

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194190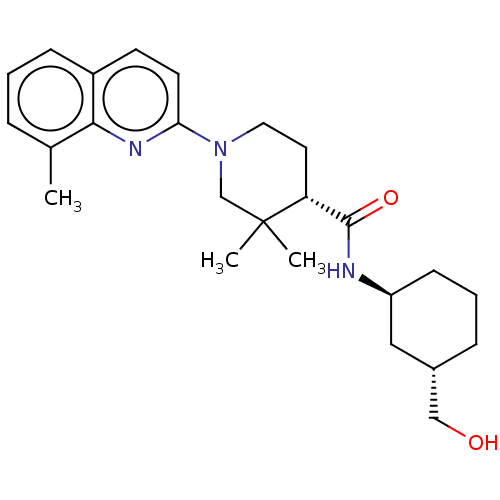 (CHEMBL3956184) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human whole blood assessed as reduction in LPS-induced PGE2 production preincubated for 30 mins followed by LPS stimulation ... | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194190 (CHEMBL3956184) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human mPGES-1 expressed in 293E cells assessed as reduction in conversion of PGH2 to PGE2 after 1.5 min by LC/MS analysis | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194141 (CHEMBL3938686) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human mPGES-1 expressed in 293E cells assessed as reduction in conversion of PGH2 to PGE2 after 1.5 min by LC/MS analysis | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194141 (CHEMBL3938686) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human whole blood assessed as reduction in LPS-induced PGE2 production preincubated for 30 mins followed by LPS stimulation ... | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194141 (CHEMBL3938686) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human A549 cells assessed as reduction in recombinant human interleukin-1 beta-induced PGE2 production preincubated for 30 m... | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194138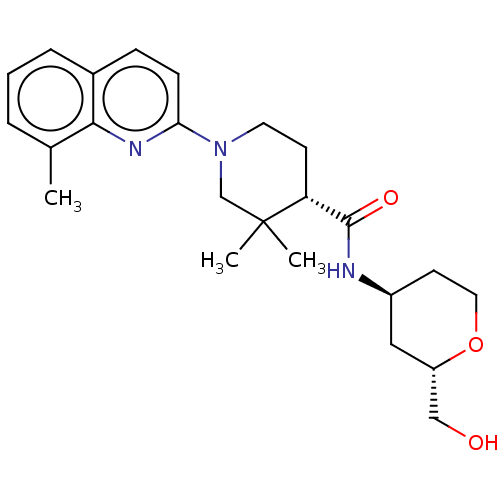 (CHEMBL3928608) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human mPGES-1 expressed in 293E cells assessed as reduction in conversion of PGH2 to PGE2 after 1.5 min by LC/MS analysis | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194190 (CHEMBL3956184) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human A549 cells assessed as reduction in recombinant human interleukin-1 beta-induced PGE2 production preincubated for 30 m... | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194140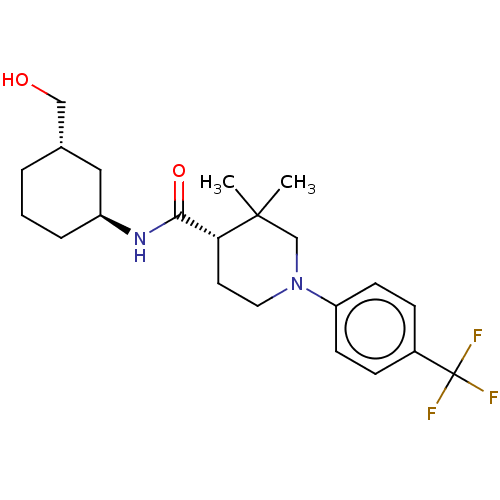 (CHEMBL3947494) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human mPGES-1 expressed in 293E cells assessed as reduction in conversion of PGH2 to PGE2 after 1.5 min by LC/MS analysis | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194137 (CHEMBL3922684) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human mPGES-1 expressed in 293E cells assessed as reduction in conversion of PGH2 to PGE2 after 1.5 min by LC/MS analysis | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194138 (CHEMBL3928608) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human A549 cells assessed as reduction in recombinant human interleukin-1 beta-induced PGE2 production preincubated for 30 m... | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194140 (CHEMBL3947494) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human whole blood assessed as reduction in LPS-induced PGE2 production preincubated for 30 mins followed by LPS stimulation ... | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194193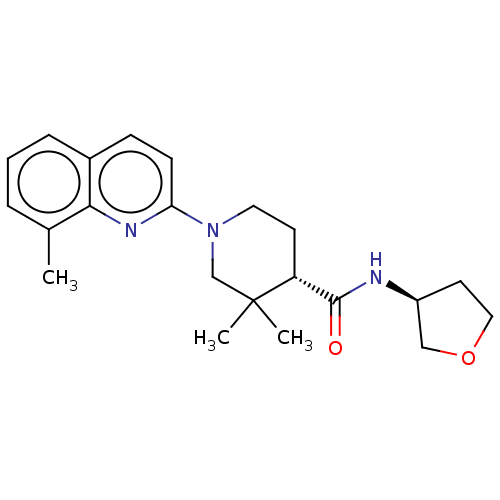 (CHEMBL3926051) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human mPGES-1 expressed in 293E cells assessed as reduction in conversion of PGH2 to PGE2 after 1.5 min by LC/MS analysis | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194138 (CHEMBL3928608) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human whole blood assessed as reduction in LPS-induced PGE2 production preincubated for 30 mins followed by LPS stimulation ... | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194192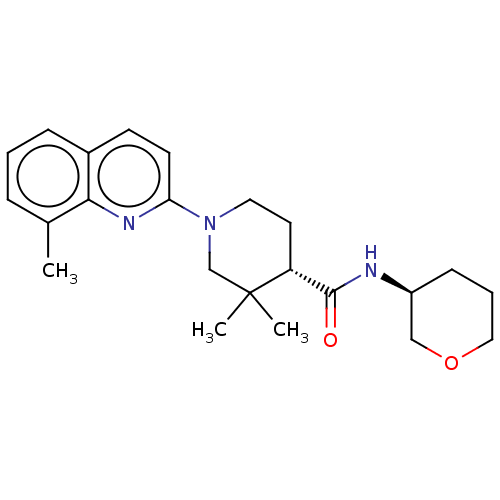 (CHEMBL3910746) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human mPGES-1 expressed in 293E cells assessed as reduction in conversion of PGH2 to PGE2 after 1.5 min by LC/MS analysis | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Canis familiaris) | BDBM50194138 (CHEMBL3928608) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of dog mPGES-1 | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194140 (CHEMBL3947494) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human A549 cells assessed as reduction in recombinant human interleukin-1 beta-induced PGE2 production preincubated for 30 m... | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194142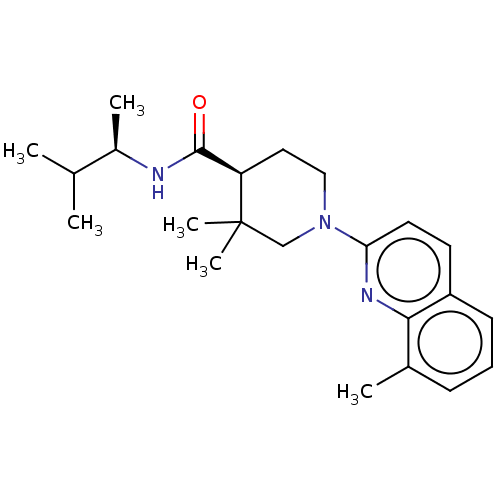 (CHEMBL3947843) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human mPGES-1 expressed in 293E cells assessed as reduction in conversion of PGH2 to PGE2 after 1.5 min by LC/MS analysis | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194193 (CHEMBL3926051) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human A549 cells assessed as reduction in recombinant human interleukin-1 beta-induced PGE2 production preincubated for 30 m... | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194137 (CHEMBL3922684) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human whole blood assessed as reduction in LPS-induced PGE2 production preincubated for 30 mins followed by LPS stimulation ... | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Canis familiaris) | BDBM50194138 (CHEMBL3928608) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in dog whole blood | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194139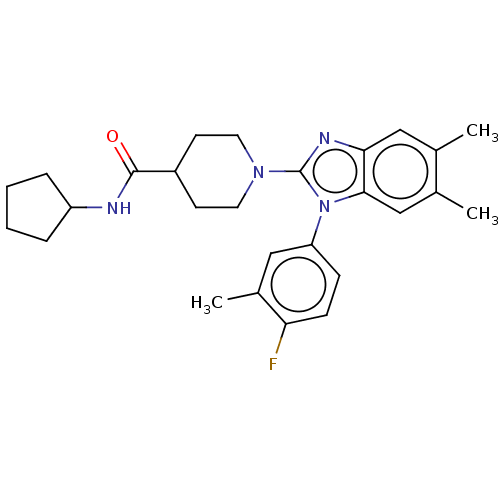 (CHEMBL3952581) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mPGES-1 (unknown origin) | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194137 (CHEMBL3922684) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human A549 cells assessed as reduction in recombinant human interleukin-1 beta-induced PGE2 production preincubated for 30 m... | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194192 (CHEMBL3910746) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human whole blood assessed as reduction in LPS-induced PGE2 production preincubated for 30 mins followed by LPS stimulation ... | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194193 (CHEMBL3926051) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human whole blood assessed as reduction in LPS-induced PGE2 production preincubated for 30 mins followed by LPS stimulation ... | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194192 (CHEMBL3910746) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human A549 cells assessed as reduction in recombinant human interleukin-1 beta-induced PGE2 production preincubated for 30 m... | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194142 (CHEMBL3947843) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human whole blood assessed as reduction in LPS-induced PGE2 production preincubated for 30 mins followed by LPS stimulation ... | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194142 (CHEMBL3947843) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human A549 cells assessed as reduction in recombinant human interleukin-1 beta-induced PGE2 production preincubated for 30 m... | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194191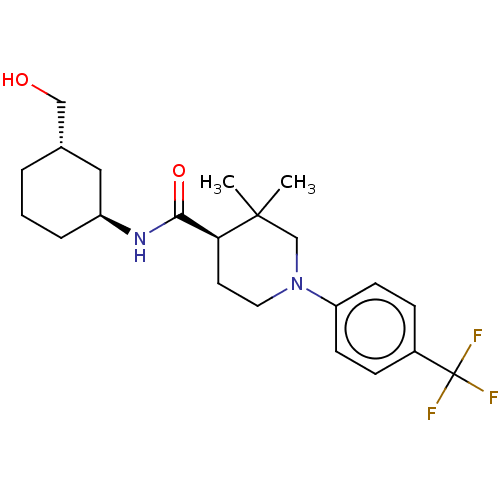 (CHEMBL3919693) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 504 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human mPGES-1 expressed in 293E cells assessed as reduction in conversion of PGH2 to PGE2 after 1.5 min by LC/MS analysis | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human whole blood assessed as reduction in LPS-induced PGE2 production preincubated for 30 mins followed by LPS stimulation ... | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194194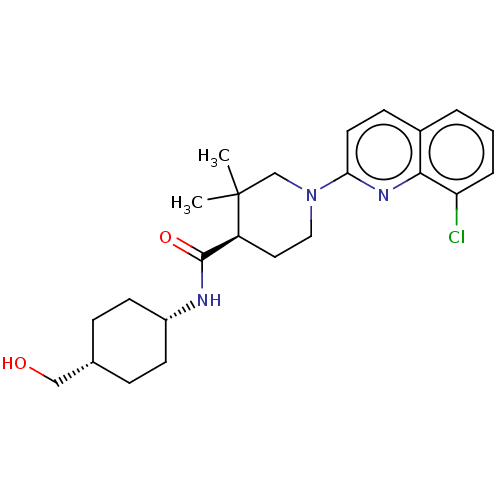 (CHEMBL3950405) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human mPGES-1 expressed in 293E cells assessed as reduction in conversion of PGH2 to PGE2 after 1.5 min by LC/MS analysis | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194191 (CHEMBL3919693) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human A549 cells assessed as reduction in recombinant human interleukin-1 beta-induced PGE2 production preincubated for 30 m... | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194194 (CHEMBL3950405) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human A549 cells assessed as reduction in recombinant human interleukin-1 beta-induced PGE2 production preincubated for 30 m... | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||