 Found 18 hits Enz. Inhib. hit(s) with all data for entry = 50048811
Found 18 hits Enz. Inhib. hit(s) with all data for entry = 50048811 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M5
(Mus musculus) | BDBM50228206
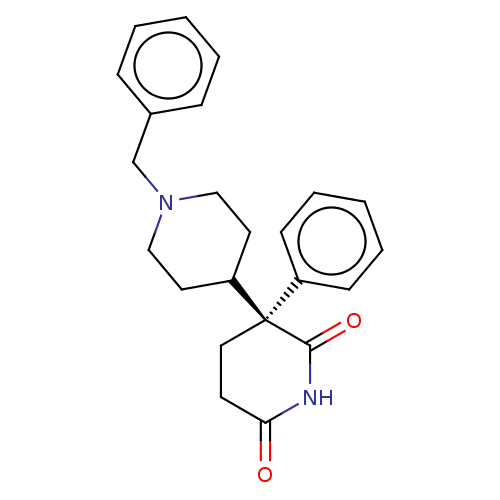
(Dexetimide | R-16470 [as hydrochloride])Show SMILES O=C1CC[C@@](C2CCN(Cc3ccccc3)CC2)(C(=O)N1)c1ccccc1 Show InChI InChI=1S/C23H26N2O2/c26-21-11-14-23(22(27)24-21,19-9-5-2-6-10-19)20-12-15-25(16-13-20)17-18-7-3-1-4-8-18/h1-10,20H,11-17H2,(H,24,26,27)/t23-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins Medical Institutions
Curated by ChEMBL
| Assay Description
Ability (10 ug/kg) to inhibit binding of [125I]iododexetimide to muscarinic receptor in mice |
J Med Chem 32: 1057-62 (1989)
BindingDB Entry DOI: 10.7270/Q29S1T87 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50018556
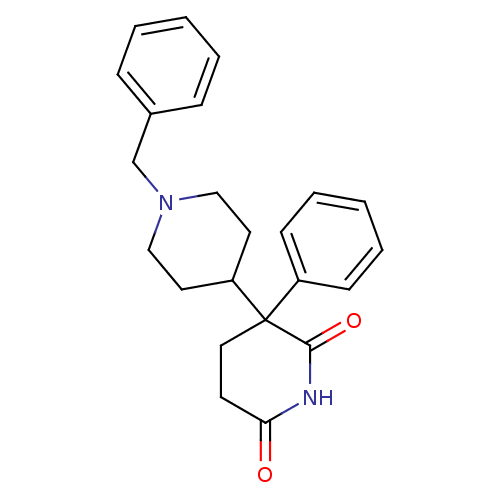
((+)-1'-benzyl-3-phenyl-3,4'-bipiperidine-2,6-dione...)Show SMILES O=C1CCC(C2CCN(Cc3ccccc3)CC2)(C(=O)N1)c1ccccc1 Show InChI InChI=1S/C23H26N2O2/c26-21-11-14-23(22(27)24-21,19-9-5-2-6-10-19)20-12-15-25(16-13-20)17-18-7-3-1-4-8-18/h1-10,20H,11-17H2,(H,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins Medical Institutions
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylscopolamine from rat brain homogenate |
J Med Chem 32: 1057-62 (1989)
BindingDB Entry DOI: 10.7270/Q29S1T87 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50228211
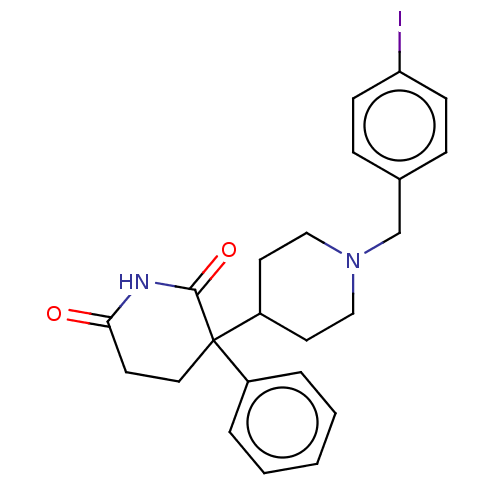
(CHEMBL274789)Show SMILES Ic1ccc(CN2CCC(CC2)C2(CCC(=O)NC2=O)c2ccccc2)cc1 Show InChI InChI=1S/C23H25IN2O2/c24-20-8-6-17(7-9-20)16-26-14-11-19(12-15-26)23(18-4-2-1-3-5-18)13-10-21(27)25-22(23)28/h1-9,19H,10-16H2,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins Medical Institutions
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylscopolamine from rat brain homogenate |
J Med Chem 32: 1057-62 (1989)
BindingDB Entry DOI: 10.7270/Q29S1T87 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50228212

(CHEMBL10086)Show SMILES Fc1cccc(CN2CCC(CC2)C2(CCC(=O)NC2=O)c2ccccc2)c1 Show InChI InChI=1S/C23H25FN2O2/c24-20-8-4-5-17(15-20)16-26-13-10-19(11-14-26)23(18-6-2-1-3-7-18)12-9-21(27)25-22(23)28/h1-8,15,19H,9-14,16H2,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins Medical Institutions
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylscopolamine from rat brain homogenate |
J Med Chem 32: 1057-62 (1989)
BindingDB Entry DOI: 10.7270/Q29S1T87 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50228214
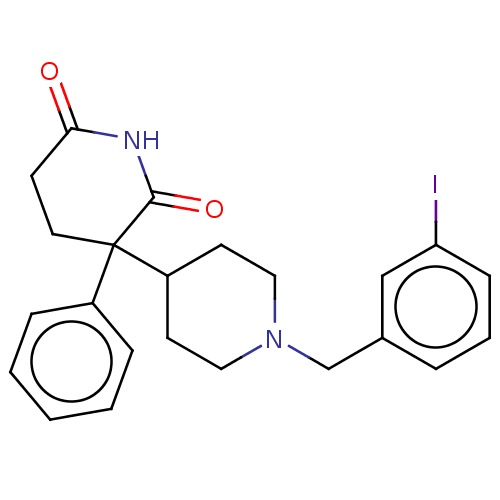
(CHEMBL10278)Show SMILES Ic1cccc(CN2CCC(CC2)C2(CCC(=O)NC2=O)c2ccccc2)c1 Show InChI InChI=1S/C23H25IN2O2/c24-20-8-4-5-17(15-20)16-26-13-10-19(11-14-26)23(18-6-2-1-3-7-18)12-9-21(27)25-22(23)28/h1-8,15,19H,9-14,16H2,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins Medical Institutions
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylscopolamine from rat brain homogenate |
J Med Chem 32: 1057-62 (1989)
BindingDB Entry DOI: 10.7270/Q29S1T87 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50228209
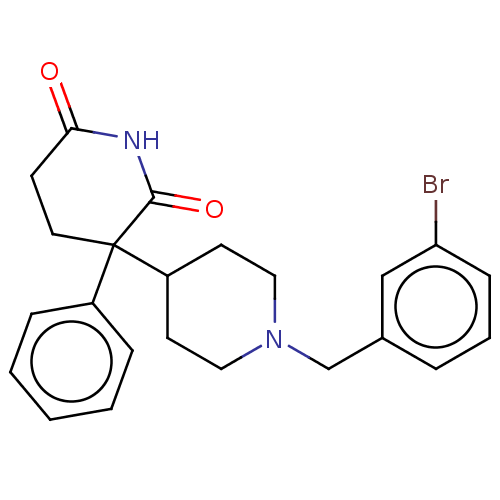
(CHEMBL10399)Show SMILES Brc1cccc(CN2CCC(CC2)C2(CCC(=O)NC2=O)c2ccccc2)c1 Show InChI InChI=1S/C23H25BrN2O2/c24-20-8-4-5-17(15-20)16-26-13-10-19(11-14-26)23(18-6-2-1-3-7-18)12-9-21(27)25-22(23)28/h1-8,15,19H,9-14,16H2,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins Medical Institutions
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylscopolamine from rat brain homogenate |
J Med Chem 32: 1057-62 (1989)
BindingDB Entry DOI: 10.7270/Q29S1T87 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50228213
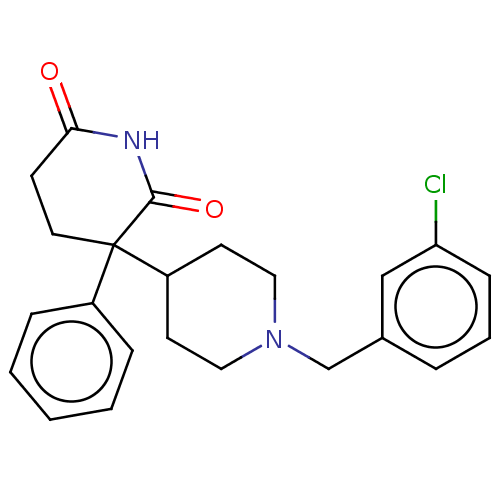
(CHEMBL273262)Show SMILES Clc1cccc(CN2CCC(CC2)C2(CCC(=O)NC2=O)c2ccccc2)c1 Show InChI InChI=1S/C23H25ClN2O2/c24-20-8-4-5-17(15-20)16-26-13-10-19(11-14-26)23(18-6-2-1-3-7-18)12-9-21(27)25-22(23)28/h1-8,15,19H,9-14,16H2,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins Medical Institutions
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylscopolamine from rat brain homogenate |
J Med Chem 32: 1057-62 (1989)
BindingDB Entry DOI: 10.7270/Q29S1T87 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50018560
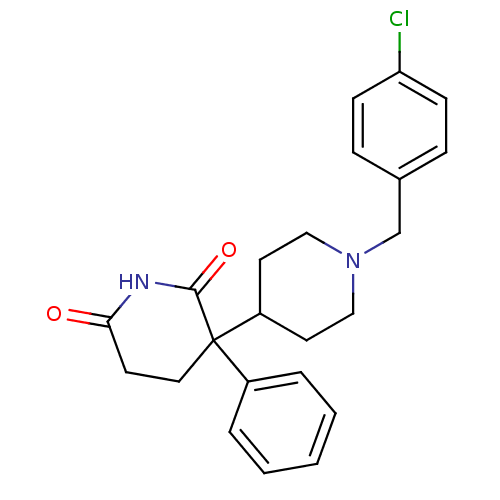
((+/-)-1'-(4-chlorobenzyl)-3-phenyl-3,4'-bipiperidi...)Show SMILES Clc1ccc(CN2CCC(CC2)C2(CCC(=O)NC2=O)c2ccccc2)cc1 Show InChI InChI=1S/C23H25ClN2O2/c24-20-8-6-17(7-9-20)16-26-14-11-19(12-15-26)23(18-4-2-1-3-5-18)13-10-21(27)25-22(23)28/h1-9,19H,10-16H2,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins Medical Institutions
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylscopolamine from rat brain homogenate |
J Med Chem 32: 1057-62 (1989)
BindingDB Entry DOI: 10.7270/Q29S1T87 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50228205
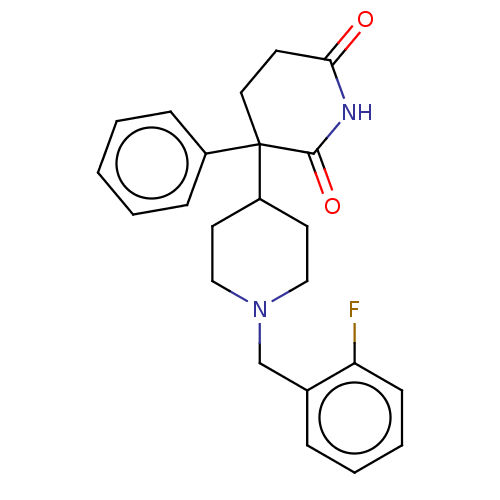
(CHEMBL10331)Show SMILES Fc1ccccc1CN1CCC(CC1)C1(CCC(=O)NC1=O)c1ccccc1 Show InChI InChI=1S/C23H25FN2O2/c24-20-9-5-4-6-17(20)16-26-14-11-19(12-15-26)23(18-7-2-1-3-8-18)13-10-21(27)25-22(23)28/h1-9,19H,10-16H2,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins Medical Institutions
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylscopolamine from rat brain homogenate |
J Med Chem 32: 1057-62 (1989)
BindingDB Entry DOI: 10.7270/Q29S1T87 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50018553
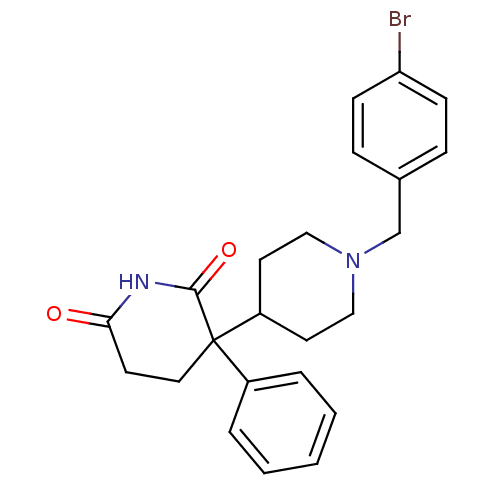
((+)-1'-(4-bromobenzyl)-3-phenyl-3,4'-bipiperidine-...)Show SMILES Brc1ccc(CN2CCC(CC2)C2(CCC(=O)NC2=O)c2ccccc2)cc1 Show InChI InChI=1S/C23H25BrN2O2/c24-20-8-6-17(7-9-20)16-26-14-11-19(12-15-26)23(18-4-2-1-3-5-18)13-10-21(27)25-22(23)28/h1-9,19H,10-16H2,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins Medical Institutions
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylscopolamine from rat brain homogenate |
J Med Chem 32: 1057-62 (1989)
BindingDB Entry DOI: 10.7270/Q29S1T87 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50018551
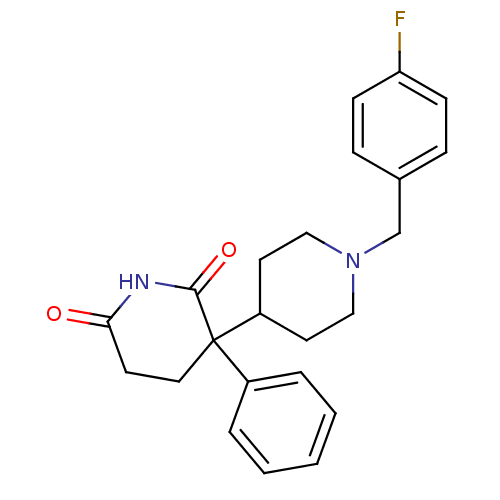
((+/-)-1'-(4-fluorobenzyl)-3-phenyl-3,4'-bipiperidi...)Show SMILES Fc1ccc(CN2CCC(CC2)C2(CCC(=O)NC2=O)c2ccccc2)cc1 Show InChI InChI=1S/C23H25FN2O2/c24-20-8-6-17(7-9-20)16-26-14-11-19(12-15-26)23(18-4-2-1-3-5-18)13-10-21(27)25-22(23)28/h1-9,19H,10-16H2,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins Medical Institutions
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylscopolamine from rat brain homogenate |
J Med Chem 32: 1057-62 (1989)
BindingDB Entry DOI: 10.7270/Q29S1T87 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50228207
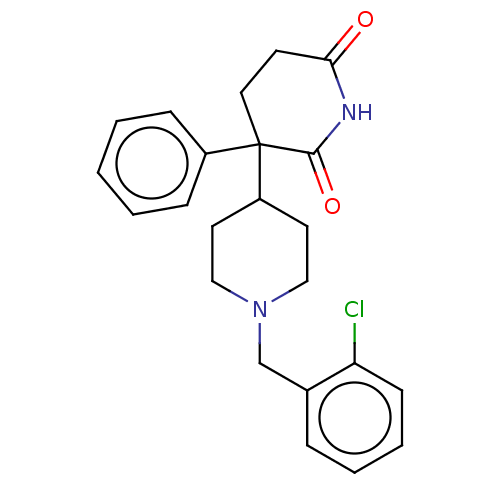
(CHEMBL9966)Show SMILES Clc1ccccc1CN1CCC(CC1)C1(CCC(=O)NC1=O)c1ccccc1 Show InChI InChI=1S/C23H25ClN2O2/c24-20-9-5-4-6-17(20)16-26-14-11-19(12-15-26)23(18-7-2-1-3-8-18)13-10-21(27)25-22(23)28/h1-9,19H,10-16H2,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins Medical Institutions
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylscopolamine from rat brain homogenate |
J Med Chem 32: 1057-62 (1989)
BindingDB Entry DOI: 10.7270/Q29S1T87 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50228210
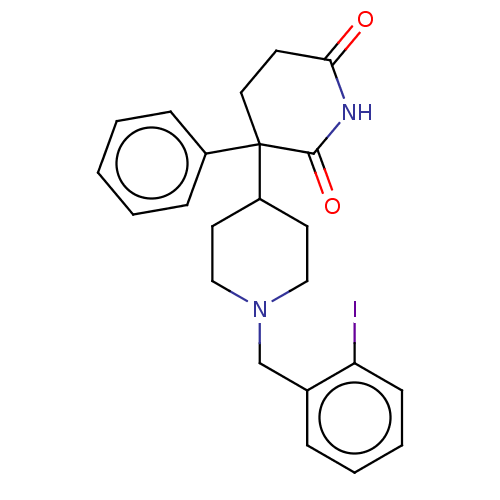
(CHEMBL276237)Show SMILES Ic1ccccc1CN1CCC(CC1)C1(CCC(=O)NC1=O)c1ccccc1 Show InChI InChI=1S/C23H25IN2O2/c24-20-9-5-4-6-17(20)16-26-14-11-19(12-15-26)23(18-7-2-1-3-8-18)13-10-21(27)25-22(23)28/h1-9,19H,10-16H2,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins Medical Institutions
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylscopolamine from rat brain homogenate |
J Med Chem 32: 1057-62 (1989)
BindingDB Entry DOI: 10.7270/Q29S1T87 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50228208
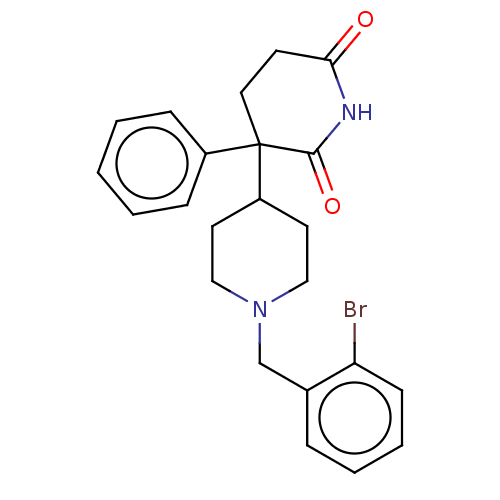
(CHEMBL274552)Show SMILES Brc1ccccc1CN1CCC(CC1)C1(CCC(=O)NC1=O)c1ccccc1 Show InChI InChI=1S/C23H25BrN2O2/c24-20-9-5-4-6-17(20)16-26-14-11-19(12-15-26)23(18-7-2-1-3-8-18)13-10-21(27)25-22(23)28/h1-9,19H,10-16H2,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins Medical Institutions
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylscopolamine from rat brain homogenate |
J Med Chem 32: 1057-62 (1989)
BindingDB Entry DOI: 10.7270/Q29S1T87 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Mus musculus) | BDBM39341

(11-[(4-methylpiperazin-1-yl)acetyl]-5,11-dihydro-6...)Show InChI InChI=1S/C19H21N5O2/c1-22-9-11-23(12-10-22)13-17(25)24-16-7-3-2-5-14(16)19(26)21-15-6-4-8-20-18(15)24/h2-8H,9-13H2,1H3,(H,21,26) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins Medical Institutions
Curated by ChEMBL
| Assay Description
Ability (10 ug/kg) to inhibit binding of [125I]iododexetimide to muscarinic receptor in mice |
J Med Chem 32: 1057-62 (1989)
BindingDB Entry DOI: 10.7270/Q29S1T87 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Mus musculus) | BDBM50018556

((+)-1'-benzyl-3-phenyl-3,4'-bipiperidine-2,6-dione...)Show SMILES O=C1CCC(C2CCN(Cc3ccccc3)CC2)(C(=O)N1)c1ccccc1 Show InChI InChI=1S/C23H26N2O2/c26-21-11-14-23(22(27)24-21,19-9-5-2-6-10-19)20-12-15-25(16-13-20)17-18-7-3-1-4-8-18/h1-10,20H,11-17H2,(H,24,26,27) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins Medical Institutions
Curated by ChEMBL
| Assay Description
Ability (10 ug/kg) to inhibit binding of [125I]iododexetimide to muscarinic receptor in mice |
J Med Chem 32: 1057-62 (1989)
BindingDB Entry DOI: 10.7270/Q29S1T87 |
More data for this
Ligand-Target Pair | |
D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor
(Mus musculus (Mouse)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins Medical Institutions
Curated by ChEMBL
| Assay Description
Ability (10 ug/kg) to inhibit binding of [125I]iododexetimide to Dopamine receptor of mice |
J Med Chem 32: 1057-62 (1989)
BindingDB Entry DOI: 10.7270/Q29S1T87 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1
(Mus musculus (Mouse)-MOUSE) | BDBM50000788

((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...)Show SMILES Oc1ccc2C[C@H]3N(CC=C)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O |r| Show InChI InChI=1S/C19H21NO4/c1-2-8-20-9-7-18-15-11-3-4-12(21)16(15)24-17(18)13(22)5-6-19(18,23)14(20)10-11/h2-4,14,17,21,23H,1,5-10H2/t14-,17+,18+,19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins Medical Institutions
Curated by ChEMBL
| Assay Description
Ability (10 ug/kg) to inhibit binding of [125I]iododexetimide to opioid receptor mice |
J Med Chem 32: 1057-62 (1989)
BindingDB Entry DOI: 10.7270/Q29S1T87 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data