

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Progesterone receptor (Homo sapiens (Human)) | BDBM50298207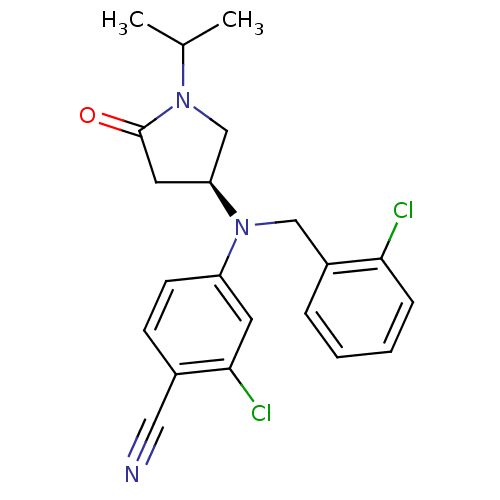 ((S)-2-chloro-4-((2-chlorobenzyl)(1-isopropyl-5-oxo...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to progesterone receptor | Bioorg Med Chem Lett 19: 4664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.081 BindingDB Entry DOI: 10.7270/Q25H7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50298210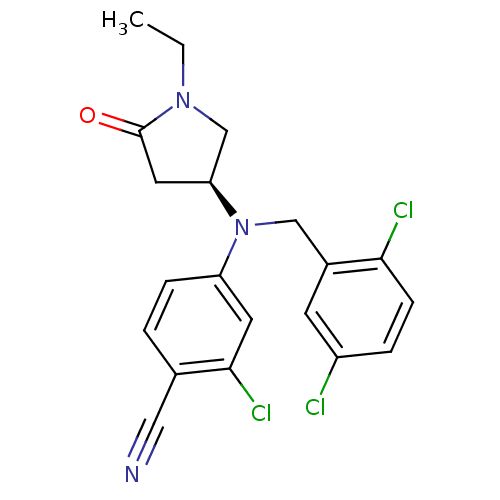 ((S)-2-chloro-4-((2,5-dichlorobenzyl)(1-ethyl-5-oxo...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to progesterone receptor | Bioorg Med Chem Lett 19: 4664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.081 BindingDB Entry DOI: 10.7270/Q25H7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50298208 ((S)-2-chloro-4-((2-chloro-5-fluorobenzyl)(1-ethyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to progesterone receptor | Bioorg Med Chem Lett 19: 4664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.081 BindingDB Entry DOI: 10.7270/Q25H7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50298209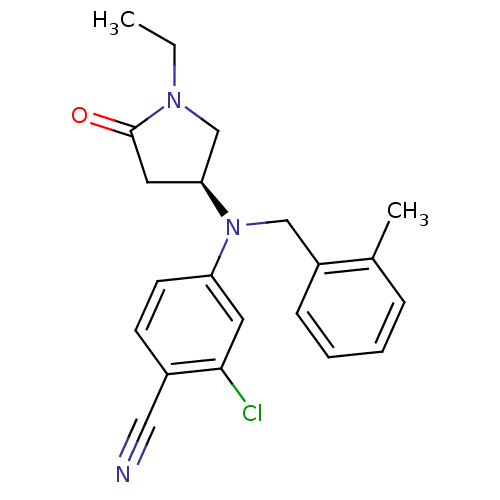 ((S)-2-chloro-4-((1-ethyl-5-oxopyrrolidin-3-yl)(2-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to progesterone receptor | Bioorg Med Chem Lett 19: 4664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.081 BindingDB Entry DOI: 10.7270/Q25H7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50298219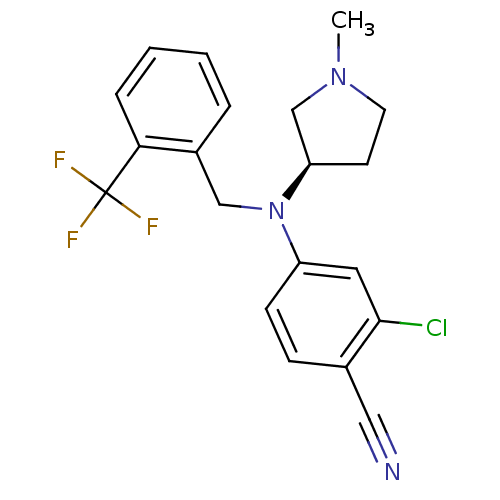 ((R)-2-chloro-4-((1-methylpyrrolidin-3-yl)(2-(trifl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to progesterone receptor | Bioorg Med Chem Lett 19: 4664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.081 BindingDB Entry DOI: 10.7270/Q25H7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50298212 ((S)-2-chloro-4-((2-fluorobenzyl)(1-methyl-5-oxopyr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to progesterone receptor | Bioorg Med Chem Lett 19: 4664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.081 BindingDB Entry DOI: 10.7270/Q25H7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50298211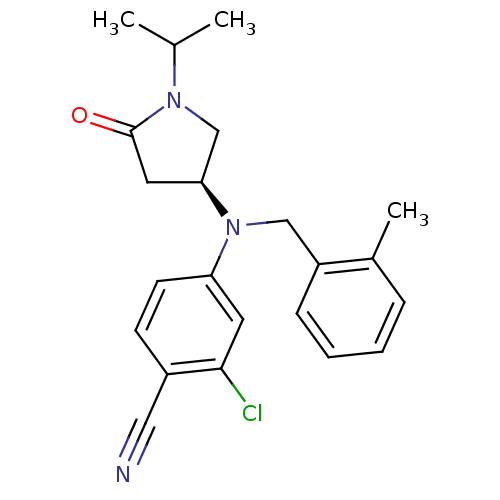 ((S)-2-chloro-4-((1-isopropyl-5-oxopyrrolidin-3-yl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to progesterone receptor | Bioorg Med Chem Lett 19: 4664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.081 BindingDB Entry DOI: 10.7270/Q25H7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50298206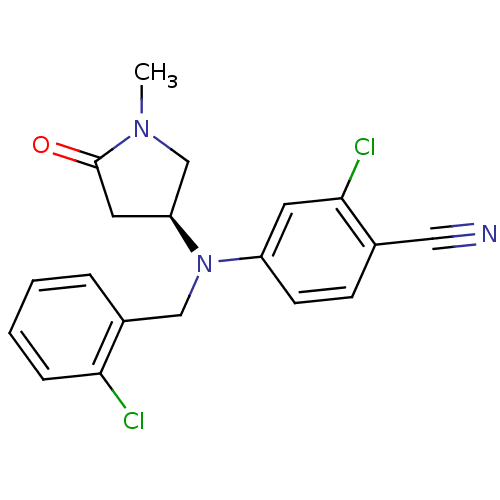 ((S)-2-chloro-4-((2-chlorobenzyl)(1-methyl-5-oxopyr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to progesterone receptor | Bioorg Med Chem Lett 19: 4664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.081 BindingDB Entry DOI: 10.7270/Q25H7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50298213 ((S)-2-chloro-4-((2,3-difluorobenzyl)(1-methyl-5-ox...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to progesterone receptor | Bioorg Med Chem Lett 19: 4664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.081 BindingDB Entry DOI: 10.7270/Q25H7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50298214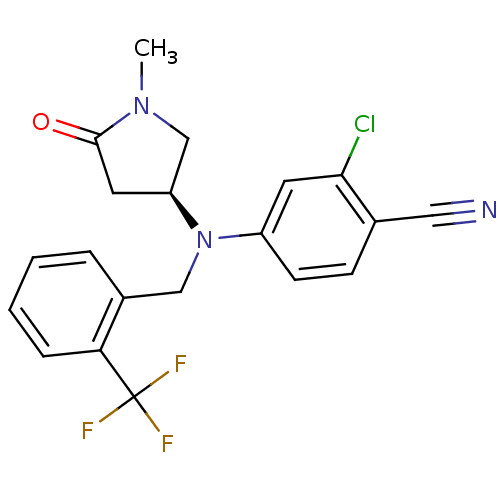 ((S)-2-chloro-4-((1-methyl-5-oxopyrrolidin-3-yl)(2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to progesterone receptor | Bioorg Med Chem Lett 19: 4664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.081 BindingDB Entry DOI: 10.7270/Q25H7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50298215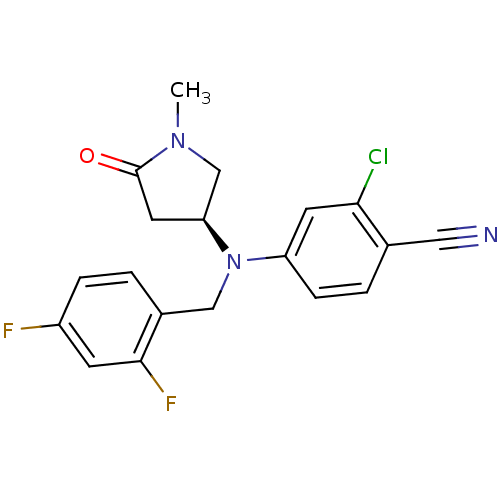 ((S)-2-chloro-4-((2,4-difluorobenzyl)(1-methyl-5-ox...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to progesterone receptor | Bioorg Med Chem Lett 19: 4664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.081 BindingDB Entry DOI: 10.7270/Q25H7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50298216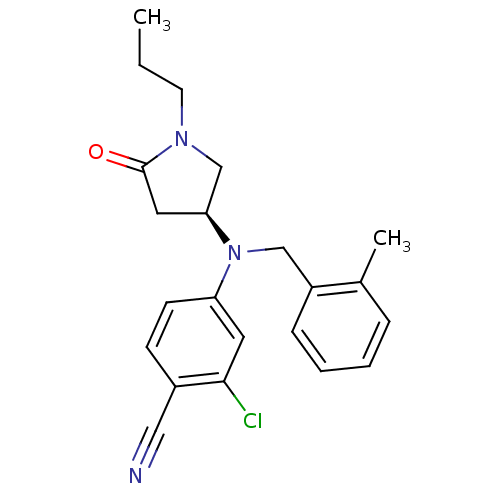 ((S)-2-chloro-4-((2-methylbenzyl)(5-oxo-1-propylpyr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to progesterone receptor | Bioorg Med Chem Lett 19: 4664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.081 BindingDB Entry DOI: 10.7270/Q25H7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50298217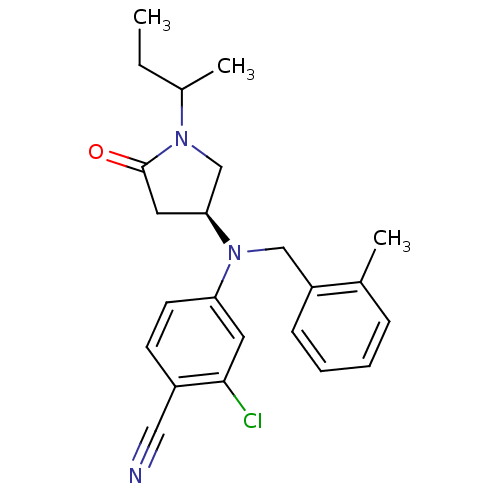 (4-(((3S)-1-sec-butyl-5-oxopyrrolidin-3-yl)(2-methy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to progesterone receptor | Bioorg Med Chem Lett 19: 4664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.081 BindingDB Entry DOI: 10.7270/Q25H7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50298218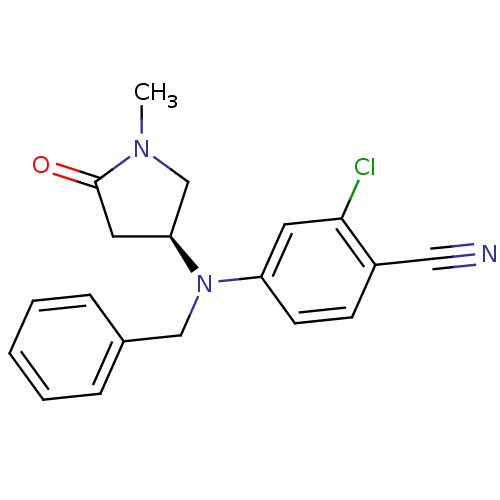 (2-Chloro-4-[[(3S)-1-methyl-5-oxo-3-pyrrolidinyl](p...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to progesterone receptor | Bioorg Med Chem Lett 19: 4664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.081 BindingDB Entry DOI: 10.7270/Q25H7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50298219 ((R)-2-chloro-4-((1-methylpyrrolidin-3-yl)(2-(trifl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to androgen receptor | Bioorg Med Chem Lett 19: 4664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.081 BindingDB Entry DOI: 10.7270/Q25H7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50298219 ((R)-2-chloro-4-((1-methylpyrrolidin-3-yl)(2-(trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human ERG by whole cell patch clamp assay | Bioorg Med Chem Lett 19: 4664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.081 BindingDB Entry DOI: 10.7270/Q25H7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50298208 ((S)-2-chloro-4-((2-chloro-5-fluorobenzyl)(1-ethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human ERG by whole cell patch clamp assay | Bioorg Med Chem Lett 19: 4664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.081 BindingDB Entry DOI: 10.7270/Q25H7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50298212 ((S)-2-chloro-4-((2-fluorobenzyl)(1-methyl-5-oxopyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human ERG by whole cell patch clamp assay | Bioorg Med Chem Lett 19: 4664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.081 BindingDB Entry DOI: 10.7270/Q25H7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50298217 (4-(((3S)-1-sec-butyl-5-oxopyrrolidin-3-yl)(2-methy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 750 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at progesterone receptor in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase activity | Bioorg Med Chem Lett 19: 4664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.081 BindingDB Entry DOI: 10.7270/Q25H7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50298218 (2-Chloro-4-[[(3S)-1-methyl-5-oxo-3-pyrrolidinyl](p...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.27E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at progesterone receptor in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase activity | Bioorg Med Chem Lett 19: 4664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.081 BindingDB Entry DOI: 10.7270/Q25H7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50298216 ((S)-2-chloro-4-((2-methylbenzyl)(5-oxo-1-propylpyr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.42E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at progesterone receptor in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase activity | Bioorg Med Chem Lett 19: 4664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.081 BindingDB Entry DOI: 10.7270/Q25H7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50298210 ((S)-2-chloro-4-((2,5-dichlorobenzyl)(1-ethyl-5-oxo...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at progesterone receptor in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase activity | Bioorg Med Chem Lett 19: 4664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.081 BindingDB Entry DOI: 10.7270/Q25H7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50298209 ((S)-2-chloro-4-((1-ethyl-5-oxopyrrolidin-3-yl)(2-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at progesterone receptor in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase activity | Bioorg Med Chem Lett 19: 4664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.081 BindingDB Entry DOI: 10.7270/Q25H7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50298208 ((S)-2-chloro-4-((2-chloro-5-fluorobenzyl)(1-ethyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at progesterone receptor in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase activity | Bioorg Med Chem Lett 19: 4664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.081 BindingDB Entry DOI: 10.7270/Q25H7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50298207 ((S)-2-chloro-4-((2-chlorobenzyl)(1-isopropyl-5-oxo...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at progesterone receptor in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase activity | Bioorg Med Chem Lett 19: 4664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.081 BindingDB Entry DOI: 10.7270/Q25H7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50298206 ((S)-2-chloro-4-((2-chlorobenzyl)(1-methyl-5-oxopyr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at progesterone receptor in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase activity | Bioorg Med Chem Lett 19: 4664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.081 BindingDB Entry DOI: 10.7270/Q25H7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50298215 ((S)-2-chloro-4-((2,4-difluorobenzyl)(1-methyl-5-ox...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 625 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at progesterone receptor in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase activity | Bioorg Med Chem Lett 19: 4664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.081 BindingDB Entry DOI: 10.7270/Q25H7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50298212 ((S)-2-chloro-4-((2-fluorobenzyl)(1-methyl-5-oxopyr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 135 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at progesterone receptor in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase activity | Bioorg Med Chem Lett 19: 4664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.081 BindingDB Entry DOI: 10.7270/Q25H7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50298219 ((R)-2-chloro-4-((1-methylpyrrolidin-3-yl)(2-(trifl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at progesterone receptor in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase activity | Bioorg Med Chem Lett 19: 4664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.081 BindingDB Entry DOI: 10.7270/Q25H7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50298213 ((S)-2-chloro-4-((2,3-difluorobenzyl)(1-methyl-5-ox...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 35 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at progesterone receptor in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase activity | Bioorg Med Chem Lett 19: 4664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.081 BindingDB Entry DOI: 10.7270/Q25H7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50298214 ((S)-2-chloro-4-((1-methyl-5-oxopyrrolidin-3-yl)(2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at progesterone receptor in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase activity | Bioorg Med Chem Lett 19: 4664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.081 BindingDB Entry DOI: 10.7270/Q25H7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50298211 ((S)-2-chloro-4-((1-isopropyl-5-oxopyrrolidin-3-yl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at progesterone receptor in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase activity | Bioorg Med Chem Lett 19: 4664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.081 BindingDB Entry DOI: 10.7270/Q25H7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||