ((S)-1-(1H-Indol-4-yloxy)-3-[(4S,6R)-2-methyl-4-(4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of 5-HT induced [35S]-GTP-gammaS, binding at human cloned 5-HT 1A receptors. | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50130160 ((S)-1-[(4R,6R)-4-(5-Fluoro-benzo[b]thiophen-2-yl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity at 5-hydroxytryptamine 1A receptor by [3H]-8-OH-DPAT displacement. | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50130164 ((S)-1-[(4S,6R)-4-(4,5-Dimethoxy-benzo[b]thiophen-2...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50130159 ((S)-1-[(4R,6S)-4-(5-Fluoro-benzo[b]thiophen-2-yl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity at 5-hydroxytryptamine 1A receptor by [3H]-8-OH-DPAT displacement. | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50130154 ((S)-1-[(4S,6R)-4-(6-Chloro-benzo[b]thiophen-2-yl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of 5-HT induced [35S]-GTP-gammaS, binding at human cloned 5-HT 1A receptors. | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50130158 ((S)-1-(1H-Indol-4-yloxy)-3-[(4S,6S)-4-(4-methoxy-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity at 5-hydroxytryptamine 1A receptor by [3H]-8-OH-DPAT displacement. | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50130171 ((S)-1-[(4S,6R)-4-(4-Fluoro-benzo[b]thiophen-2-yl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity at 5-hydroxytryptamine 1A receptor by [3H]-8-OH-DPAT displacement. | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50130167 ((S)-1-(1H-Indol-4-yloxy)-3-[(4S,6R)-4-(6-methoxy-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity at 5-hydroxytryptamine 1A receptor by [3H]-8-OH-DPAT displacement. | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 64 total ) | Next | Last >> |