 Found 21 hits Enz. Inhib. hit(s) with all data for entry = 50048065
Found 21 hits Enz. Inhib. hit(s) with all data for entry = 50048065 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50131550
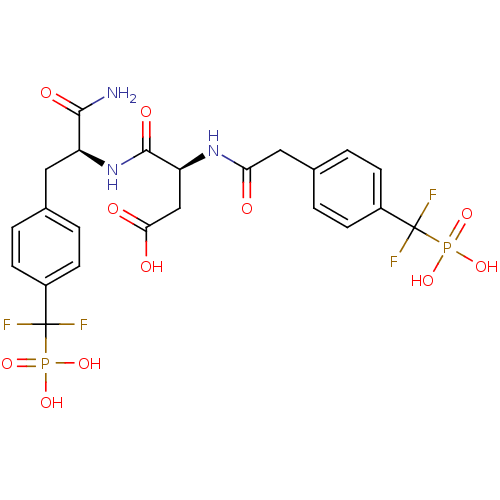
((3S)-3-{[(1S)-1-carbamoyl-2-{4-[difluoro(phosphono...)Show SMILES NC(=O)[C@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)Cc1ccc(cc1)C(F)(F)P(O)(O)=O |r| Show InChI InChI=1S/C23H25F4N3O11P2/c24-22(25,42(36,37)38)14-5-1-12(2-6-14)9-16(20(28)34)30-21(35)17(11-19(32)33)29-18(31)10-13-3-7-15(8-4-13)23(26,27)43(39,40)41/h1-8,16-17H,9-11H2,(H2,28,34)(H,29,31)(H,30,35)(H,32,33)(H2,36,37,38)(H2,39,40,41)/t16-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Eur J Med Chem 122: 756-769 (2016)
Article DOI: 10.1016/j.ejmech.2016.05.060
BindingDB Entry DOI: 10.7270/Q25Q4Z2D |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50308854

(CHEMBL590235 | [7-(4-{1-Benzotriazol-1-yl-2-[4-(di...)Show SMILES COC(CC(C)C)c1ccc2ccc(-c3ccc(cc3)C(Cc3ccc(cc3)C(F)(F)P(O)(O)=O)(c3ccccc3)n3nnc4ccccc34)c(c2n1)P(O)(O)=O Show InChI InChI=1S/C42H40F2N4O7P2/c1-27(2)25-38(55-3)36-24-18-30-17-23-34(40(39(30)45-36)56(49,50)51)29-15-21-32(22-16-29)41(31-9-5-4-6-10-31,48-37-12-8-7-11-35(37)46-47-48)26-28-13-19-33(20-14-28)42(43,44)57(52,53)54/h4-24,27,38H,25-26H2,1-3H3,(H2,49,50,51)(H2,52,53,54) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Eur J Med Chem 122: 756-769 (2016)
Article DOI: 10.1016/j.ejmech.2016.05.060
BindingDB Entry DOI: 10.7270/Q25Q4Z2D |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086955
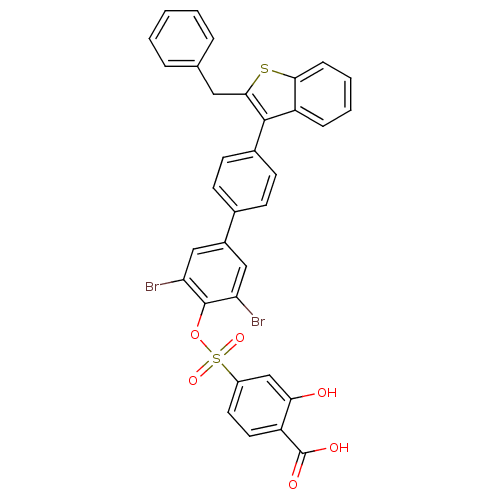
(4-[4''-(2-Benzyl-benzo[b]thiophen-3-yl)-3,5-dibrom...)Show SMILES OC(=O)c1ccc(cc1O)S(=O)(=O)Oc1c(Br)cc(cc1Br)-c1ccc(cc1)-c1c(Cc2ccccc2)sc2ccccc12 Show InChI InChI=1S/C34H22Br2O6S2/c35-27-17-23(18-28(36)33(27)42-44(40,41)24-14-15-25(34(38)39)29(37)19-24)21-10-12-22(13-11-21)32-26-8-4-5-9-30(26)43-31(32)16-20-6-2-1-3-7-20/h1-15,17-19,37H,16H2,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Eur J Med Chem 122: 756-769 (2016)
Article DOI: 10.1016/j.ejmech.2016.05.060
BindingDB Entry DOI: 10.7270/Q25Q4Z2D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50308846
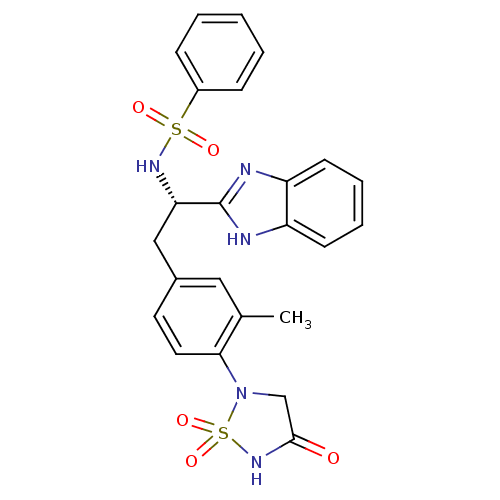
(CHEMBL592245 | N-{(S)-1-(1H-Benzoimidazol-2-yl)-2-...)Show SMILES Cc1cc(C[C@H](NS(=O)(=O)c2ccccc2)c2nc3ccccc3[nH]2)ccc1N1CC(=O)NS1(=O)=O |r| Show InChI InChI=1S/C24H23N5O5S2/c1-16-13-17(11-12-22(16)29-15-23(30)28-36(29,33)34)14-21(24-25-19-9-5-6-10-20(19)26-24)27-35(31,32)18-7-3-2-4-8-18/h2-13,21,27H,14-15H2,1H3,(H,25,26)(H,28,30)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Eur J Med Chem 122: 756-769 (2016)
Article DOI: 10.1016/j.ejmech.2016.05.060
BindingDB Entry DOI: 10.7270/Q25Q4Z2D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50201800
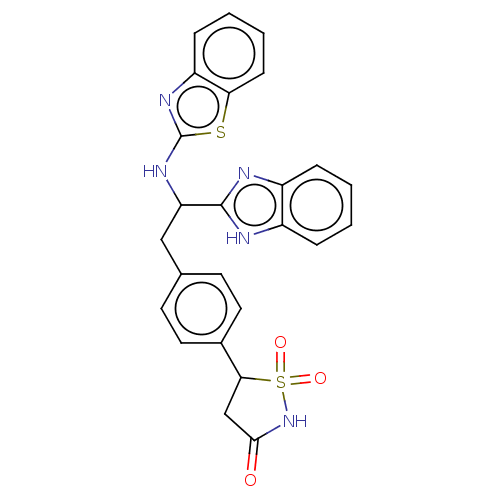
(CHEMBL3901092)Show SMILES O=C1CC(c2ccc(CC(Nc3nc4ccccc4s3)c3nc4ccccc4[nH]3)cc2)S(=O)(=O)N1 Show InChI InChI=1S/C25H21N5O3S2/c31-23-14-22(35(32,33)30-23)16-11-9-15(10-12-16)13-20(24-26-17-5-1-2-6-18(17)27-24)29-25-28-19-7-3-4-8-21(19)34-25/h1-12,20,22H,13-14H2,(H,26,27)(H,28,29)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Eur J Med Chem 122: 756-769 (2016)
Article DOI: 10.1016/j.ejmech.2016.05.060
BindingDB Entry DOI: 10.7270/Q25Q4Z2D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50209683
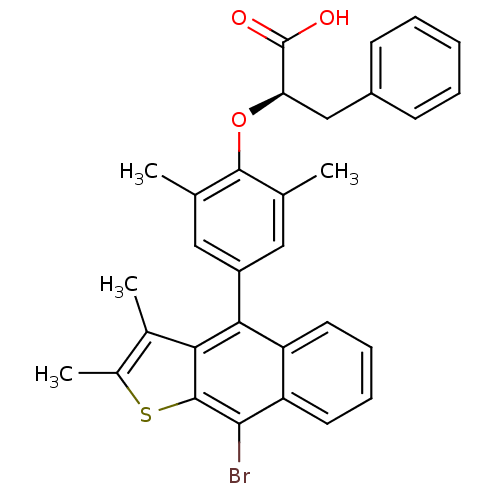
((R)-2-(4-(9-bromo-2,3-dimethylnaphtho[2,3-b]thioph...)Show SMILES Cc1sc2c(Br)c3ccccc3c(-c3cc(C)c(O[C@H](Cc4ccccc4)C(O)=O)c(C)c3)c2c1C Show InChI InChI=1S/C31H27BrO3S/c1-17-14-22(15-18(2)29(17)35-25(31(33)34)16-21-10-6-5-7-11-21)27-23-12-8-9-13-24(23)28(32)30-26(27)19(3)20(4)36-30/h5-15,25H,16H2,1-4H3,(H,33,34)/t25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 384 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Eur J Med Chem 122: 756-769 (2016)
Article DOI: 10.1016/j.ejmech.2016.05.060
BindingDB Entry DOI: 10.7270/Q25Q4Z2D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50201806
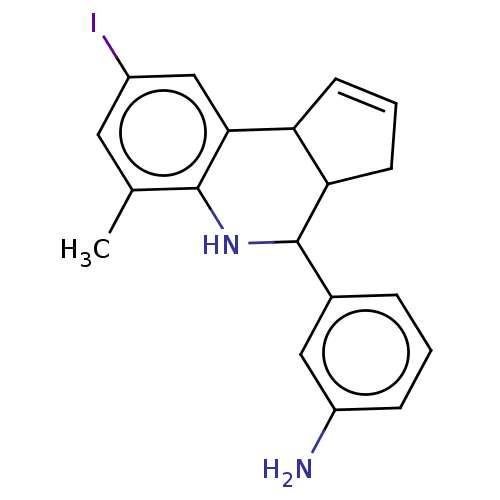
(CHEMBL3905288)Show SMILES Cc1cc(I)cc2C3C=CCC3C(Nc12)c1cccc(N)c1 |c:8| Show InChI InChI=1S/C19H19IN2/c1-11-8-13(20)10-17-15-6-3-7-16(15)19(22-18(11)17)12-4-2-5-14(21)9-12/h2-6,8-10,15-16,19,22H,7,21H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Eur J Med Chem 122: 756-769 (2016)
Article DOI: 10.1016/j.ejmech.2016.05.060
BindingDB Entry DOI: 10.7270/Q25Q4Z2D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50308851
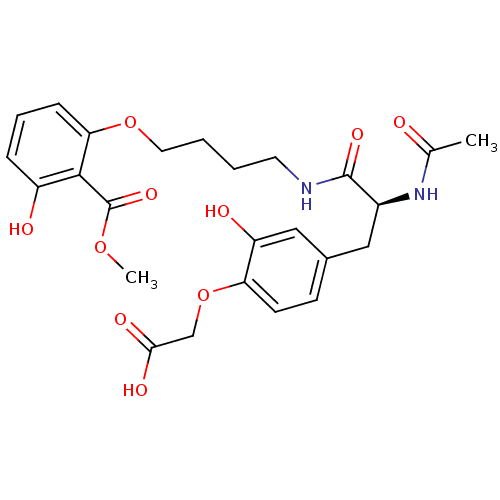
((S)-2-(4-(2-acetamido-3-(4-(3-hydroxy-2-(methoxyca...)Show SMILES COC(=O)c1c(O)cccc1OCCCCNC(=O)[C@H](Cc1ccc(OCC(O)=O)c(O)c1)NC(C)=O |r| Show InChI InChI=1S/C25H30N2O10/c1-15(28)27-17(12-16-8-9-20(19(30)13-16)37-14-22(31)32)24(33)26-10-3-4-11-36-21-7-5-6-18(29)23(21)25(34)35-2/h5-9,13,17,29-30H,3-4,10-12,14H2,1-2H3,(H,26,33)(H,27,28)(H,31,32)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Eur J Med Chem 122: 756-769 (2016)
Article DOI: 10.1016/j.ejmech.2016.05.060
BindingDB Entry DOI: 10.7270/Q25Q4Z2D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50341986
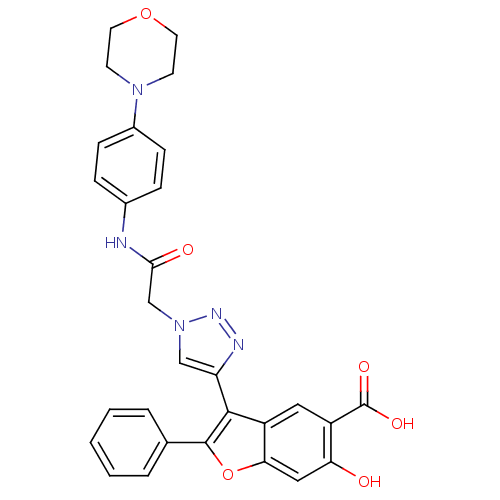
(6-hydroxy-3-(1-(2-(4-morpholinophenylamino)-2-oxoe...)Show SMILES OC(=O)c1cc2c(-c3cn(CC(=O)Nc4ccc(cc4)N4CCOCC4)nn3)c(oc2cc1O)-c1ccccc1 Show InChI InChI=1S/C29H25N5O6/c35-24-15-25-22(14-21(24)29(37)38)27(28(40-25)18-4-2-1-3-5-18)23-16-34(32-31-23)17-26(36)30-19-6-8-20(9-7-19)33-10-12-39-13-11-33/h1-9,14-16,35H,10-13,17H2,(H,30,36)(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Eur J Med Chem 122: 756-769 (2016)
Article DOI: 10.1016/j.ejmech.2016.05.060
BindingDB Entry DOI: 10.7270/Q25Q4Z2D |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50391109

(CHEMBL179166 | Sodium orthovanadate (SOV) | Vanada...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged CDC25B assessed as reduction in dephosphorylation of florigenic phosphatase substrate preincubated for 1 h... |
Eur J Med Chem 122: 756-769 (2016)
Article DOI: 10.1016/j.ejmech.2016.05.060
BindingDB Entry DOI: 10.7270/Q25Q4Z2D |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50201805

(CHEMBL3903987)Show SMILES CCOC(=O)c1c(C)[nH]c(\C=C2/S\C(=N/c3ccccc3)N(C2=O)c2ccccc2)c1C Show InChI InChI=1S/C25H23N3O3S/c1-4-31-24(30)22-16(2)20(26-17(22)3)15-21-23(29)28(19-13-9-6-10-14-19)25(32-21)27-18-11-7-5-8-12-18/h5-15,26H,4H2,1-3H3/b21-15-,27-25- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.66E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged CDC25B assessed as reduction in dephosphorylation of florigenic phosphatase substrate preincubated for 1 h... |
Eur J Med Chem 122: 756-769 (2016)
Article DOI: 10.1016/j.ejmech.2016.05.060
BindingDB Entry DOI: 10.7270/Q25Q4Z2D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50346601

(NSC-114945 | OLEANOLIC_ACID | Oleanolic acid | Ole...)Show SMILES CC1(C)CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2C1)C(O)=O |r,c:10| Show InChI InChI=1S/C30H48O3/c1-25(2)14-16-30(24(32)33)17-15-28(6)19(20(30)18-25)8-9-22-27(5)12-11-23(31)26(3,4)21(27)10-13-29(22,28)7/h8,20-23,31H,9-18H2,1-7H3,(H,32,33)/t20-,21-,22+,23-,27-,28+,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.39E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged PTP1B catalytic domain expressed in Escherichia coli BL21-Conden plus (DE3) using pNPP as substrate |
Eur J Med Chem 122: 756-769 (2016)
Article DOI: 10.1016/j.ejmech.2016.05.060
BindingDB Entry DOI: 10.7270/Q25Q4Z2D |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50201807

(CHEMBL3934071)Show SMILES CC(C)N1\C(S\C(=C/c2ccc(OCCN(C)c3ccccn3)cc2)C1=O)=N\O Show InChI InChI=1S/C21H24N4O3S/c1-15(2)25-20(26)18(29-21(25)23-27)14-16-7-9-17(10-8-16)28-13-12-24(3)19-6-4-5-11-22-19/h4-11,14-15,27H,12-13H2,1-3H3/b18-14-,23-21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.47E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged CDC25B assessed as reduction in dephosphorylation of florigenic phosphatase substrate preincubated for 1 h... |
Eur J Med Chem 122: 756-769 (2016)
Article DOI: 10.1016/j.ejmech.2016.05.060
BindingDB Entry DOI: 10.7270/Q25Q4Z2D |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50201802
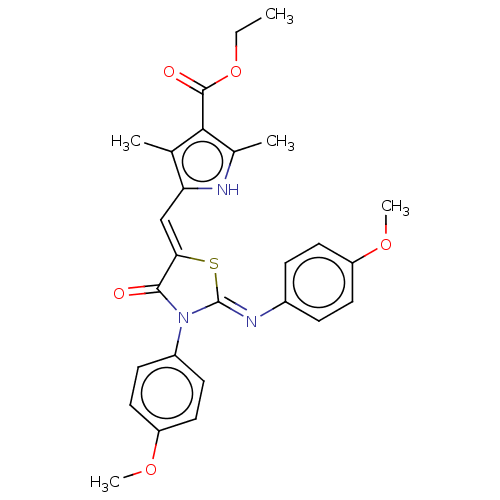
(CHEMBL3981128)Show SMILES CCOC(=O)c1c(C)[nH]c(\C=C2/S\C(=N/c3ccc(OC)cc3)N(C2=O)c2ccc(OC)cc2)c1C Show InChI InChI=1S/C27H27N3O5S/c1-6-35-26(32)24-16(2)22(28-17(24)3)15-23-25(31)30(19-9-13-21(34-5)14-10-19)27(36-23)29-18-7-11-20(33-4)12-8-18/h7-15,28H,6H2,1-5H3/b23-15-,29-27- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.74E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged CDC25B assessed as reduction in dephosphorylation of florigenic phosphatase substrate preincubated for 1 h... |
Eur J Med Chem 122: 756-769 (2016)
Article DOI: 10.1016/j.ejmech.2016.05.060
BindingDB Entry DOI: 10.7270/Q25Q4Z2D |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50201801
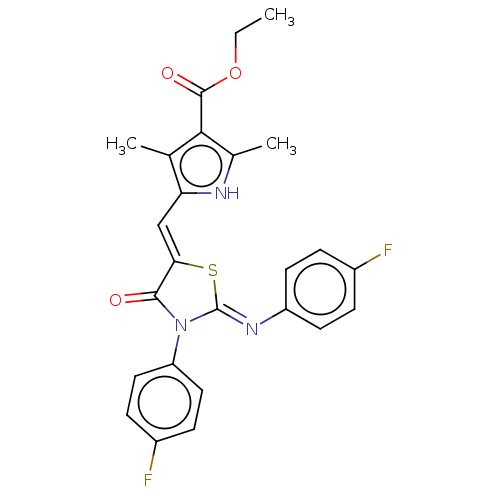
(CHEMBL3942806)Show SMILES CCOC(=O)c1c(C)[nH]c(\C=C2/S\C(=N/c3ccc(F)cc3)N(C2=O)c2ccc(F)cc2)c1C Show InChI InChI=1S/C25H21F2N3O3S/c1-4-33-24(32)22-14(2)20(28-15(22)3)13-21-23(31)30(19-11-7-17(27)8-12-19)25(34-21)29-18-9-5-16(26)6-10-18/h5-13,28H,4H2,1-3H3/b21-13-,29-25- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.87E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged CDC25B assessed as reduction in dephosphorylation of florigenic phosphatase substrate preincubated for 1 h... |
Eur J Med Chem 122: 756-769 (2016)
Article DOI: 10.1016/j.ejmech.2016.05.060
BindingDB Entry DOI: 10.7270/Q25Q4Z2D |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50201798
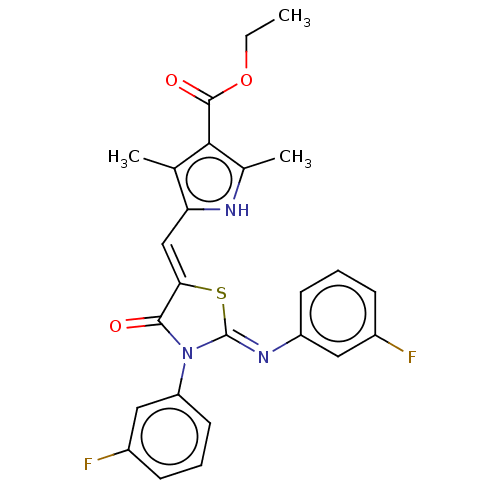
(CHEMBL3944187)Show SMILES CCOC(=O)c1c(C)[nH]c(\C=C2/S\C(=N/c3cccc(F)c3)N(C2=O)c2cccc(F)c2)c1C Show InChI InChI=1S/C25H21F2N3O3S/c1-4-33-24(32)22-14(2)20(28-15(22)3)13-21-23(31)30(19-10-6-8-17(27)12-19)25(34-21)29-18-9-5-7-16(26)11-18/h5-13,28H,4H2,1-3H3/b21-13-,29-25- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.39E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged CDC25B assessed as reduction in dephosphorylation of florigenic phosphatase substrate preincubated for 1 h... |
Eur J Med Chem 122: 756-769 (2016)
Article DOI: 10.1016/j.ejmech.2016.05.060
BindingDB Entry DOI: 10.7270/Q25Q4Z2D |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50201804
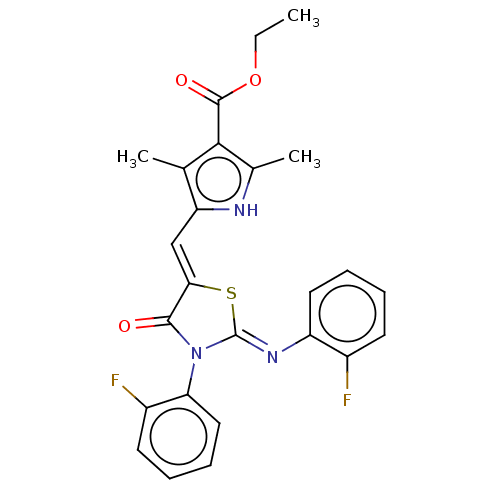
(CHEMBL3952328)Show SMILES CCOC(=O)c1c(C)[nH]c(\C=C2/S\C(=N/c3ccccc3F)N(C2=O)c2ccccc2F)c1C Show InChI InChI=1S/C25H21F2N3O3S/c1-4-33-24(32)22-14(2)19(28-15(22)3)13-21-23(31)30(20-12-8-6-10-17(20)27)25(34-21)29-18-11-7-5-9-16(18)26/h5-13,28H,4H2,1-3H3/b21-13-,29-25- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.66E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged CDC25B assessed as reduction in dephosphorylation of florigenic phosphatase substrate preincubated for 1 h... |
Eur J Med Chem 122: 756-769 (2016)
Article DOI: 10.1016/j.ejmech.2016.05.060
BindingDB Entry DOI: 10.7270/Q25Q4Z2D |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50201803
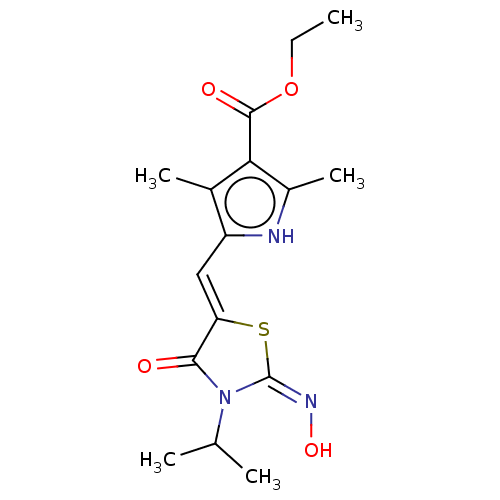
(CHEMBL3908947)Show SMILES CCOC(=O)c1c(C)[nH]c(\C=C2/S\C(=N\O)N(C(C)C)C2=O)c1C Show InChI InChI=1S/C16H21N3O4S/c1-6-23-15(21)13-9(4)11(17-10(13)5)7-12-14(20)19(8(2)3)16(18-22)24-12/h7-8,17,22H,6H2,1-5H3/b12-7-,18-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.83E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged CDC25B assessed as reduction in dephosphorylation of florigenic phosphatase substrate preincubated for 1 h... |
Eur J Med Chem 122: 756-769 (2016)
Article DOI: 10.1016/j.ejmech.2016.05.060
BindingDB Entry DOI: 10.7270/Q25Q4Z2D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50201801

(CHEMBL3942806)Show SMILES CCOC(=O)c1c(C)[nH]c(\C=C2/S\C(=N/c3ccc(F)cc3)N(C2=O)c2ccc(F)cc2)c1C Show InChI InChI=1S/C25H21F2N3O3S/c1-4-33-24(32)22-14(2)20(28-15(22)3)13-21-23(31)30(19-11-7-17(27)8-12-19)25(34-21)29-18-9-5-16(26)6-10-18/h5-13,28H,4H2,1-3H3/b21-13-,29-25- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.09E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged PTP1B catalytic domain expressed in Escherichia coli BL21-Conden plus (DE3) using pNPP as substrate |
Eur J Med Chem 122: 756-769 (2016)
Article DOI: 10.1016/j.ejmech.2016.05.060
BindingDB Entry DOI: 10.7270/Q25Q4Z2D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50201798

(CHEMBL3944187)Show SMILES CCOC(=O)c1c(C)[nH]c(\C=C2/S\C(=N/c3cccc(F)c3)N(C2=O)c2cccc(F)c2)c1C Show InChI InChI=1S/C25H21F2N3O3S/c1-4-33-24(32)22-14(2)20(28-15(22)3)13-21-23(31)30(19-10-6-8-17(27)12-19)25(34-21)29-18-9-5-7-16(26)11-18/h5-13,28H,4H2,1-3H3/b21-13-,29-25- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.83E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged PTP1B catalytic domain expressed in Escherichia coli BL21-Conden plus (DE3) using pNPP as substrate |
Eur J Med Chem 122: 756-769 (2016)
Article DOI: 10.1016/j.ejmech.2016.05.060
BindingDB Entry DOI: 10.7270/Q25Q4Z2D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50201805

(CHEMBL3903987)Show SMILES CCOC(=O)c1c(C)[nH]c(\C=C2/S\C(=N/c3ccccc3)N(C2=O)c2ccccc2)c1C Show InChI InChI=1S/C25H23N3O3S/c1-4-31-24(30)22-16(2)20(26-17(22)3)15-21-23(29)28(19-13-9-6-10-14-19)25(32-21)27-18-11-7-5-8-12-18/h5-15,26H,4H2,1-3H3/b21-15-,27-25- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.66E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged PTP1B catalytic domain expressed in Escherichia coli BL21-Conden plus (DE3) using pNPP as substrate |
Eur J Med Chem 122: 756-769 (2016)
Article DOI: 10.1016/j.ejmech.2016.05.060
BindingDB Entry DOI: 10.7270/Q25Q4Z2D |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data