

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50056333 (CHEMBL3322470) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H] CP-55,940 from human CB2 receptor expressed in HEK cells at 10 uM after 3 hrs by scintillation counting | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50056338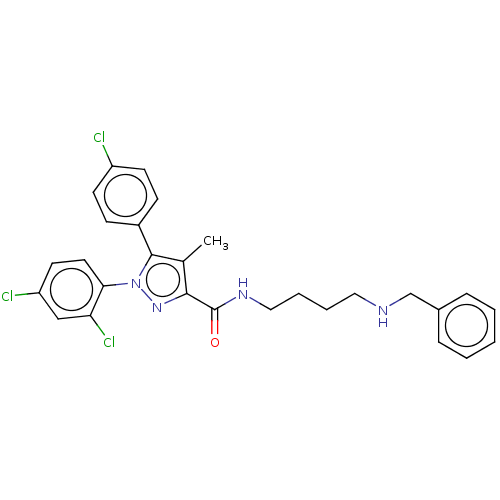 (CHEMBL3322475) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H] CP-55,940 from human CB2 receptor expressed in HEK cells at 10 uM after 3 hrs by scintillation counting | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50056339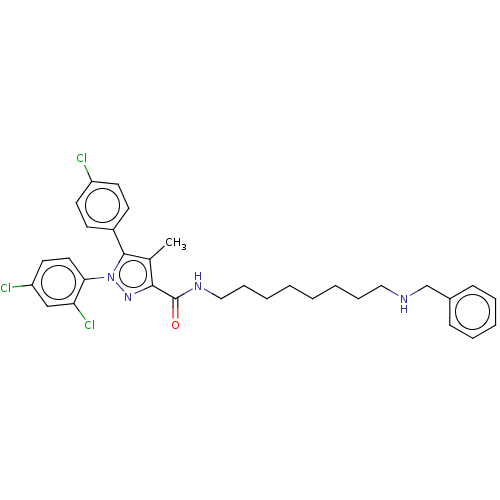 (CHEMBL3322476) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H] CP-55,940 from human CB2 receptor expressed in HEK cells at 10 uM after 3 hrs by scintillation counting | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056319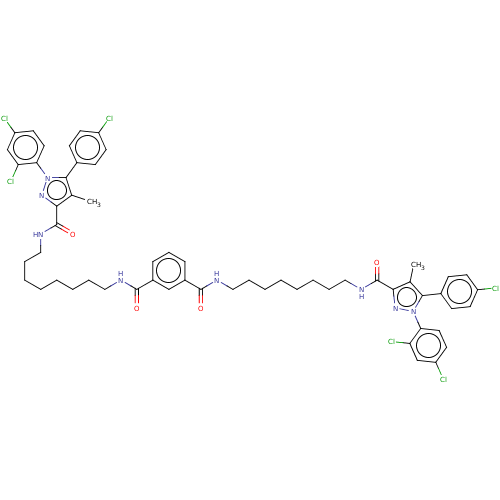 (CHEMBL3322458) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056320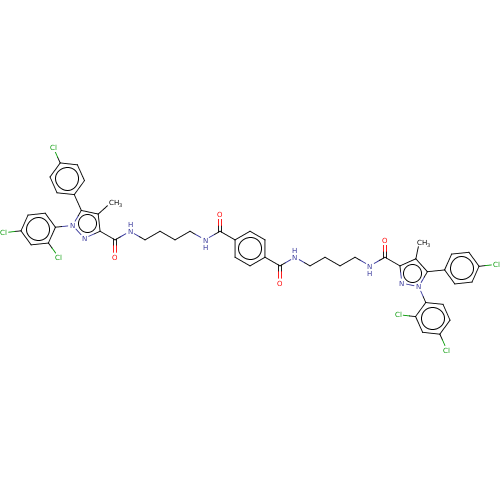 (CHEMBL3322454) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056321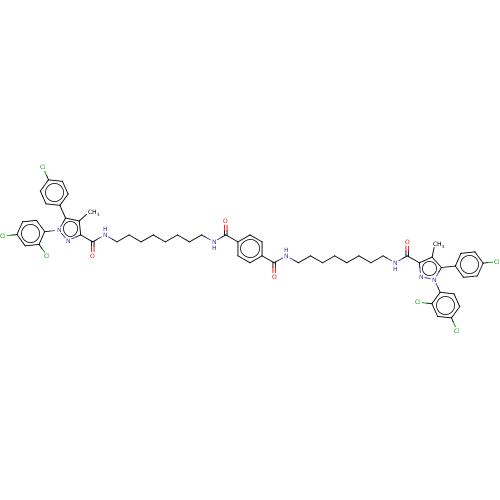 (CHEMBL3322455) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.07E+4 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056322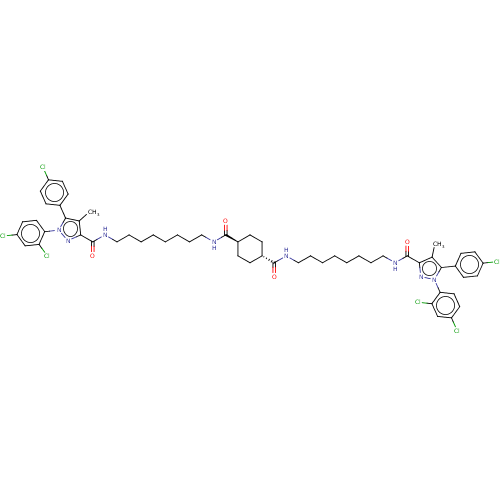 (CHEMBL3322457) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.55E+3 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056323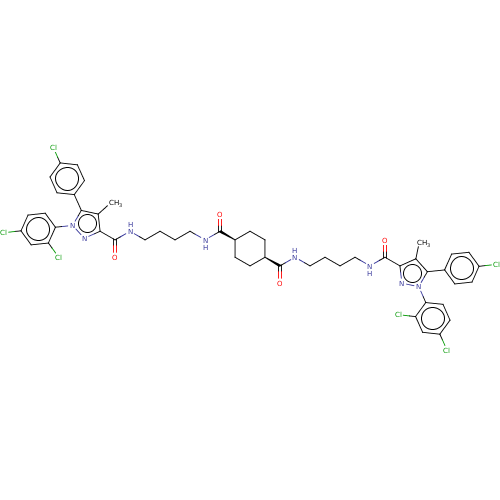 (CHEMBL3322459) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.66E+3 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056324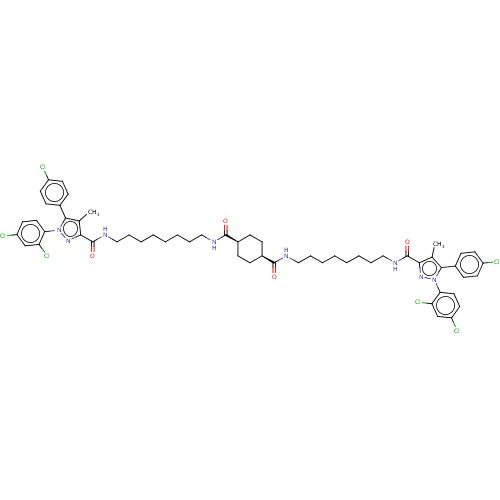 (CHEMBL3322460) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.71E+3 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056325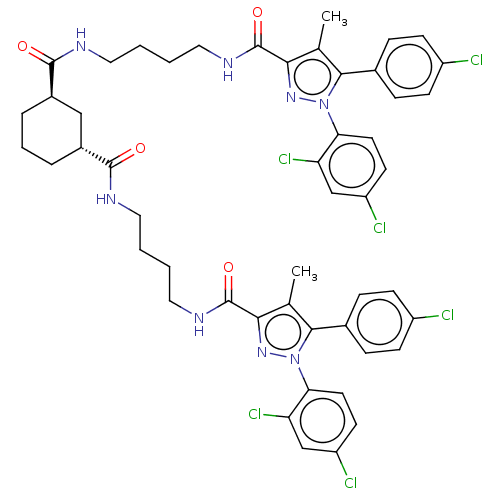 (CHEMBL3322461) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.55E+3 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056326 (CHEMBL3322462) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056327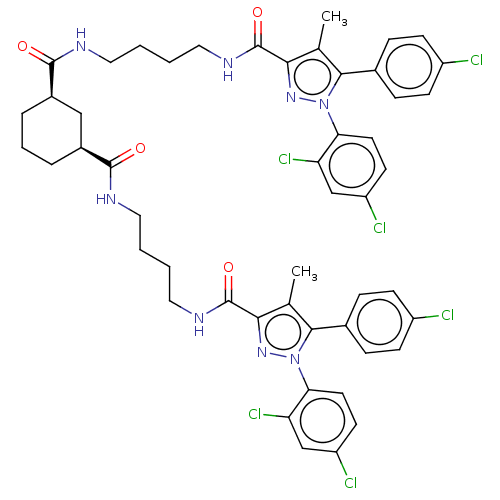 (CHEMBL3322463) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.43E+4 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056328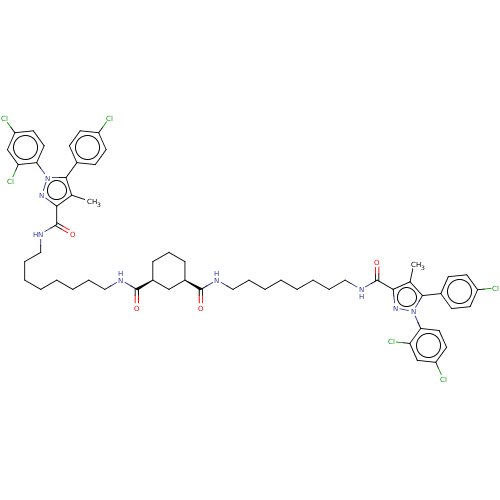 (CHEMBL3322464) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056329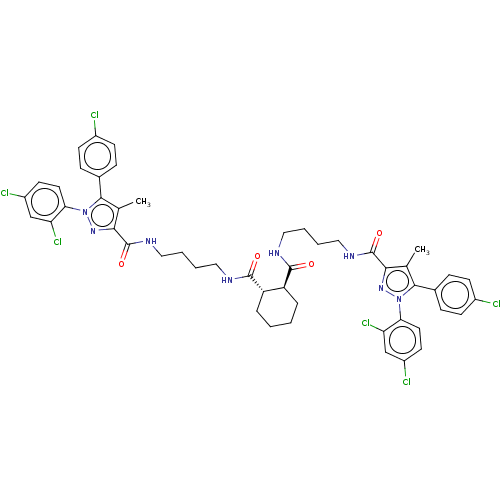 (CHEMBL3322465) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056330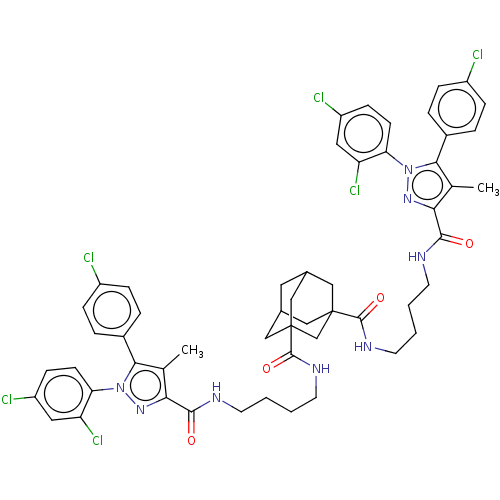 (CHEMBL3322467) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.62E+4 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056331 (CHEMBL3322468) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.24E+3 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056332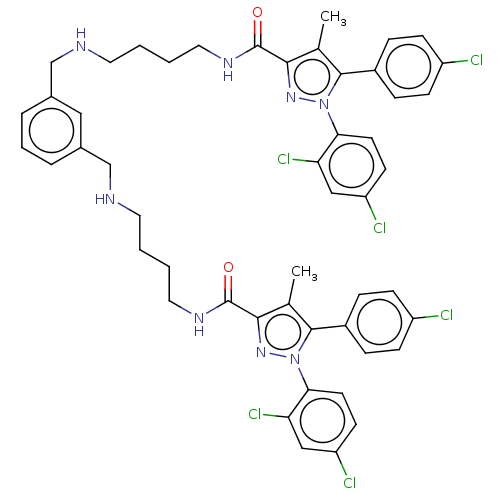 (CHEMBL3322469) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.85E+3 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056333 (CHEMBL3322470) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.95E+3 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056334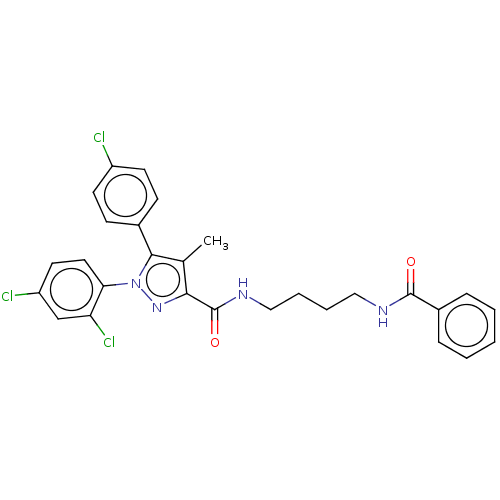 (CHEMBL3322471) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 776 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056335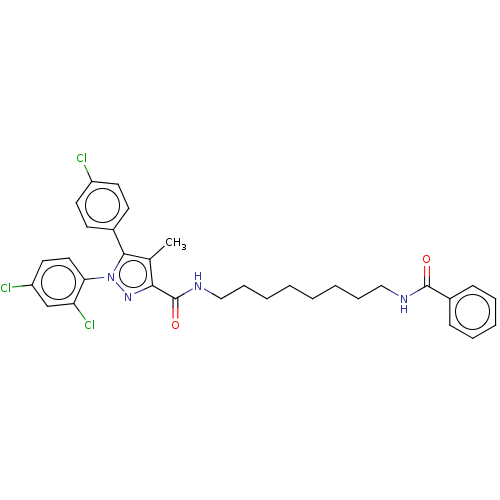 (CHEMBL3322472) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 501 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056336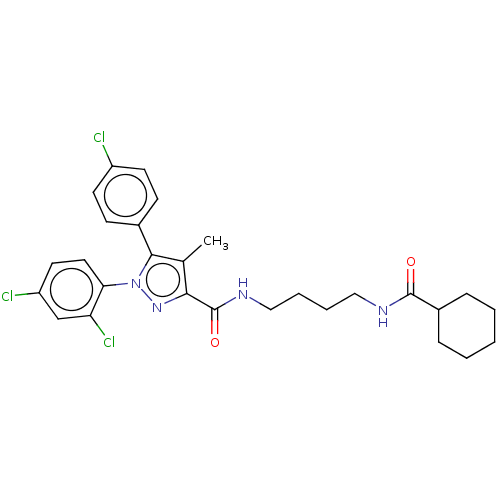 (CHEMBL3322473) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056337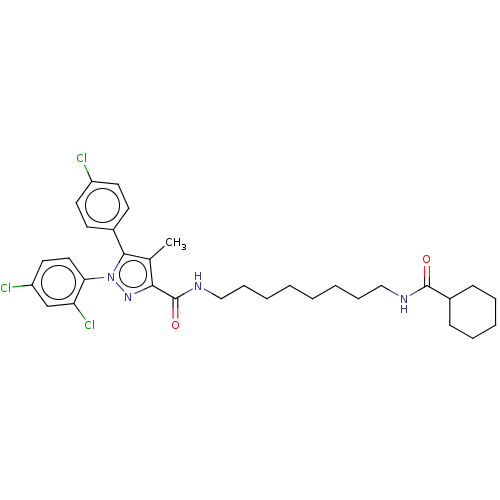 (CHEMBL3322474) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056338 (CHEMBL3322475) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 661 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056339 (CHEMBL3322476) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 123 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056340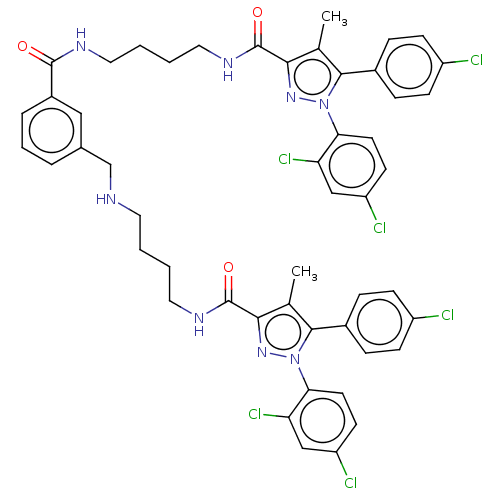 (CHEMBL3322478) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.62E+3 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056341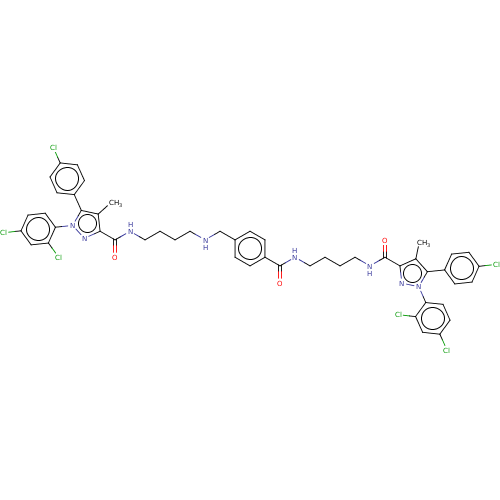 (CHEMBL3322479) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.91E+3 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21279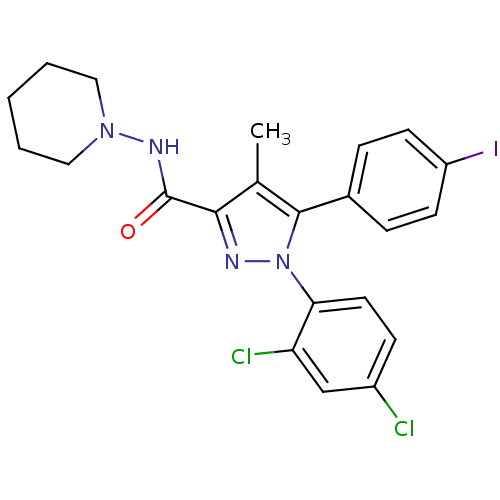 (1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 68 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056316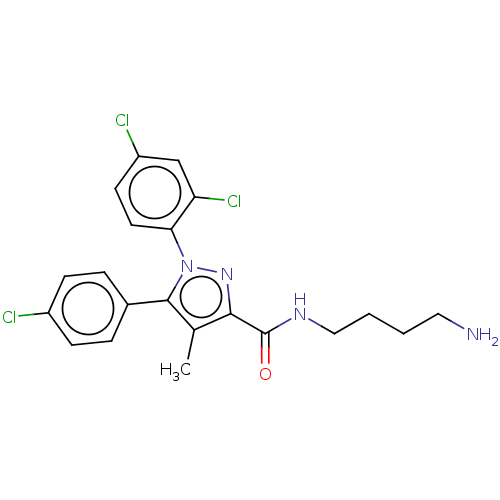 (CHEMBL3322348) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.82E+3 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056317 (CHEMBL3322349) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 112 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056318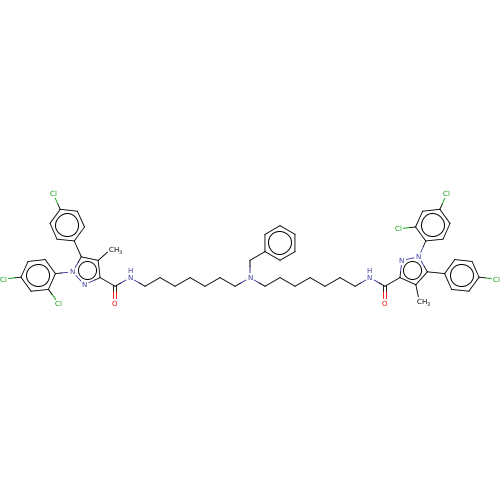 (CHEMBL3322350) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.89E+3 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50328952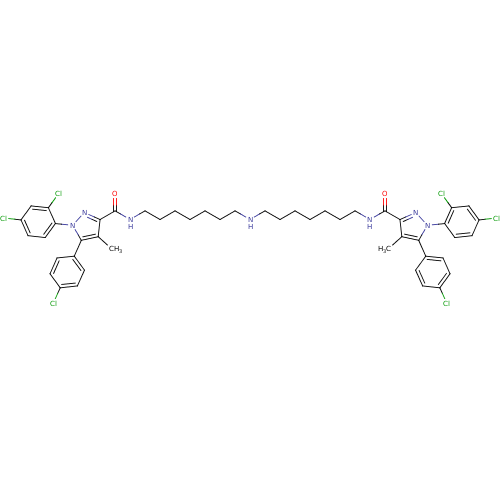 (CHEMBL1269766 | N,N'-(Iminodiheptane-7,1-diyl)bis[...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 871 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056319 (CHEMBL3322458) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056320 (CHEMBL3322454) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.51E+4 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056321 (CHEMBL3322455) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.07E+4 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056322 (CHEMBL3322457) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.55E+3 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056323 (CHEMBL3322459) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.55E+3 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056324 (CHEMBL3322460) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.71E+3 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056325 (CHEMBL3322461) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.51E+3 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056326 (CHEMBL3322462) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.59E+3 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056327 (CHEMBL3322463) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056328 (CHEMBL3322464) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056329 (CHEMBL3322465) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056330 (CHEMBL3322467) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.62E+4 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056331 (CHEMBL3322468) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.24E+3 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056332 (CHEMBL3322469) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.91E+3 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056333 (CHEMBL3322470) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.95E+3 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056334 (CHEMBL3322471) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 776 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056335 (CHEMBL3322472) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 501 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50056336 (CHEMBL3322473) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 63 total ) | Next | Last >> |