 Found 17 hits Enz. Inhib. hit(s) with all data for entry = 50025936
Found 17 hits Enz. Inhib. hit(s) with all data for entry = 50025936 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glutathione hydrolase 1 proenzyme
(Homo sapiens (Human)) | BDBM50248772
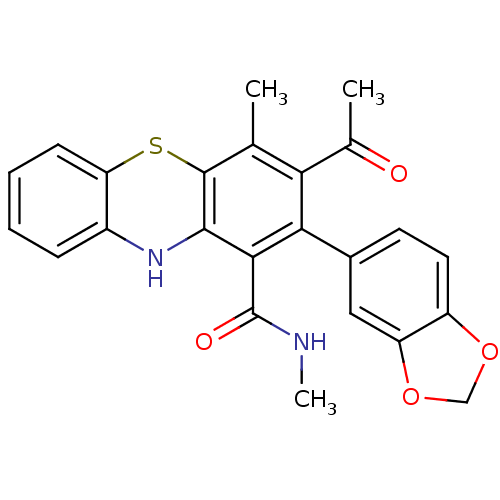
(2-(1,3-benzodioxol-5-yl)-N,N',4-trimethyl-10Hpheno...)Show SMILES CNC(=O)c1c2Nc3ccccc3Sc2c(C)c(C(C)=O)c1-c1ccc2OCOc2c1 Show InChI InChI=1S/C24H20N2O4S/c1-12-19(13(2)27)20(14-8-9-16-17(10-14)30-11-29-16)21(24(28)25-3)22-23(12)31-18-7-5-4-6-15(18)26-22/h4-10,26H,11H2,1-3H3,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of gamma-glutamyltranspeptidase (unknown origin) by colorimetric assay |
Eur J Med Chem 44: 197-202 (2008)
Article DOI: 10.1016/j.ejmech.2008.02.028
BindingDB Entry DOI: 10.7270/Q20K28B1 |
More data for this
Ligand-Target Pair | |
Glutathione hydrolase 1 proenzyme
(Homo sapiens (Human)) | BDBM50248738
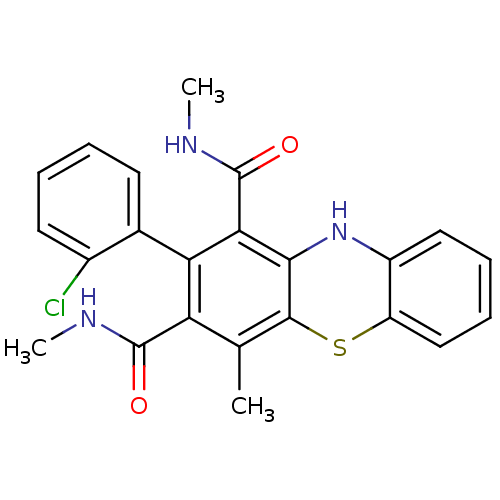
(2-(2-Chlorophenyl)-N,N',4-trimethyl-10Hphenothiazi...)Show SMILES CNC(=O)c1c(C)c2Sc3ccccc3Nc2c(C(=O)NC)c1-c1ccccc1Cl |(29.29,-26.84,;27.96,-27.61,;27.97,-29.15,;29.31,-29.91,;26.64,-29.93,;25.3,-29.17,;25.28,-27.63,;23.96,-29.95,;22.61,-29.17,;21.28,-29.96,;19.94,-29.2,;18.61,-29.97,;18.61,-31.51,;19.95,-32.28,;21.28,-31.51,;22.62,-32.28,;23.97,-31.5,;25.31,-32.27,;25.32,-33.81,;26.66,-34.57,;23.99,-34.58,;24,-36.12,;26.66,-31.49,;27.99,-32.25,;28,-33.78,;29.33,-34.55,;30.66,-33.77,;30.65,-32.22,;29.31,-31.47,;30.63,-30.69,)| Show InChI InChI=1S/C23H20ClN3O2S/c1-12-17(22(28)25-2)18(13-8-4-5-9-14(13)24)19(23(29)26-3)20-21(12)30-16-11-7-6-10-15(16)27-20/h4-11,27H,1-3H3,(H,25,28)(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of gamma-glutamyltranspeptidase (unknown origin) by colorimetric assay |
Eur J Med Chem 44: 197-202 (2008)
Article DOI: 10.1016/j.ejmech.2008.02.028
BindingDB Entry DOI: 10.7270/Q20K28B1 |
More data for this
Ligand-Target Pair | |
Glutathione hydrolase 1 proenzyme
(Homo sapiens (Human)) | BDBM50248626
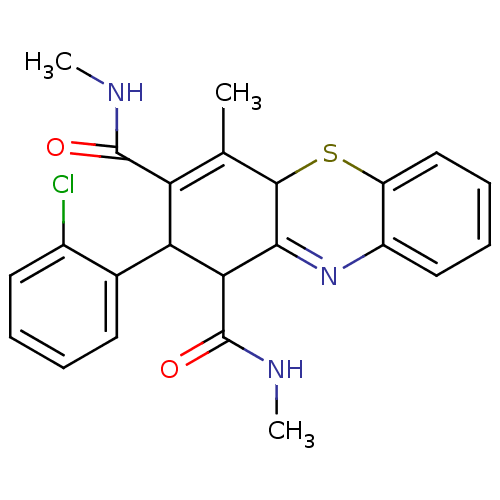
(2-(2-Chlorophenyl)-N,N',4-trimethyl-2,10-dihydro-1...)Show SMILES CNC(=O)C1C(C(C(=O)NC)=C(C)C2Sc3ccccc3N=C12)c1ccccc1Cl |t:10,22| Show InChI InChI=1S/C23H22ClN3O2S/c1-12-17(22(28)25-2)18(13-8-4-5-9-14(13)24)19(23(29)26-3)20-21(12)30-16-11-7-6-10-15(16)27-20/h4-11,18-19,21H,1-3H3,(H,25,28)(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of gamma-glutamyltranspeptidase (unknown origin) by colorimetric assay |
Eur J Med Chem 44: 197-202 (2008)
Article DOI: 10.1016/j.ejmech.2008.02.028
BindingDB Entry DOI: 10.7270/Q20K28B1 |
More data for this
Ligand-Target Pair | |
Glutathione hydrolase 1 proenzyme
(Homo sapiens (Human)) | BDBM50248624
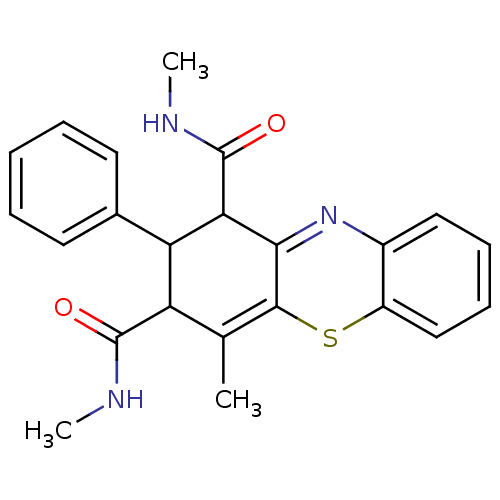
(2-Phenyl-N,N',4-trimethyl-2,10-dihydro-1Hphenothia...)Show SMILES CNC(=O)C1C(C(C(=O)NC)C2=Nc3ccccc3SC2=C1C)c1ccccc1 |c:22,t:11| Show InChI InChI=1S/C23H23N3O2S/c1-13-17(22(27)24-2)18(14-9-5-4-6-10-14)19(23(28)25-3)20-21(13)29-16-12-8-7-11-15(16)26-20/h4-12,17-19H,1-3H3,(H,24,27)(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of gamma-glutamyltranspeptidase (unknown origin) by colorimetric assay |
Eur J Med Chem 44: 197-202 (2008)
Article DOI: 10.1016/j.ejmech.2008.02.028
BindingDB Entry DOI: 10.7270/Q20K28B1 |
More data for this
Ligand-Target Pair | |
Glutathione hydrolase 1 proenzyme
(Homo sapiens (Human)) | BDBM50248739
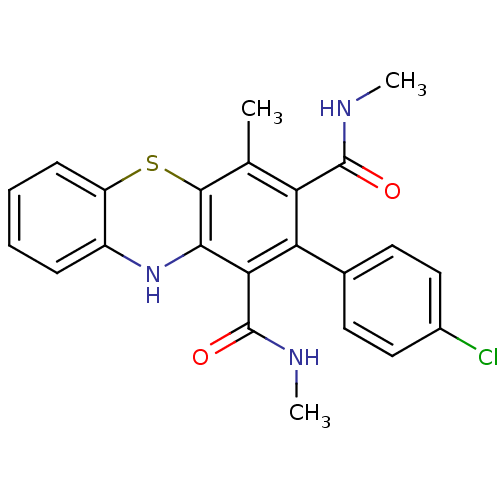
(2-(4-Chlorophenyl)-N,N',4-trimethyl-10Hphenothiazi...)Show SMILES CNC(=O)c1c(C)c2Sc3ccccc3Nc2c(C(=O)NC)c1-c1ccc(Cl)cc1 Show InChI InChI=1S/C23H20ClN3O2S/c1-12-17(22(28)25-2)18(13-8-10-14(24)11-9-13)19(23(29)26-3)20-21(12)30-16-7-5-4-6-15(16)27-20/h4-11,27H,1-3H3,(H,25,28)(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of gamma-glutamyltranspeptidase (unknown origin) by colorimetric assay |
Eur J Med Chem 44: 197-202 (2008)
Article DOI: 10.1016/j.ejmech.2008.02.028
BindingDB Entry DOI: 10.7270/Q20K28B1 |
More data for this
Ligand-Target Pair | |
Glutathione hydrolase 1 proenzyme
(Homo sapiens (Human)) | BDBM50248736

(2-Phenyl-N,N',4-trimethyl-10H-phenothiazine-1,3-di...)Show SMILES CNC(=O)c1c(C)c2Sc3ccccc3Nc2c(C(=O)NC)c1-c1ccccc1 Show InChI InChI=1S/C23H21N3O2S/c1-13-17(22(27)24-2)18(14-9-5-4-6-10-14)19(23(28)25-3)20-21(13)29-16-12-8-7-11-15(16)26-20/h4-12,26H,1-3H3,(H,24,27)(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of gamma-glutamyltranspeptidase (unknown origin) by colorimetric assay |
Eur J Med Chem 44: 197-202 (2008)
Article DOI: 10.1016/j.ejmech.2008.02.028
BindingDB Entry DOI: 10.7270/Q20K28B1 |
More data for this
Ligand-Target Pair | |
Glutathione hydrolase 1 proenzyme
(Homo sapiens (Human)) | BDBM50248771

(2-(4-Methoxyphenyl)-N,N',4-trimethyl-10Hphenothiaz...)Show SMILES CNC(=O)c1c(C)c2Sc3ccccc3Nc2c(C(=O)NC)c1-c1ccc(OC)cc1 Show InChI InChI=1S/C24H23N3O3S/c1-13-18(23(28)25-2)19(14-9-11-15(30-4)12-10-14)20(24(29)26-3)21-22(13)31-17-8-6-5-7-16(17)27-21/h5-12,27H,1-4H3,(H,25,28)(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of gamma-glutamyltranspeptidase (unknown origin) by colorimetric assay |
Eur J Med Chem 44: 197-202 (2008)
Article DOI: 10.1016/j.ejmech.2008.02.028
BindingDB Entry DOI: 10.7270/Q20K28B1 |
More data for this
Ligand-Target Pair | |
Glutathione hydrolase 1 proenzyme
(Homo sapiens (Human)) | BDBM50248625
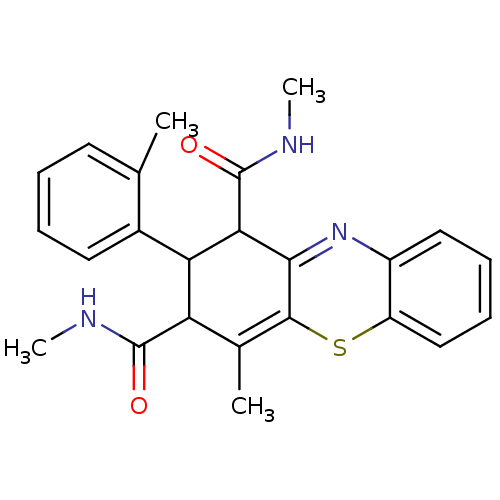
(2-(2-Methylphenyl)-N,N',4-trimethyl-2,10-dihydro-1...)Show SMILES CNC(=O)C1C(C(C(=O)NC)C2=Nc3ccccc3SC2=C1C)c1ccccc1C |c:22,t:11| Show InChI InChI=1S/C24H25N3O2S/c1-13-9-5-6-10-15(13)19-18(23(28)25-3)14(2)22-21(20(19)24(29)26-4)27-16-11-7-8-12-17(16)30-22/h5-12,18-20H,1-4H3,(H,25,28)(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of gamma-glutamyltranspeptidase (unknown origin) by colorimetric assay |
Eur J Med Chem 44: 197-202 (2008)
Article DOI: 10.1016/j.ejmech.2008.02.028
BindingDB Entry DOI: 10.7270/Q20K28B1 |
More data for this
Ligand-Target Pair | |
Glutathione hydrolase 1 proenzyme
(Homo sapiens (Human)) | BDBM50248773
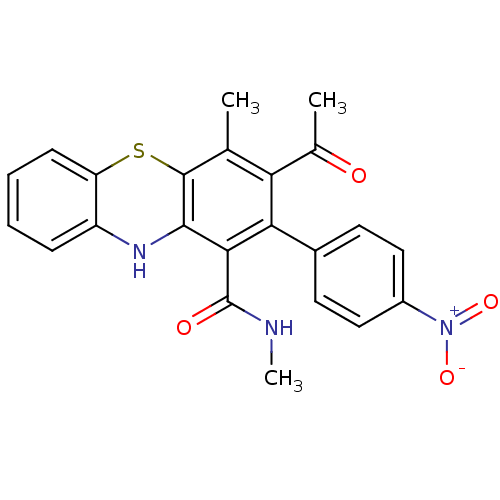
(2-(4-Nitrophenyl)-N,N',4-trimethyl-10Hphenothiazin...)Show SMILES CNC(=O)c1c2Nc3ccccc3Sc2c(C)c(C(C)=O)c1-c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C23H19N3O4S/c1-12-18(13(2)27)19(14-8-10-15(11-9-14)26(29)30)20(23(28)24-3)21-22(12)31-17-7-5-4-6-16(17)25-21/h4-11,25H,1-3H3,(H,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of gamma-glutamyltranspeptidase (unknown origin) by colorimetric assay |
Eur J Med Chem 44: 197-202 (2008)
Article DOI: 10.1016/j.ejmech.2008.02.028
BindingDB Entry DOI: 10.7270/Q20K28B1 |
More data for this
Ligand-Target Pair | |
Glutathione hydrolase 1 proenzyme
(Homo sapiens (Human)) | BDBM50248737
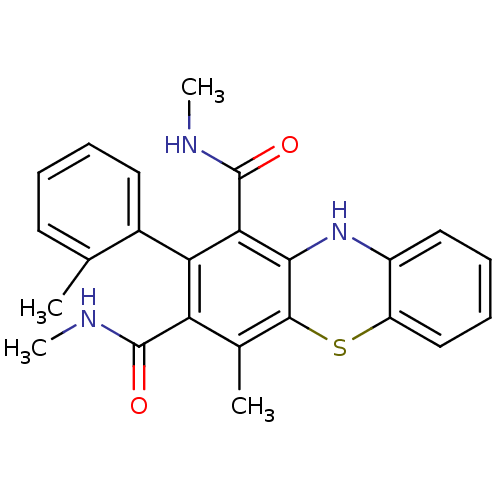
(2-(2-Methylphenyl)-N,N',4-trimethyl-10Hphenothiazi...)Show SMILES CNC(=O)c1c(C)c2Sc3ccccc3Nc2c(C(=O)NC)c1-c1ccccc1C |(14.18,-27.19,;12.85,-27.96,;12.86,-29.49,;14.2,-30.25,;11.53,-30.28,;10.19,-29.51,;10.17,-27.97,;8.85,-30.3,;7.5,-29.52,;6.17,-30.31,;4.83,-29.54,;3.5,-30.31,;3.5,-31.86,;4.84,-32.63,;6.17,-31.86,;7.52,-32.63,;8.86,-31.85,;10.2,-32.62,;10.21,-34.16,;11.55,-34.92,;8.88,-34.93,;8.89,-36.47,;11.55,-31.84,;12.88,-32.6,;12.89,-34.13,;14.22,-34.89,;15.55,-34.12,;15.54,-32.57,;14.2,-31.81,;15.53,-31.04,)| Show InChI InChI=1S/C24H23N3O2S/c1-13-9-5-6-10-15(13)19-18(23(28)25-3)14(2)22-21(20(19)24(29)26-4)27-16-11-7-8-12-17(16)30-22/h5-12,27H,1-4H3,(H,25,28)(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of gamma-glutamyltranspeptidase (unknown origin) by colorimetric assay |
Eur J Med Chem 44: 197-202 (2008)
Article DOI: 10.1016/j.ejmech.2008.02.028
BindingDB Entry DOI: 10.7270/Q20K28B1 |
More data for this
Ligand-Target Pair | |
Glutathione hydrolase 1 proenzyme
(Homo sapiens (Human)) | BDBM50248701

(2-(4-Methoxyphenyl)-N,N',4-trimethyl-2,10-dihydro-...)Show SMILES CNC(=O)C1C(C(C(=O)NC)=C(C)C2Sc3ccccc3N=C12)c1ccc(OC)cc1 |t:10,22| Show InChI InChI=1S/C24H25N3O3S/c1-13-18(23(28)25-2)19(14-9-11-15(30-4)12-10-14)20(24(29)26-3)21-22(13)31-17-8-6-5-7-16(17)27-21/h5-12,19-20,22H,1-4H3,(H,25,28)(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of gamma-glutamyltranspeptidase (unknown origin) by colorimetric assay |
Eur J Med Chem 44: 197-202 (2008)
Article DOI: 10.1016/j.ejmech.2008.02.028
BindingDB Entry DOI: 10.7270/Q20K28B1 |
More data for this
Ligand-Target Pair | |
Glutathione hydrolase 1 proenzyme
(Homo sapiens (Human)) | BDBM50248770
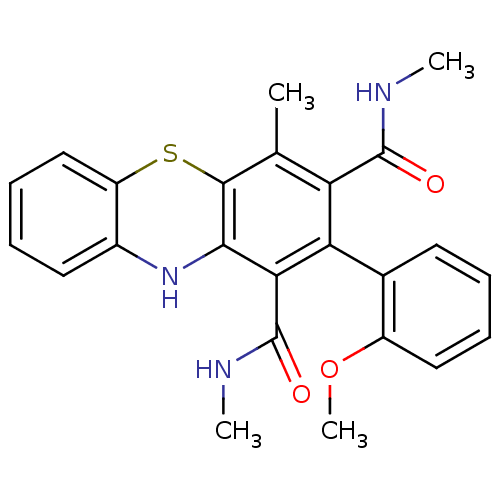
(2-(2-Methoxyphenyl)-N,N',4-trimethyl-10Hphenothiaz...)Show SMILES CNC(=O)c1c(C)c2Sc3ccccc3Nc2c(C(=O)NC)c1-c1ccccc1OC |(14.81,-40.77,;13.29,-41.54,;13.28,-43.08,;14.61,-43.85,;11.94,-43.85,;10.6,-43.08,;10.6,-41.54,;9.27,-43.85,;7.94,-43.08,;6.6,-43.85,;5.27,-43.08,;3.93,-43.85,;3.93,-45.39,;5.27,-46.16,;6.6,-45.39,;7.94,-46.16,;9.27,-45.39,;10.6,-46.16,;10.6,-47.7,;11.93,-48.47,;9.26,-48.47,;9.26,-50.02,;11.93,-45.39,;13.4,-45.86,;14.21,-44.5,;15.75,-44.54,;16.49,-45.89,;15.68,-47.21,;14.14,-47.16,;13.46,-48.47,;14.45,-49.92,)| Show InChI InChI=1S/C24H23N3O3S/c1-13-18(23(28)25-2)19(14-9-5-7-11-16(14)30-4)20(24(29)26-3)21-22(13)31-17-12-8-6-10-15(17)27-21/h5-12,27H,1-4H3,(H,25,28)(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of gamma-glutamyltranspeptidase (unknown origin) by colorimetric assay |
Eur J Med Chem 44: 197-202 (2008)
Article DOI: 10.1016/j.ejmech.2008.02.028
BindingDB Entry DOI: 10.7270/Q20K28B1 |
More data for this
Ligand-Target Pair | |
Glutathione hydrolase 1 proenzyme
(Homo sapiens (Human)) | BDBM50248700
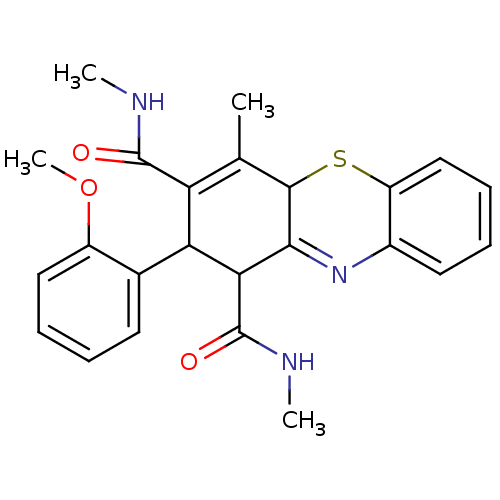
(2-(2-Methoxyphenyl)-N,N',4-trimethyl-2,10-dihydro-...)Show SMILES CNC(=O)C1C(C(C(=O)NC)=C(C)C2Sc3ccccc3N=C12)c1ccccc1OC |t:10,22| Show InChI InChI=1S/C24H25N3O3S/c1-13-18(23(28)25-2)19(14-9-5-7-11-16(14)30-4)20(24(29)26-3)21-22(13)31-17-12-8-6-10-15(17)27-21/h5-12,19-20,22H,1-4H3,(H,25,28)(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of gamma-glutamyltranspeptidase (unknown origin) by colorimetric assay |
Eur J Med Chem 44: 197-202 (2008)
Article DOI: 10.1016/j.ejmech.2008.02.028
BindingDB Entry DOI: 10.7270/Q20K28B1 |
More data for this
Ligand-Target Pair | |
Glutathione hydrolase 1 proenzyme
(Homo sapiens (Human)) | BDBM50248702
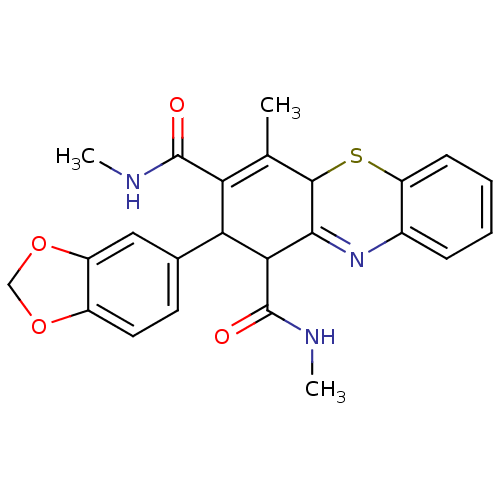
(2-(1,3-Benzodioxol-5-yl)-N,N',4-trimethyl-2,10-dih...)Show SMILES CNC(=O)C1C(C(C(=O)NC)=C(C)C2Sc3ccccc3N=C12)c1ccc2OCOc2c1 |t:10,22| Show InChI InChI=1S/C24H23N3O4S/c1-12-18(23(28)25-2)19(13-8-9-15-16(10-13)31-11-30-15)20(24(29)26-3)21-22(12)32-17-7-5-4-6-14(17)27-21/h4-10,19-20,22H,11H2,1-3H3,(H,25,28)(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of gamma-glutamyltranspeptidase (unknown origin) by colorimetric assay |
Eur J Med Chem 44: 197-202 (2008)
Article DOI: 10.1016/j.ejmech.2008.02.028
BindingDB Entry DOI: 10.7270/Q20K28B1 |
More data for this
Ligand-Target Pair | |
Glutathione hydrolase 1 proenzyme
(Homo sapiens (Human)) | BDBM50248655
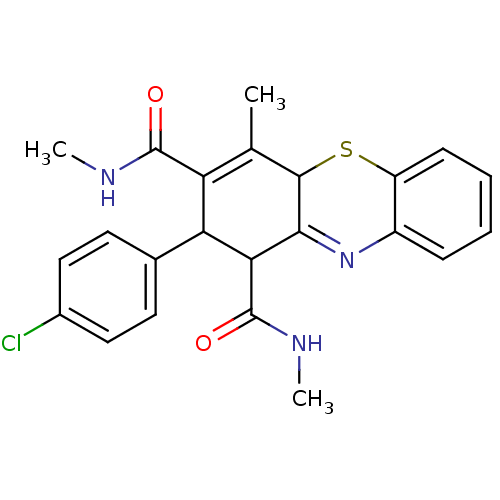
(2-(4-Chlorophenyl)-N,N',4-trimethyl-2,10-dihydro-1...)Show SMILES CNC(=O)C1C(C(C(=O)NC)=C(C)C2Sc3ccccc3N=C12)c1ccc(Cl)cc1 |t:10,22| Show InChI InChI=1S/C23H22ClN3O2S/c1-12-17(22(28)25-2)18(13-8-10-14(24)11-9-13)19(23(29)26-3)20-21(12)30-16-7-5-4-6-15(16)27-20/h4-11,18-19,21H,1-3H3,(H,25,28)(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of gamma-glutamyltranspeptidase (unknown origin) by colorimetric assay |
Eur J Med Chem 44: 197-202 (2008)
Article DOI: 10.1016/j.ejmech.2008.02.028
BindingDB Entry DOI: 10.7270/Q20K28B1 |
More data for this
Ligand-Target Pair | |
Glutathione hydrolase 1 proenzyme
(Homo sapiens (Human)) | BDBM22360
(2-(acetyloxy)benzoate | 2-(acetyloxy)benzoic acid ...)Show InChI InChI=1S/C9H8O4/c1-6(10)13-8-5-3-2-4-7(8)9(11)12/h2-5H,1H3,(H,11,12) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of gamma-glutamyltranspeptidase (unknown origin) by colorimetric assay |
Eur J Med Chem 44: 197-202 (2008)
Article DOI: 10.1016/j.ejmech.2008.02.028
BindingDB Entry DOI: 10.7270/Q20K28B1 |
More data for this
Ligand-Target Pair | |
Glutathione hydrolase 1 proenzyme
(Homo sapiens (Human)) | BDBM50248703

(2-(4-Nitrophenyl)-N,N',4-trimethyl-2,10-dihydro-1H...)Show SMILES CNC(=O)C1C(C(C(=O)NC)=C(C)C2Sc3ccccc3N=C12)c1ccc(cc1)[N+]([O-])=O |t:10,22| Show InChI InChI=1S/C23H22N4O4S/c1-12-17(22(28)24-2)18(13-8-10-14(11-9-13)27(30)31)19(23(29)25-3)20-21(12)32-16-7-5-4-6-15(16)26-20/h4-11,18-19,21H,1-3H3,(H,24,28)(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of gamma-glutamyltranspeptidase (unknown origin) by colorimetric assay |
Eur J Med Chem 44: 197-202 (2008)
Article DOI: 10.1016/j.ejmech.2008.02.028
BindingDB Entry DOI: 10.7270/Q20K28B1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data