 Found 51 hits Enz. Inhib. hit(s) with all data for entry = 50031199
Found 51 hits Enz. Inhib. hit(s) with all data for entry = 50031199 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50131550
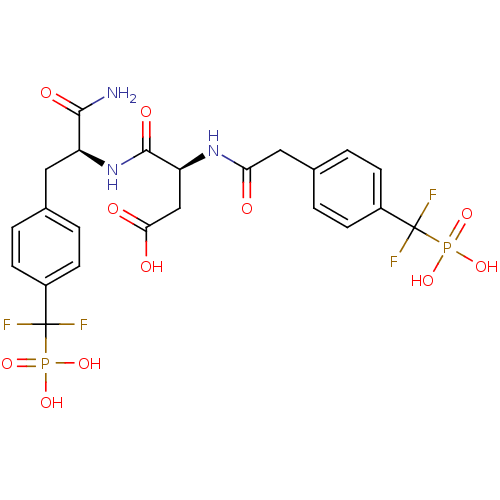
((3S)-3-{[(1S)-1-carbamoyl-2-{4-[difluoro(phosphono...)Show SMILES NC(=O)[C@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)Cc1ccc(cc1)C(F)(F)P(O)(O)=O |r| Show InChI InChI=1S/C23H25F4N3O11P2/c24-22(25,42(36,37)38)14-5-1-12(2-6-14)9-16(20(28)34)30-21(35)17(11-19(32)33)29-18(31)10-13-3-7-15(8-4-13)23(26,27)43(39,40)41/h1-8,16-17H,9-11H2,(H2,28,34)(H,29,31)(H,30,35)(H,32,33)(H2,36,37,38)(H2,39,40,41)/t16-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50219566
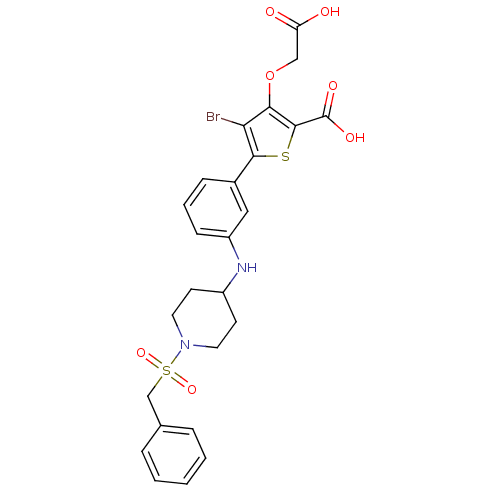
(4-bromo-3-carboxymethoxy-5-[3-(1-phenylmethanesulf...)Show SMILES OC(=O)COc1c(Br)c(sc1C(O)=O)-c1cccc(NC2CCN(CC2)S(=O)(=O)Cc2ccccc2)c1 Show InChI InChI=1S/C25H25BrN2O7S2/c26-21-22(35-14-20(29)30)24(25(31)32)36-23(21)17-7-4-8-19(13-17)27-18-9-11-28(12-10-18)37(33,34)15-16-5-2-1-3-6-16/h1-8,13,18,27H,9-12,14-15H2,(H,29,30)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50219566

(4-bromo-3-carboxymethoxy-5-[3-(1-phenylmethanesulf...)Show SMILES OC(=O)COc1c(Br)c(sc1C(O)=O)-c1cccc(NC2CCN(CC2)S(=O)(=O)Cc2ccccc2)c1 Show InChI InChI=1S/C25H25BrN2O7S2/c26-21-22(35-14-20(29)30)24(25(31)32)36-23(21)17-7-4-8-19(13-17)27-18-9-11-28(12-10-18)37(33,34)15-16-5-2-1-3-6-16/h1-8,13,18,27H,9-12,14-15H2,(H,29,30)(H,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50308852

((S)-2-(N-(4-(2-acetamido-3-(4-(3-hydroxy-2-(methox...)Show SMILES CCc1cc(C[C@H](NC(C)=O)C(=O)NCCCCOc2cccc(O)c2C(=O)OC)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O |r| Show InChI InChI=1S/C34H37N3O11/c1-4-22-18-21(14-15-25(22)37(31(41)33(44)45)26-11-6-5-10-23(26)32(42)43)19-24(36-20(2)38)30(40)35-16-7-8-17-48-28-13-9-12-27(39)29(28)34(46)47-3/h5-6,9-15,18,24,39H,4,7-8,16-17,19H2,1-3H3,(H,35,40)(H,36,38)(H,42,43)(H,44,45)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50308849

((S)-2-(carboxyformamido)-7-((5-fluoro-1H-indole-2-...)Show SMILES OC(=O)C(=O)Nc1sc2[C@H](CNC(=O)c3cc4cc(F)ccc4[nH]3)NCCc2c1C(O)=O |r| Show InChI InChI=1S/C20H17FN4O6S/c21-9-1-2-11-8(5-9)6-12(24-11)16(26)23-7-13-15-10(3-4-22-13)14(19(28)29)18(32-15)25-17(27)20(30)31/h1-2,5-6,13,22,24H,3-4,7H2,(H,23,26)(H,25,27)(H,28,29)(H,30,31)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50131550

((3S)-3-{[(1S)-1-carbamoyl-2-{4-[difluoro(phosphono...)Show SMILES NC(=O)[C@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)Cc1ccc(cc1)C(F)(F)P(O)(O)=O |r| Show InChI InChI=1S/C23H25F4N3O11P2/c24-22(25,42(36,37)38)14-5-1-12(2-6-14)9-16(20(28)34)30-21(35)17(11-19(32)33)29-18(31)10-13-3-7-15(8-4-13)23(26,27)43(39,40)41/h1-8,16-17H,9-11H2,(H2,28,34)(H,29,31)(H,30,35)(H,32,33)(H2,36,37,38)(H2,39,40,41)/t16-,17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50308852

((S)-2-(N-(4-(2-acetamido-3-(4-(3-hydroxy-2-(methox...)Show SMILES CCc1cc(C[C@H](NC(C)=O)C(=O)NCCCCOc2cccc(O)c2C(=O)OC)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O |r| Show InChI InChI=1S/C34H37N3O11/c1-4-22-18-21(14-15-25(22)37(31(41)33(44)45)26-11-6-5-10-23(26)32(42)43)19-24(36-20(2)38)30(40)35-16-7-8-17-48-28-13-9-12-27(39)29(28)34(46)47-3/h5-6,9-15,18,24,39H,4,7-8,16-17,19H2,1-3H3,(H,35,40)(H,36,38)(H,42,43)(H,44,45)/t24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50308836
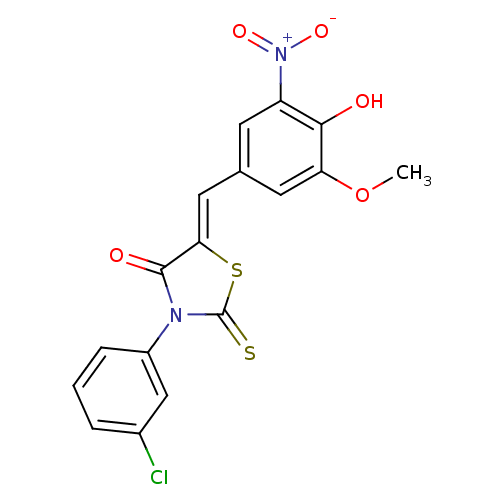
(3-(3-Chloro-phenyl)-5-[1-(4-hydroxy-3-methoxy-5-ni...)Show SMILES COc1cc(\C=C2/SC(=S)N(C2=O)c2cccc(Cl)c2)cc(c1O)[N+]([O-])=O Show InChI InChI=1S/C17H11ClN2O5S2/c1-25-13-6-9(5-12(15(13)21)20(23)24)7-14-16(22)19(17(26)27-14)11-4-2-3-10(18)8-11/h2-8,21H,1H3/b14-7- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B by cell-based competitive inhibition assay |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13996
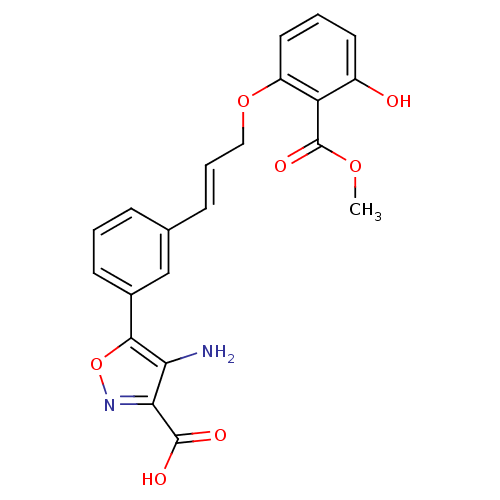
(4-amino-5-{3-[(1E)-3-[3-hydroxy-2-(methoxycarbonyl...)Show SMILES COC(=O)c1c(O)cccc1OC\C=C\c1cccc(c1)-c1onc(C(O)=O)c1N Show InChI InChI=1S/C21H18N2O7/c1-28-21(27)16-14(24)8-3-9-15(16)29-10-4-6-12-5-2-7-13(11-12)19-17(22)18(20(25)26)23-30-19/h2-9,11,24H,10,22H2,1H3,(H,25,26)/b6-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50308843
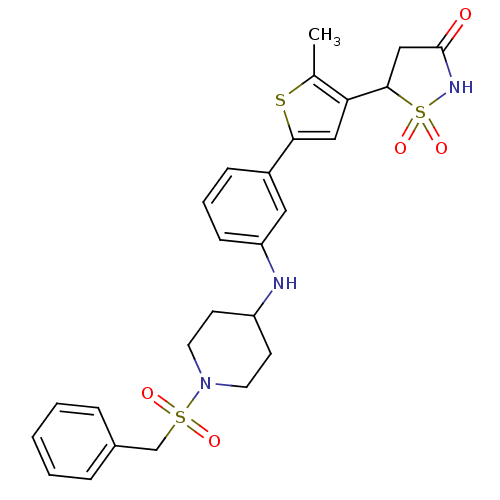
(5-{2-Methyl-5-[3-(1-phenylmethanesulfonyl-piperidi...)Show SMILES Cc1sc(cc1C1CC(=O)NS1(=O)=O)-c1cccc(NC2CCN(CC2)S(=O)(=O)Cc2ccccc2)c1 Show InChI InChI=1S/C26H29N3O5S3/c1-18-23(25-16-26(30)28-37(25,33)34)15-24(35-18)20-8-5-9-22(14-20)27-21-10-12-29(13-11-21)36(31,32)17-19-6-3-2-4-7-19/h2-9,14-15,21,25,27H,10-13,16-17H2,1H3,(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50308850
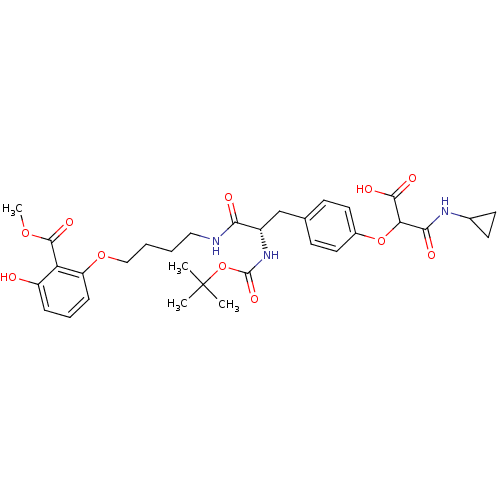
(2-(4-((S)-2-(tert-butoxycarbonylamino)-3-(4-(3-hyd...)Show SMILES COC(=O)c1c(O)cccc1OCCCCNC(=O)[C@H](Cc1ccc(OC(C(O)=O)C(=O)NC2CC2)cc1)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C32H41N3O11/c1-32(2,3)46-31(42)35-22(18-19-10-14-21(15-11-19)45-26(29(39)40)28(38)34-20-12-13-20)27(37)33-16-5-6-17-44-24-9-7-8-23(36)25(24)30(41)43-4/h7-11,14-15,20,22,26,36H,5-6,12-13,16-18H2,1-4H3,(H,33,37)(H,34,38)(H,35,42)(H,39,40)/t22-,26?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50308851
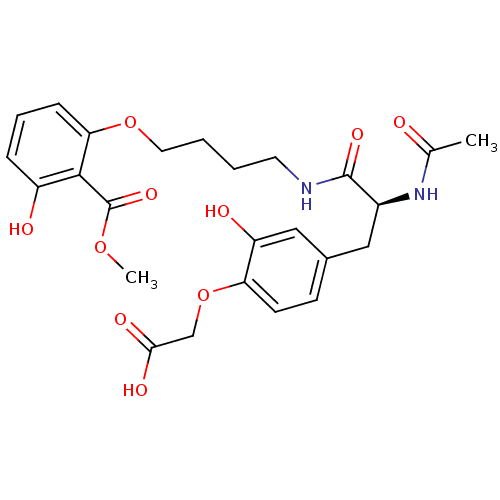
((S)-2-(4-(2-acetamido-3-(4-(3-hydroxy-2-(methoxyca...)Show SMILES COC(=O)c1c(O)cccc1OCCCCNC(=O)[C@H](Cc1ccc(OCC(O)=O)c(O)c1)NC(C)=O |r| Show InChI InChI=1S/C25H30N2O10/c1-15(28)27-17(12-16-8-9-20(19(30)13-16)37-14-22(31)32)24(33)26-10-3-4-11-36-21-7-5-6-18(29)23(21)25(34)35-2/h5-9,13,17,29-30H,3-4,10-12,14H2,1-2H3,(H,26,33)(H,27,28)(H,31,32)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50308851

((S)-2-(4-(2-acetamido-3-(4-(3-hydroxy-2-(methoxyca...)Show SMILES COC(=O)c1c(O)cccc1OCCCCNC(=O)[C@H](Cc1ccc(OCC(O)=O)c(O)c1)NC(C)=O |r| Show InChI InChI=1S/C25H30N2O10/c1-15(28)27-17(12-16-8-9-20(19(30)13-16)37-14-22(31)32)24(33)26-10-3-4-11-36-21-7-5-6-18(29)23(21)25(34)35-2/h5-9,13,17,29-30H,3-4,10-12,14H2,1-2H3,(H,26,33)(H,27,28)(H,31,32)/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.82E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50308850

(2-(4-((S)-2-(tert-butoxycarbonylamino)-3-(4-(3-hyd...)Show SMILES COC(=O)c1c(O)cccc1OCCCCNC(=O)[C@H](Cc1ccc(OC(C(O)=O)C(=O)NC2CC2)cc1)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C32H41N3O11/c1-32(2,3)46-31(42)35-22(18-19-10-14-21(15-11-19)45-26(29(39)40)28(38)34-20-12-13-20)27(37)33-16-5-6-17-44-24-9-7-8-23(36)25(24)30(41)43-4/h7-11,14-15,20,22,26,36H,5-6,12-13,16-18H2,1-4H3,(H,33,37)(H,34,38)(H,35,42)(H,39,40)/t22-,26?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase beta
(Homo sapiens (Human)) | BDBM50308835

(4-((S)-2-(4-ethylthiazol-2-yl)-2-((S)-2-(methoxyca...)Show SMILES CCc1csc(n1)[C@H](Cc1ccc(NS(O)(=O)=O)cc1)NC(=O)[C@H](CC(C)C)C(=O)OC |r| Show InChI InChI=1S/C21H29N3O6S2/c1-5-15-12-31-20(22-15)18(23-19(25)17(10-13(2)3)21(26)30-4)11-14-6-8-16(9-7-14)24-32(27,28)29/h6-9,12-13,17-18,24H,5,10-11H2,1-4H3,(H,23,25)(H,27,28,29)/t17-,18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human protein-tyrosine phosphatase beta |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50308854

(CHEMBL590235 | [7-(4-{1-Benzotriazol-1-yl-2-[4-(di...)Show SMILES COC(CC(C)C)c1ccc2ccc(-c3ccc(cc3)C(Cc3ccc(cc3)C(F)(F)P(O)(O)=O)(c3ccccc3)n3nnc4ccccc34)c(c2n1)P(O)(O)=O Show InChI InChI=1S/C42H40F2N4O7P2/c1-27(2)25-38(55-3)36-24-18-30-17-23-34(40(39(30)45-36)56(49,50)51)29-15-21-32(22-16-29)41(31-9-5-4-6-10-31,48-37-12-8-7-11-35(37)46-47-48)26-28-13-19-33(20-14-28)42(43,44)57(52,53)54/h4-24,27,38H,25-26H2,1-3H3,(H2,49,50,51)(H2,52,53,54) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50308841
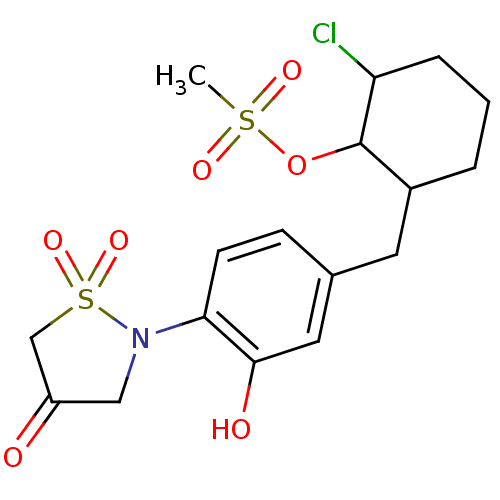
(CHEMBL604457 | Methanesulfonic acid 2-chloro-6-[3-...)Show SMILES CS(=O)(=O)OC1C(Cl)CCCC1Cc1ccc(N2CC(=O)CS2(=O)=O)c(O)c1 Show InChI InChI=1S/C17H22ClNO7S2/c1-27(22,23)26-17-12(3-2-4-14(17)18)7-11-5-6-15(16(21)8-11)19-9-13(20)10-28(19,24)25/h5-6,8,12,14,17,21H,2-4,7,9-10H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50308842
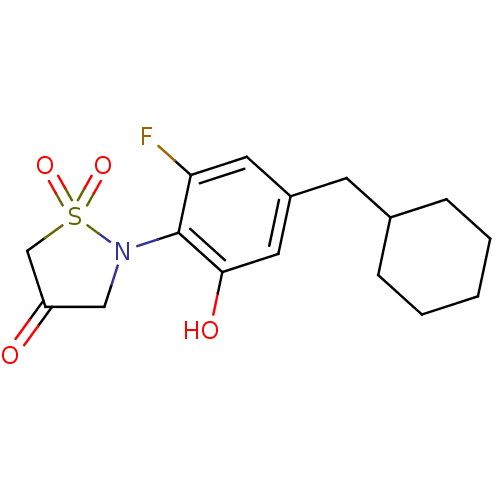
(2-(4-Cyclohexylmethyl-2-fluoro-6-hydroxy-phenyl)-1...)Show InChI InChI=1S/C16H20FNO4S/c17-14-7-12(6-11-4-2-1-3-5-11)8-15(20)16(14)18-9-13(19)10-23(18,21)22/h7-8,11,20H,1-6,9-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
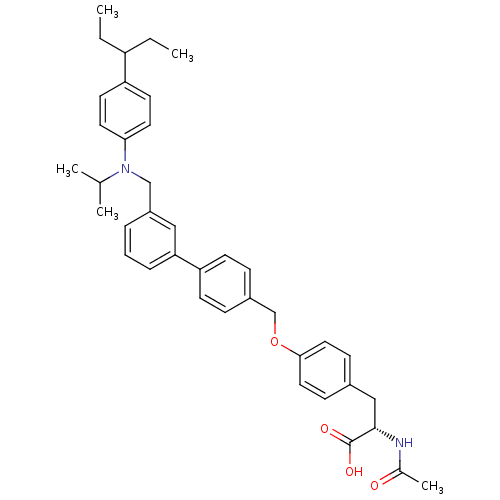
(Homo sapiens (Human)) | BDBM50308847
((S)-2-acetamido-3-(4-((3'-((isopropyl(4-(pentan-3-...)Show SMILES CCC(CC)c1ccc(cc1)N(Cc1cccc(c1)-c1ccc(COc2ccc(C[C@H](NC(C)=O)C(O)=O)cc2)cc1)C(C)C |r| Show InChI InChI=1S/C39H46N2O4/c1-6-32(7-2)33-17-19-36(20-18-33)41(27(3)4)25-31-9-8-10-35(23-31)34-15-11-30(12-16-34)26-45-37-21-13-29(14-22-37)24-38(39(43)44)40-28(5)42/h8-23,27,32,38H,6-7,24-26H2,1-5H3,(H,40,42)(H,43,44)/t38-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50308844
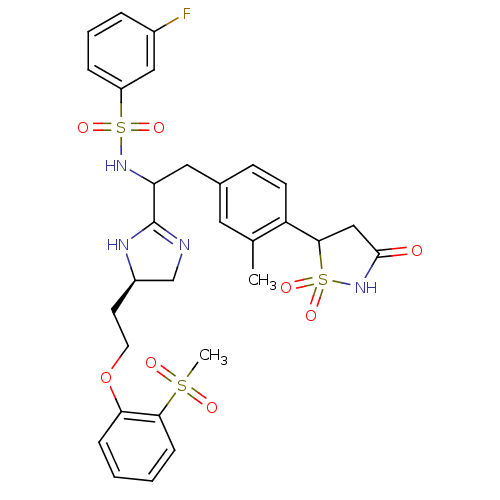
(3-Fluoro-N-{1-{(R)-4-[2-(2-methanesulfonyl-phenoxy...)Show SMILES Cc1cc(CC(NS(=O)(=O)c2cccc(F)c2)C2=NC[C@@H](CCOc3ccccc3S(C)(=O)=O)N2)ccc1C1CC(=O)NS1(=O)=O |r,t:18| Show InChI InChI=1S/C30H33FN4O8S3/c1-19-14-20(10-11-24(19)28-17-29(36)35-46(28,41)42)15-25(34-45(39,40)23-7-5-6-21(31)16-23)30-32-18-22(33-30)12-13-43-26-8-3-4-9-27(26)44(2,37)38/h3-11,14,16,22,25,28,34H,12-13,15,17-18H2,1-2H3,(H,32,33)(H,35,36)/t22-,25?,28?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50308844

(3-Fluoro-N-{1-{(R)-4-[2-(2-methanesulfonyl-phenoxy...)Show SMILES Cc1cc(CC(NS(=O)(=O)c2cccc(F)c2)C2=NC[C@@H](CCOc3ccccc3S(C)(=O)=O)N2)ccc1C1CC(=O)NS1(=O)=O |r,t:18| Show InChI InChI=1S/C30H33FN4O8S3/c1-19-14-20(10-11-24(19)28-17-29(36)35-46(28,41)42)15-25(34-45(39,40)23-7-5-6-21(31)16-23)30-32-18-22(33-30)12-13-43-26-8-3-4-9-27(26)44(2,37)38/h3-11,14,16,22,25,28,34H,12-13,15,17-18H2,1-2H3,(H,32,33)(H,35,36)/t22-,25?,28?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM14269
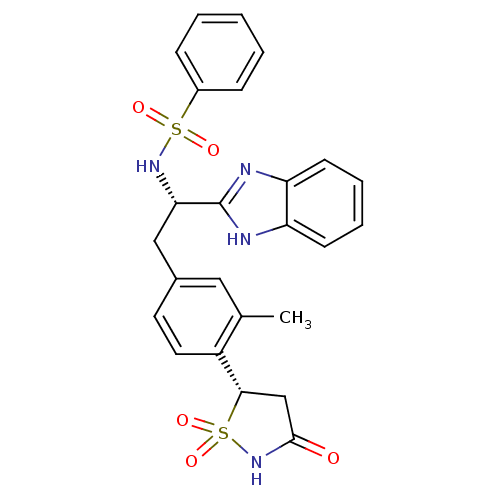
((S)-isothiazolidinone | IZD deriv. 2 | N-[(1S)-1-(...)Show SMILES Cc1cc(C[C@H](NS(=O)(=O)c2ccccc2)c2nc3ccccc3[nH]2)ccc1[C@@H]1CC(=O)NS1(=O)=O |r| Show InChI InChI=1S/C25H24N4O5S2/c1-16-13-17(11-12-19(16)23-15-24(30)29-36(23,33)34)14-22(25-26-20-9-5-6-10-21(20)27-25)28-35(31,32)18-7-3-2-4-8-18/h2-13,22-23,28H,14-15H2,1H3,(H,26,27)(H,29,30)/t22-,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50308845
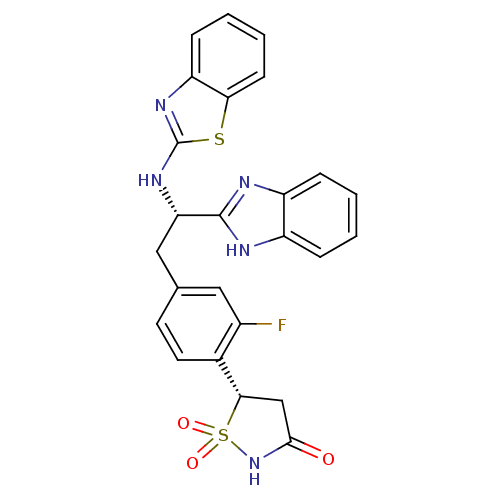
((S)-5-{4-[(S)-2-(1H-Benzoimidazol-2-yl)-2-(benzoth...)Show SMILES Fc1cc(C[C@H](Nc2nc3ccccc3s2)c2nc3ccccc3[nH]2)ccc1[C@@H]1CC(=O)NS1(=O)=O |r| Show InChI InChI=1S/C25H20FN5O3S2/c26-16-11-14(9-10-15(16)22-13-23(32)31-36(22,33)34)12-20(24-27-17-5-1-2-6-18(17)28-24)30-25-29-19-7-3-4-8-21(19)35-25/h1-11,20,22H,12-13H2,(H,27,28)(H,29,30)(H,31,32)/t20-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171117

(2-(4-(N-(3-bromo-4-(difluoro(phosphono)methyl)benz...)Show SMILES CN(C)S(=O)(=O)c1ccc(CN(Cc2ccc(c(Br)c2)C(F)(F)P(O)(O)=O)S(=O)(=O)c2ccc(OCC(O)=O)cc2)cc1 Show InChI InChI=1S/C25H26BrF2N2O10PS2/c1-29(2)42(36,37)20-8-3-17(4-9-20)14-30(43(38,39)21-10-6-19(7-11-21)40-16-24(31)32)15-18-5-12-22(23(26)13-18)25(27,28)41(33,34)35/h3-13H,14-16H2,1-2H3,(H,31,32)(H2,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50171117

(2-(4-(N-(3-bromo-4-(difluoro(phosphono)methyl)benz...)Show SMILES CN(C)S(=O)(=O)c1ccc(CN(Cc2ccc(c(Br)c2)C(F)(F)P(O)(O)=O)S(=O)(=O)c2ccc(OCC(O)=O)cc2)cc1 Show InChI InChI=1S/C25H26BrF2N2O10PS2/c1-29(2)42(36,37)20-8-3-17(4-9-20)14-30(43(38,39)21-10-6-19(7-11-21)40-16-24(31)32)15-18-5-12-22(23(26)13-18)25(27,28)41(33,34)35/h3-13H,14-16H2,1-2H3,(H,31,32)(H2,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50025835
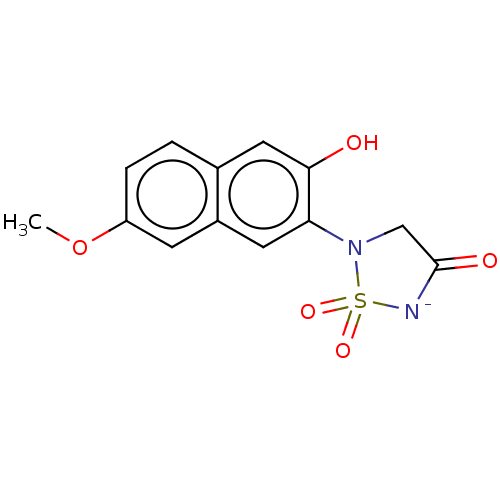
(CHEMBL592291)Show SMILES [K+].COc1ccc2cc(O)c(cc2c1)N1CC(=O)[N-]S1(=O)=O Show InChI InChI=1S/C13H12N2O5S/c1-20-10-3-2-8-6-12(16)11(5-9(8)4-10)15-7-13(17)14-21(15,18)19/h2-6H,7H2,1H3,(H2,14,16,17)/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <30 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM14269

((S)-isothiazolidinone | IZD deriv. 2 | N-[(1S)-1-(...)Show SMILES Cc1cc(C[C@H](NS(=O)(=O)c2ccccc2)c2nc3ccccc3[nH]2)ccc1[C@@H]1CC(=O)NS1(=O)=O |r| Show InChI InChI=1S/C25H24N4O5S2/c1-16-13-17(11-12-19(16)23-15-24(30)29-36(23,33)34)14-22(25-26-20-9-5-6-10-21(20)27-25)28-35(31,32)18-7-3-2-4-8-18/h2-13,22-23,28H,14-15H2,1H3,(H,26,27)(H,29,30)/t22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50308854

(CHEMBL590235 | [7-(4-{1-Benzotriazol-1-yl-2-[4-(di...)Show SMILES COC(CC(C)C)c1ccc2ccc(-c3ccc(cc3)C(Cc3ccc(cc3)C(F)(F)P(O)(O)=O)(c3ccccc3)n3nnc4ccccc34)c(c2n1)P(O)(O)=O Show InChI InChI=1S/C42H40F2N4O7P2/c1-27(2)25-38(55-3)36-24-18-30-17-23-34(40(39(30)45-36)56(49,50)51)29-15-21-32(22-16-29)41(31-9-5-4-6-10-31,48-37-12-8-7-11-35(37)46-47-48)26-28-13-19-33(20-14-28)42(43,44)57(52,53)54/h4-24,27,38H,25-26H2,1-3H3,(H2,49,50,51)(H2,52,53,54) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50308835

(4-((S)-2-(4-ethylthiazol-2-yl)-2-((S)-2-(methoxyca...)Show SMILES CCc1csc(n1)[C@H](Cc1ccc(NS(O)(=O)=O)cc1)NC(=O)[C@H](CC(C)C)C(=O)OC |r| Show InChI InChI=1S/C21H29N3O6S2/c1-5-15-12-31-20(22-15)18(23-19(25)17(10-13(2)3)21(26)30-4)11-14-6-8-16(9-7-14)24-32(27,28)29/h6-9,12-13,17-18,24H,5,10-11H2,1-4H3,(H,23,25)(H,27,28,29)/t17-,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50308840
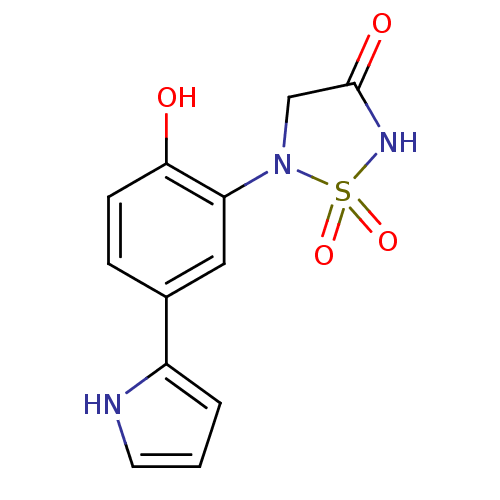
(5-[2-Hydroxy-5-(1H-pyrrol-2-yl)-phenyl]-1,1-dioxo-...)Show InChI InChI=1S/C12H11N3O4S/c16-11-4-3-8(9-2-1-5-13-9)6-10(11)15-7-12(17)14-20(15,18)19/h1-6,13,16H,7H2,(H,14,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50239833
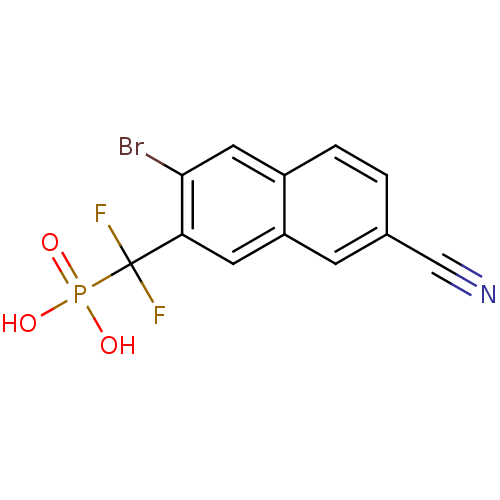
((3-bromo-7-cyanonaphthalen-2-yl)difluoromethylphos...)Show InChI InChI=1S/C12H7BrF2NO3P/c13-11-5-8-2-1-7(6-16)3-9(8)4-10(11)12(14,15)20(17,18)19/h1-5H,(H2,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50239833

((3-bromo-7-cyanonaphthalen-2-yl)difluoromethylphos...)Show InChI InChI=1S/C12H7BrF2NO3P/c13-11-5-8-2-1-7(6-16)3-9(8)4-10(11)12(14,15)20(17,18)19/h1-5H,(H2,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50142317
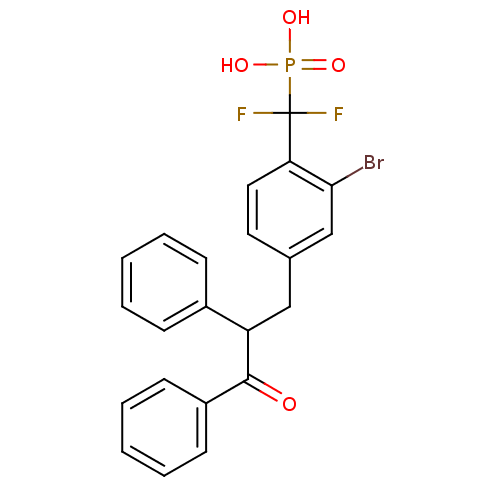
((2-bromo-4-(3-oxo-2,3-diphenylpropyl)phenyl)difluo...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(C(=O)c2ccccc2)c2ccccc2)cc1Br Show InChI InChI=1S/C22H18BrF2O4P/c23-20-14-15(11-12-19(20)22(24,25)30(27,28)29)13-18(16-7-3-1-4-8-16)21(26)17-9-5-2-6-10-17/h1-12,14,18H,13H2,(H2,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50142317

((2-bromo-4-(3-oxo-2,3-diphenylpropyl)phenyl)difluo...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(C(=O)c2ccccc2)c2ccccc2)cc1Br Show InChI InChI=1S/C22H18BrF2O4P/c23-20-14-15(11-12-19(20)22(24,25)30(27,28)29)13-18(16-7-3-1-4-8-16)21(26)17-9-5-2-6-10-17/h1-12,14,18H,13H2,(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50308839
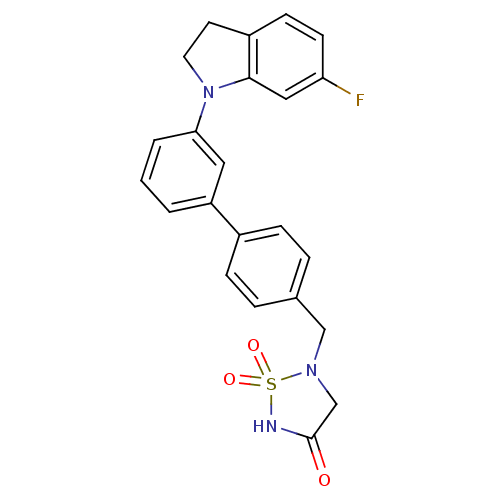
(5-[3'-(6-Fluoro-2,3-dihydro-indol-1-yl)-biphenyl-4...)Show SMILES Fc1ccc2CCN(c2c1)c1cccc(c1)-c1ccc(CN2CC(=O)NS2(=O)=O)cc1 Show InChI InChI=1S/C23H20FN3O3S/c24-20-9-8-18-10-11-27(22(18)13-20)21-3-1-2-19(12-21)17-6-4-16(5-7-17)14-26-15-23(28)25-31(26,29)30/h1-9,12-13H,10-11,14-15H2,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50308846
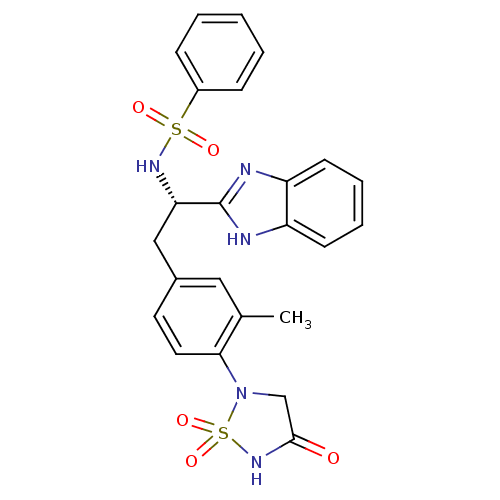
(CHEMBL592245 | N-{(S)-1-(1H-Benzoimidazol-2-yl)-2-...)Show SMILES Cc1cc(C[C@H](NS(=O)(=O)c2ccccc2)c2nc3ccccc3[nH]2)ccc1N1CC(=O)NS1(=O)=O |r| Show InChI InChI=1S/C24H23N5O5S2/c1-16-13-17(11-12-22(16)29-15-23(30)28-36(29,33)34)14-21(24-25-19-9-5-6-10-20(19)26-24)27-35(31,32)18-7-3-2-4-8-18/h2-13,21,27H,14-15H2,1H3,(H,25,26)(H,28,30)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50308846

(CHEMBL592245 | N-{(S)-1-(1H-Benzoimidazol-2-yl)-2-...)Show SMILES Cc1cc(C[C@H](NS(=O)(=O)c2ccccc2)c2nc3ccccc3[nH]2)ccc1N1CC(=O)NS1(=O)=O |r| Show InChI InChI=1S/C24H23N5O5S2/c1-16-13-17(11-12-22(16)29-15-23(30)28-36(29,33)34)14-21(24-25-19-9-5-6-10-20(19)26-24)27-35(31,32)18-7-3-2-4-8-18/h2-13,21,27H,14-15H2,1H3,(H,25,26)(H,28,30)/t21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50308845

((S)-5-{4-[(S)-2-(1H-Benzoimidazol-2-yl)-2-(benzoth...)Show SMILES Fc1cc(C[C@H](Nc2nc3ccccc3s2)c2nc3ccccc3[nH]2)ccc1[C@@H]1CC(=O)NS1(=O)=O |r| Show InChI InChI=1S/C25H20FN5O3S2/c26-16-11-14(9-10-15(16)22-13-23(32)31-36(22,33)34)12-20(24-27-17-5-1-2-6-18(17)28-24)30-25-29-19-7-3-4-8-21(19)35-25/h1-11,20,22H,12-13H2,(H,27,28)(H,29,30)(H,31,32)/t20-,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50308853
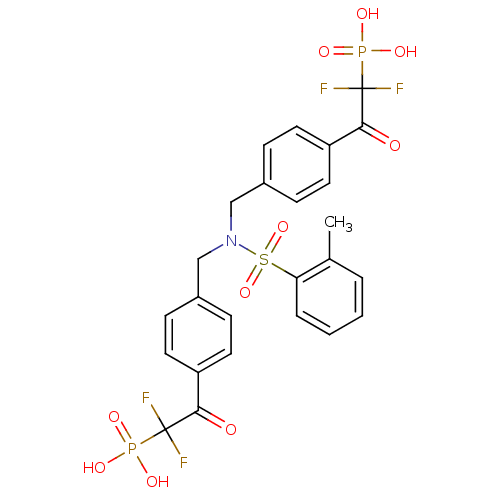
(2,2'-(4,4'-(o-tolylsulfonylazanediyl)bis(methylene...)Show SMILES Cc1ccccc1S(=O)(=O)N(Cc1ccc(cc1)C(=O)C(F)(F)P(O)(O)=O)Cc1ccc(cc1)C(=O)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C25H23F4NO10P2S/c1-16-4-2-3-5-21(16)43(39,40)30(14-17-6-10-19(11-7-17)22(31)24(26,27)41(33,34)35)15-18-8-12-20(13-9-18)23(32)25(28,29)42(36,37)38/h2-13H,14-15H2,1H3,(H2,33,34,35)(H2,36,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50166434
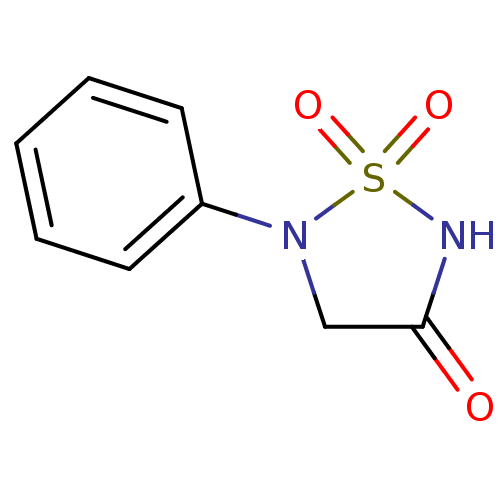
(1,1-Dioxo-5-phenyl-1lambda*6*-[1,2,5]thiadiazolidi...)Show InChI InChI=1S/C8H8N2O3S/c11-8-6-10(14(12,13)9-8)7-4-2-1-3-5-7/h1-5H,6H2,(H,9,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
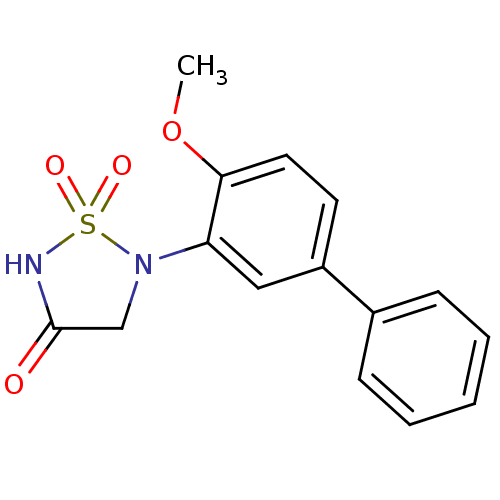
(Homo sapiens (Human)) | BDBM50166435
(5-(4-METHOXYBIPHENYL-3-YL)-1,2,5-THIADIAZOLIDIN-3-...)Show InChI InChI=1S/C15H14N2O4S/c1-21-14-8-7-12(11-5-3-2-4-6-11)9-13(14)17-10-15(18)16-22(17,19)20/h2-9H,10H2,1H3,(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
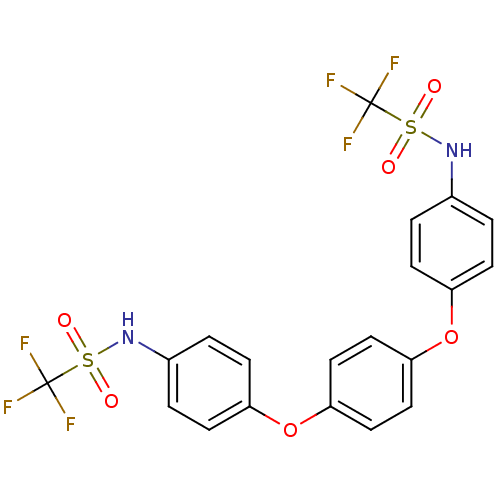
(Homo sapiens (Human)) | BDBM50308838
(CHEMBL605283 | N,N'-(4,4'-(1,4-phenylenebis(oxy))b...)Show SMILES FC(F)(F)S(=O)(=O)Nc1ccc(Oc2ccc(Oc3ccc(NS(=O)(=O)C(F)(F)F)cc3)cc2)cc1 Show InChI InChI=1S/C20H14F6N2O6S2/c21-19(22,23)35(29,30)27-13-1-5-15(6-2-13)33-17-9-11-18(12-10-17)34-16-7-3-14(4-8-16)28-36(31,32)20(24,25)26/h1-12,27-28H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50166434

(1,1-Dioxo-5-phenyl-1lambda*6*-[1,2,5]thiadiazolidi...)Show InChI InChI=1S/C8H8N2O3S/c11-8-6-10(14(12,13)9-8)7-4-2-1-3-5-7/h1-5H,6H2,(H,9,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50308848

(2-(3-(2-(2-(4-chlorophenyl)-4-methylthiazol-5-yl)e...)Show SMILES Cc1nc(sc1CCc1cccc(c1)C(O)(C(O)=O)C(F)(F)F)-c1ccc(Cl)cc1 Show InChI InChI=1S/C21H17ClF3NO3S/c1-12-17(30-18(26-12)14-6-8-16(22)9-7-14)10-5-13-3-2-4-15(11-13)20(29,19(27)28)21(23,24)25/h2-4,6-9,11,29H,5,10H2,1H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50308836

(3-(3-Chloro-phenyl)-5-[1-(4-hydroxy-3-methoxy-5-ni...)Show SMILES COc1cc(\C=C2/SC(=S)N(C2=O)c2cccc(Cl)c2)cc(c1O)[N+]([O-])=O Show InChI InChI=1S/C17H11ClN2O5S2/c1-25-13-6-9(5-12(15(13)21)20(23)24)7-14-16(22)19(17(26)27-14)11-4-2-3-10(18)8-11/h2-8,21H,1H3/b14-7- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
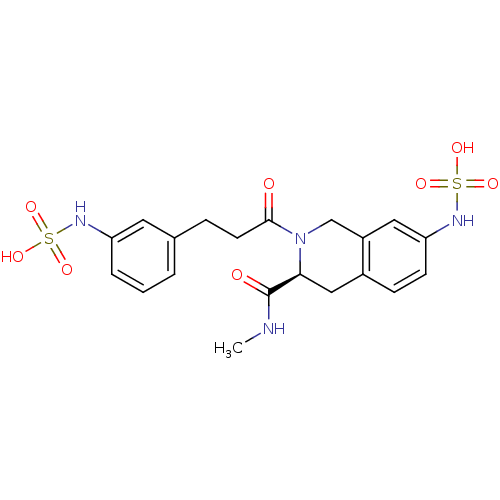
(Homo sapiens (Human)) | BDBM13461
(N-[(3S)-3-(methylcarbamoyl)-2-{3-[3-(sulfoamino)ph...)Show SMILES CNC(=O)[C@@H]1Cc2ccc(NS(O)(=O)=O)cc2CN1C(=O)CCc1cccc(NS(O)(=O)=O)c1 |r| Show InChI InChI=1S/C20H24N4O8S2/c1-21-20(26)18-11-14-6-7-17(23-34(30,31)32)10-15(14)12-24(18)19(25)8-5-13-3-2-4-16(9-13)22-33(27,28)29/h2-4,6-7,9-10,18,22-23H,5,8,11-12H2,1H3,(H,21,26)(H,27,28,29)(H,30,31,32)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50308836

(3-(3-Chloro-phenyl)-5-[1-(4-hydroxy-3-methoxy-5-ni...)Show SMILES COc1cc(\C=C2/SC(=S)N(C2=O)c2cccc(Cl)c2)cc(c1O)[N+]([O-])=O Show InChI InChI=1S/C17H11ClN2O5S2/c1-25-13-6-9(5-12(15(13)21)20(23)24)7-14-16(22)19(17(26)27-14)11-4-2-3-10(18)8-11/h2-8,21H,1H3/b14-7- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50308837
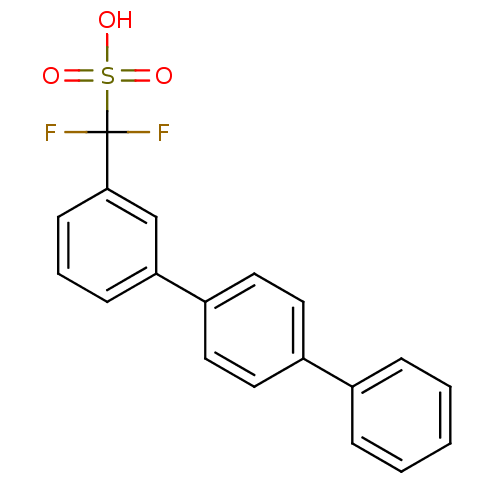
(CHEMBL602117 | Difluoro-[1,1',4',1'']terphenyl-3-y...)Show SMILES OS(=O)(=O)C(F)(F)c1cccc(c1)-c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C19H14F2O3S/c20-19(21,25(22,23)24)18-8-4-7-17(13-18)16-11-9-15(10-12-16)14-5-2-1-3-6-14/h1-13H,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13461

(N-[(3S)-3-(methylcarbamoyl)-2-{3-[3-(sulfoamino)ph...)Show SMILES CNC(=O)[C@@H]1Cc2ccc(NS(O)(=O)=O)cc2CN1C(=O)CCc1cccc(NS(O)(=O)=O)c1 |r| Show InChI InChI=1S/C20H24N4O8S2/c1-21-20(26)18-11-14-6-7-17(23-34(30,31)32)10-15(14)12-24(18)19(25)8-5-13-3-2-4-16(9-13)22-33(27,28)29/h2-4,6-7,9-10,18,22-23H,5,8,11-12H2,1H3,(H,21,26)(H,27,28,29)(H,30,31,32)/t18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50308855
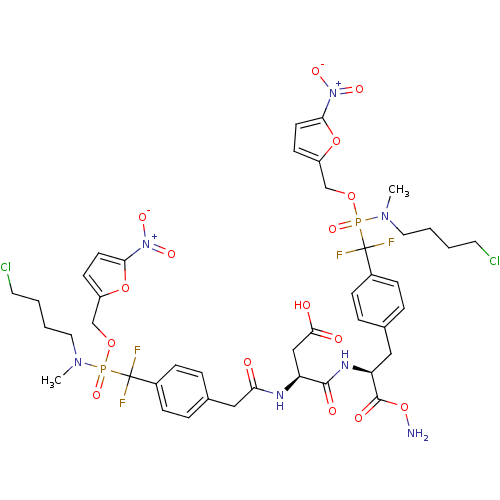
((2R)-3-((2S)-1-(aminooxy)-3-(4-((((4-chlorobutyl)(...)Show SMILES CN(CCCCCl)P(=O)(OCc1ccc(o1)[N+]([O-])=O)C(F)(F)c1ccc(C[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)Cc2ccc(cc2)C(F)(F)P(=O)(OCc2ccc(o2)[N+]([O-])=O)N(C)CCCCCl)C(=O)ON)cc1 |r| Show InChI InChI=1S/C43H51Cl2F4N7O16P2/c1-53(21-5-3-19-44)73(66,68-26-32-15-17-37(70-32)55(62)63)42(46,47)30-11-7-28(8-12-30)23-35(41(61)72-50)52-40(60)34(25-39(58)59)51-36(57)24-29-9-13-31(14-10-29)43(48,49)74(67,54(2)22-6-4-20-45)69-27-33-16-18-38(71-33)56(64)65/h7-18,34-35H,3-6,19-27,50H2,1-2H3,(H,51,57)(H,52,60)(H,58,59)/t34-,35-,73?,74?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data