 Found 48 hits Enz. Inhib. hit(s) with all data for entry = 50034170
Found 48 hits Enz. Inhib. hit(s) with all data for entry = 50034170 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50358203
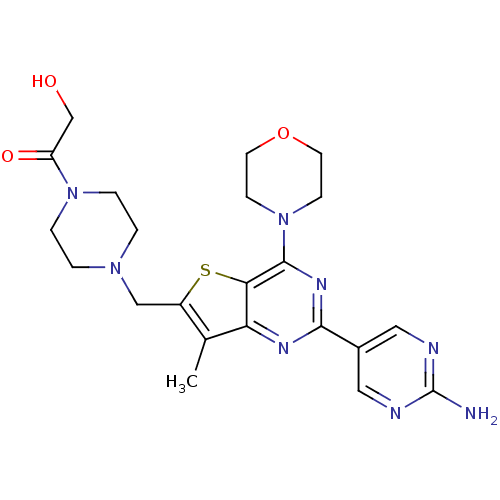
(CHEMBL1922093)Show SMILES Cc1c(CN2CCN(CC2)C(=O)CO)sc2c(nc(nc12)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C22H28N8O3S/c1-14-16(12-28-2-4-29(5-3-28)17(32)13-31)34-19-18(14)26-20(15-10-24-22(23)25-11-15)27-21(19)30-6-8-33-9-7-30/h10-11,31H,2-9,12-13H2,1H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K subunit p110alpha assessed as formation of phosphatidylinositide-3-phosphate product formation after 30 mins by fluorescence polar... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50358206
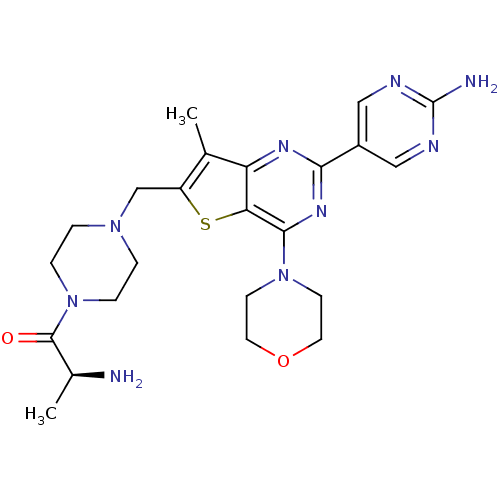
(CHEMBL1922091)Show SMILES C[C@H](N)C(=O)N1CCN(Cc2sc3c(nc(nc3c2C)-c2cnc(N)nc2)N2CCOCC2)CC1 |r| Show InChI InChI=1S/C23H31N9O2S/c1-14-17(13-30-3-5-32(6-4-30)22(33)15(2)24)35-19-18(14)28-20(16-11-26-23(25)27-12-16)29-21(19)31-7-9-34-10-8-31/h11-12,15H,3-10,13,24H2,1-2H3,(H2,25,26,27)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR expressed in insect cells assessed as phosphorylation of recombinant (GFP)-4-EBP1 measured after 30 mins by fluo... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50358205
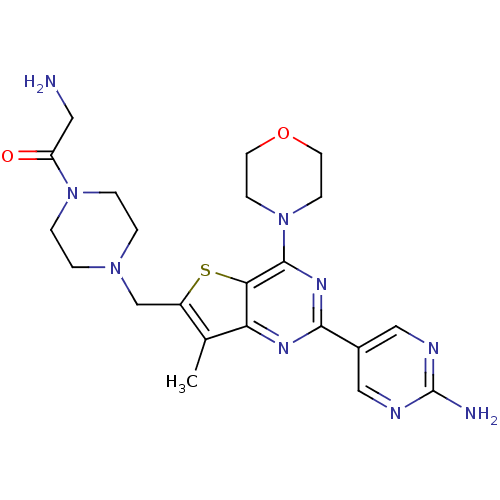
(CHEMBL1922090)Show SMILES Cc1c(CN2CCN(CC2)C(=O)CN)sc2c(nc(nc12)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C22H29N9O2S/c1-14-16(13-29-2-4-30(5-3-29)17(32)10-23)34-19-18(14)27-20(15-11-25-22(24)26-12-15)28-21(19)31-6-8-33-9-7-31/h11-12H,2-10,13,23H2,1H3,(H2,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR expressed in insect cells assessed as phosphorylation of recombinant (GFP)-4-EBP1 measured after 30 mins by fluo... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50358207

(CHEMBL1922092)Show SMILES Cc1c(CN2CCN(CC2)C(=O)C(C)(C)N)sc2c(nc(nc12)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C24H33N9O2S/c1-15-17(14-31-4-6-33(7-5-31)22(34)24(2,3)26)36-19-18(15)29-20(16-12-27-23(25)28-13-16)30-21(19)32-8-10-35-11-9-32/h12-13H,4-11,14,26H2,1-3H3,(H2,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR expressed in insect cells assessed as phosphorylation of recombinant (GFP)-4-EBP1 measured after 30 mins by fluo... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50358209

(CHEMBL1922095)Show SMILES Cc1c(CN2CCN(CC2)C(=O)C(C)(C)O)sc2c(nc(nc12)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C24H32N8O3S/c1-15-17(14-30-4-6-32(7-5-30)22(33)24(2,3)34)36-19-18(15)28-20(16-12-26-23(25)27-13-16)29-21(19)31-8-10-35-11-9-31/h12-13,34H,4-11,14H2,1-3H3,(H2,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR expressed in insect cells assessed as phosphorylation of recombinant (GFP)-4-EBP1 measured after 30 mins by fluo... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50358204

(CHEMBL1922094)Show SMILES C[C@H](O)C(=O)N1CCN(Cc2sc3c(nc(nc3c2C)-c2cnc(N)nc2)N2CCOCC2)CC1 |r| Show InChI InChI=1S/C23H30N8O3S/c1-14-17(13-29-3-5-31(6-4-29)22(33)15(2)32)35-19-18(14)27-20(16-11-25-23(24)26-12-16)28-21(19)30-7-9-34-10-8-30/h11-12,15,32H,3-10,13H2,1-2H3,(H2,24,25,26)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR expressed in insect cells assessed as phosphorylation of recombinant (GFP)-4-EBP1 measured after 30 mins by fluo... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315907
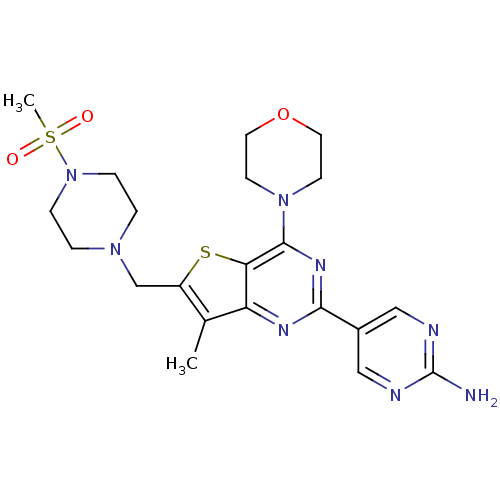
(5-(7-methyl-6-((4-(methylsulfonyl)piperazin-1-yl)m...)Show SMILES Cc1c(CN2CCN(CC2)S(C)(=O)=O)sc2c(nc(nc12)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C21H28N8O3S2/c1-14-16(13-27-3-5-29(6-4-27)34(2,30)31)33-18-17(14)25-19(15-11-23-21(22)24-12-15)26-20(18)28-7-9-32-10-8-28/h11-12H,3-10,13H2,1-2H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR expressed in insect cells assessed as phosphorylation of recombinant (GFP)-4-EBP1 measured after 30 mins by fluo... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50312606
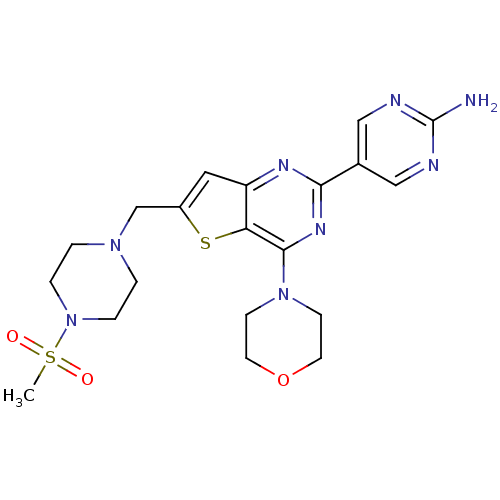
(5-(6-((4-(methylsulfonyl)piperazin-1-yl)methyl)-4-...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)CC1 Show InChI InChI=1S/C20H26N8O3S2/c1-33(29,30)28-4-2-26(3-5-28)13-15-10-16-17(32-15)19(27-6-8-31-9-7-27)25-18(24-16)14-11-22-20(21)23-12-14/h10-12H,2-9,13H2,1H3,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR expressed in insect cells assessed as phosphorylation of recombinant (GFP)-4-EBP1 measured after 30 mins by fluo... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM25028
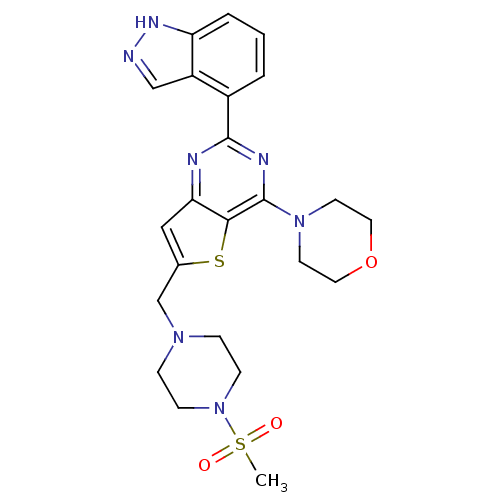
(4-[2-(1H-indazol-4-yl)-6-[(4-methanesulfonylpipera...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C23H27N7O3S2/c1-35(31,32)30-7-5-28(6-8-30)15-16-13-20-21(34-16)23(29-9-11-33-12-10-29)26-22(25-20)17-3-2-4-19-18(17)14-24-27-19/h2-4,13-14H,5-12,15H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR expressed in insect cells assessed as phosphorylation of recombinant (GFP)-4-EBP1 measured after 30 mins by fluo... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50358205

(CHEMBL1922090)Show SMILES Cc1c(CN2CCN(CC2)C(=O)CN)sc2c(nc(nc12)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C22H29N9O2S/c1-14-16(13-29-2-4-30(5-3-29)17(32)10-23)34-19-18(14)27-20(15-11-25-22(24)26-12-15)28-21(19)31-6-8-33-9-7-31/h11-12H,2-10,13,23H2,1H3,(H2,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K subunit p110alpha assessed as formation of phosphatidylinositide-3-phosphate product formation after 30 mins by fluorescence polar... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50358207

(CHEMBL1922092)Show SMILES Cc1c(CN2CCN(CC2)C(=O)C(C)(C)N)sc2c(nc(nc12)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C24H33N9O2S/c1-15-17(14-31-4-6-33(7-5-31)22(34)24(2,3)26)36-19-18(15)29-20(16-12-27-23(25)28-13-16)30-21(19)32-8-10-35-11-9-32/h12-13H,4-11,14,26H2,1-3H3,(H2,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K subunit p110alpha assessed as formation of phosphatidylinositide-3-phosphate product formation after 30 mins by fluorescence polar... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50358207

(CHEMBL1922092)Show SMILES Cc1c(CN2CCN(CC2)C(=O)C(C)(C)N)sc2c(nc(nc12)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C24H33N9O2S/c1-15-17(14-31-4-6-33(7-5-31)22(34)24(2,3)26)36-19-18(15)29-20(16-12-27-23(25)28-13-16)30-21(19)32-8-10-35-11-9-32/h12-13H,4-11,14,26H2,1-3H3,(H2,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K subunit p110delta assessed as formation of phosphatidylinositide-3-phosphate product formation after 30 mins by fluorescence polar... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50358209

(CHEMBL1922095)Show SMILES Cc1c(CN2CCN(CC2)C(=O)C(C)(C)O)sc2c(nc(nc12)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C24H32N8O3S/c1-15-17(14-30-4-6-32(7-5-30)22(33)24(2,3)34)36-19-18(15)28-20(16-12-26-23(25)27-13-16)29-21(19)31-8-10-35-11-9-31/h12-13,34H,4-11,14H2,1-3H3,(H2,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K subunit p110alpha assessed as formation of phosphatidylinositide-3-phosphate product formation after 30 mins by fluorescence polar... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50312606

(5-(6-((4-(methylsulfonyl)piperazin-1-yl)methyl)-4-...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)CC1 Show InChI InChI=1S/C20H26N8O3S2/c1-33(29,30)28-4-2-26(3-5-28)13-15-10-16-17(32-15)19(27-6-8-31-9-7-27)25-18(24-16)14-11-22-20(21)23-12-14/h10-12H,2-9,13H2,1H3,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K subunit p110alpha assessed as formation of phosphatidylinositide-3-phosphate product formation after 30 mins by fluorescence polar... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50358206

(CHEMBL1922091)Show SMILES C[C@H](N)C(=O)N1CCN(Cc2sc3c(nc(nc3c2C)-c2cnc(N)nc2)N2CCOCC2)CC1 |r| Show InChI InChI=1S/C23H31N9O2S/c1-14-17(13-30-3-5-32(6-4-30)22(33)15(2)24)35-19-18(14)28-20(16-11-26-23(25)27-12-16)29-21(19)31-7-9-34-10-8-31/h11-12,15H,3-10,13,24H2,1-2H3,(H2,25,26,27)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K subunit p110alpha assessed as formation of phosphatidylinositide-3-phosphate product formation after 30 mins by fluorescence polar... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50358206

(CHEMBL1922091)Show SMILES C[C@H](N)C(=O)N1CCN(Cc2sc3c(nc(nc3c2C)-c2cnc(N)nc2)N2CCOCC2)CC1 |r| Show InChI InChI=1S/C23H31N9O2S/c1-14-17(13-30-3-5-32(6-4-30)22(33)15(2)24)35-19-18(14)28-20(16-11-26-23(25)27-12-16)29-21(19)31-7-9-34-10-8-31/h11-12,15H,3-10,13,24H2,1-2H3,(H2,25,26,27)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K subunit p110delta assessed as formation of phosphatidylinositide-3-phosphate product formation after 30 mins by fluorescence polar... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25028

(4-[2-(1H-indazol-4-yl)-6-[(4-methanesulfonylpipera...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C23H27N7O3S2/c1-35(31,32)30-7-5-28(6-8-30)15-16-13-20-21(34-16)23(29-9-11-33-12-10-29)26-22(25-20)17-3-2-4-19-18(17)14-24-27-19/h2-4,13-14H,5-12,15H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K subunit p110alpha assessed as formation of phosphatidylinositide-3-phosphate product formation after 30 mins by fluorescence polar... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50315907

(5-(7-methyl-6-((4-(methylsulfonyl)piperazin-1-yl)m...)Show SMILES Cc1c(CN2CCN(CC2)S(C)(=O)=O)sc2c(nc(nc12)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C21H28N8O3S2/c1-14-16(13-27-3-5-29(6-4-27)34(2,30)31)33-18-17(14)25-19(15-11-23-21(22)24-12-15)26-20(18)28-7-9-32-10-8-28/h11-12H,3-10,13H2,1-2H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K subunit p110alpha assessed as formation of phosphatidylinositide-3-phosphate product formation after 30 mins by fluorescence polar... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50358205

(CHEMBL1922090)Show SMILES Cc1c(CN2CCN(CC2)C(=O)CN)sc2c(nc(nc12)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C22H29N9O2S/c1-14-16(13-29-2-4-30(5-3-29)17(32)10-23)34-19-18(14)27-20(15-11-25-22(24)26-12-15)28-21(19)31-6-8-33-9-7-31/h11-12H,2-10,13,23H2,1H3,(H2,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K subunit p110delta assessed as formation of phosphatidylinositide-3-phosphate product formation after 30 mins by fluorescence polar... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50358204

(CHEMBL1922094)Show SMILES C[C@H](O)C(=O)N1CCN(Cc2sc3c(nc(nc3c2C)-c2cnc(N)nc2)N2CCOCC2)CC1 |r| Show InChI InChI=1S/C23H30N8O3S/c1-14-17(13-29-3-5-31(6-4-29)22(33)15(2)32)35-19-18(14)27-20(16-11-25-23(24)26-12-16)28-21(19)30-7-9-34-10-8-30/h11-12,15,32H,3-10,13H2,1-2H3,(H2,24,25,26)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K subunit p110alpha assessed as formation of phosphatidylinositide-3-phosphate product formation after 30 mins by fluorescence polar... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50358209

(CHEMBL1922095)Show SMILES Cc1c(CN2CCN(CC2)C(=O)C(C)(C)O)sc2c(nc(nc12)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C24H32N8O3S/c1-15-17(14-30-4-6-32(7-5-30)22(33)24(2,3)34)36-19-18(15)28-20(16-12-26-23(25)27-13-16)29-21(19)31-8-10-35-11-9-31/h12-13,34H,4-11,14H2,1-3H3,(H2,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K subunit p110delta assessed as formation of phosphatidylinositide-3-phosphate product formation after 30 mins by fluorescence polar... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50358203

(CHEMBL1922093)Show SMILES Cc1c(CN2CCN(CC2)C(=O)CO)sc2c(nc(nc12)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C22H28N8O3S/c1-14-16(12-28-2-4-29(5-3-28)17(32)13-31)34-19-18(14)26-20(15-10-24-22(23)25-11-15)27-21(19)30-6-8-33-9-7-30/h10-11,31H,2-9,12-13H2,1H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K subunit p110delta assessed as formation of phosphatidylinositide-3-phosphate product formation after 30 mins by fluorescence polar... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50358204

(CHEMBL1922094)Show SMILES C[C@H](O)C(=O)N1CCN(Cc2sc3c(nc(nc3c2C)-c2cnc(N)nc2)N2CCOCC2)CC1 |r| Show InChI InChI=1S/C23H30N8O3S/c1-14-17(13-29-3-5-31(6-4-29)22(33)15(2)32)35-19-18(14)27-20(16-11-25-23(24)26-12-16)28-21(19)30-7-9-34-10-8-30/h11-12,15,32H,3-10,13H2,1-2H3,(H2,24,25,26)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K subunit p110delta assessed as formation of phosphatidylinositide-3-phosphate product formation after 30 mins by fluorescence polar... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50358208
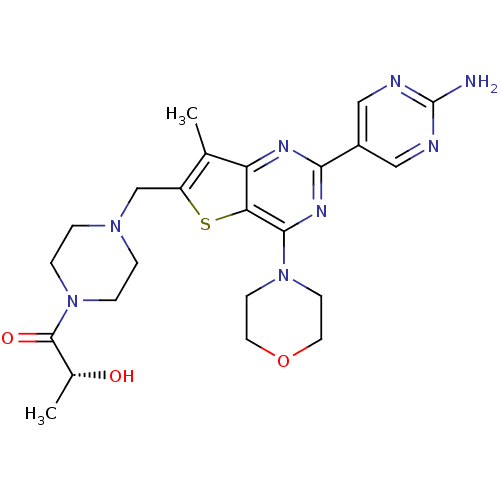
(CHEMBL1921986)Show SMILES C[C@@H](O)C(=O)N1CCN(Cc2sc3c(nc(nc3c2C)-c2cnc(N)nc2)N2CCOCC2)CC1 |r| Show InChI InChI=1S/C23H30N8O3S/c1-14-17(13-29-3-5-31(6-4-29)22(33)15(2)32)35-19-18(14)27-20(16-11-25-23(24)26-12-16)28-21(19)30-7-9-34-10-8-30/h11-12,15,32H,3-10,13H2,1-2H3,(H2,24,25,26)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K subunit p110alpha assessed as formation of phosphatidylinositide-3-phosphate product formation after 30 mins by fluorescence polar... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50358203

(CHEMBL1922093)Show SMILES Cc1c(CN2CCN(CC2)C(=O)CO)sc2c(nc(nc12)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C22H28N8O3S/c1-14-16(12-28-2-4-29(5-3-28)17(32)13-31)34-19-18(14)26-20(15-10-24-22(23)25-11-15)27-21(19)30-6-8-33-9-7-30/h10-11,31H,2-9,12-13H2,1H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K subunit p110gamma assessed as formation of phosphatidylinositide-3-phosphate product formation after 30 mins by fluorescence polar... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50358208

(CHEMBL1921986)Show SMILES C[C@@H](O)C(=O)N1CCN(Cc2sc3c(nc(nc3c2C)-c2cnc(N)nc2)N2CCOCC2)CC1 |r| Show InChI InChI=1S/C23H30N8O3S/c1-14-17(13-29-3-5-31(6-4-29)22(33)15(2)32)35-19-18(14)27-20(16-11-25-23(24)26-12-16)28-21(19)30-7-9-34-10-8-30/h11-12,15,32H,3-10,13H2,1-2H3,(H2,24,25,26)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K subunit p110delta assessed as formation of phosphatidylinositide-3-phosphate product formation after 30 mins by fluorescence polar... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50358205

(CHEMBL1922090)Show SMILES Cc1c(CN2CCN(CC2)C(=O)CN)sc2c(nc(nc12)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C22H29N9O2S/c1-14-16(13-29-2-4-30(5-3-29)17(32)10-23)34-19-18(14)27-20(15-11-25-22(24)26-12-15)28-21(19)31-6-8-33-9-7-31/h11-12H,2-10,13,23H2,1H3,(H2,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K subunit p110beta assessed as formation of phosphatidylinositide-3-phosphate product formation after 30 mins by fluorescence polari... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50358205

(CHEMBL1922090)Show SMILES Cc1c(CN2CCN(CC2)C(=O)CN)sc2c(nc(nc12)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C22H29N9O2S/c1-14-16(13-29-2-4-30(5-3-29)17(32)10-23)34-19-18(14)27-20(15-11-25-22(24)26-12-15)28-21(19)31-6-8-33-9-7-31/h11-12H,2-10,13,23H2,1H3,(H2,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K subunit p110gamma assessed as formation of phosphatidylinositide-3-phosphate product formation after 30 mins by fluorescence polar... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50358203

(CHEMBL1922093)Show SMILES Cc1c(CN2CCN(CC2)C(=O)CO)sc2c(nc(nc12)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C22H28N8O3S/c1-14-16(12-28-2-4-29(5-3-28)17(32)13-31)34-19-18(14)26-20(15-10-24-22(23)25-11-15)27-21(19)30-6-8-33-9-7-30/h10-11,31H,2-9,12-13H2,1H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K subunit p110beta assessed as formation of phosphatidylinositide-3-phosphate product formation after 30 mins by fluorescence polari... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50358207

(CHEMBL1922092)Show SMILES Cc1c(CN2CCN(CC2)C(=O)C(C)(C)N)sc2c(nc(nc12)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C24H33N9O2S/c1-15-17(14-31-4-6-33(7-5-31)22(34)24(2,3)26)36-19-18(15)29-20(16-12-27-23(25)28-13-16)30-21(19)32-8-10-35-11-9-32/h12-13H,4-11,14,26H2,1-3H3,(H2,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K subunit p110gamma assessed as formation of phosphatidylinositide-3-phosphate product formation after 30 mins by fluorescence polar... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50358209

(CHEMBL1922095)Show SMILES Cc1c(CN2CCN(CC2)C(=O)C(C)(C)O)sc2c(nc(nc12)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C24H32N8O3S/c1-15-17(14-30-4-6-32(7-5-30)22(33)24(2,3)34)36-19-18(15)28-20(16-12-26-23(25)27-13-16)29-21(19)31-8-10-35-11-9-31/h12-13,34H,4-11,14H2,1-3H3,(H2,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K subunit p110gamma assessed as formation of phosphatidylinositide-3-phosphate product formation after 30 mins by fluorescence polar... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50358204

(CHEMBL1922094)Show SMILES C[C@H](O)C(=O)N1CCN(Cc2sc3c(nc(nc3c2C)-c2cnc(N)nc2)N2CCOCC2)CC1 |r| Show InChI InChI=1S/C23H30N8O3S/c1-14-17(13-29-3-5-31(6-4-29)22(33)15(2)32)35-19-18(14)27-20(16-11-25-23(24)26-12-16)28-21(19)30-7-9-34-10-8-30/h11-12,15,32H,3-10,13H2,1-2H3,(H2,24,25,26)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K subunit p110beta assessed as formation of phosphatidylinositide-3-phosphate product formation after 30 mins by fluorescence polari... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50358206

(CHEMBL1922091)Show SMILES C[C@H](N)C(=O)N1CCN(Cc2sc3c(nc(nc3c2C)-c2cnc(N)nc2)N2CCOCC2)CC1 |r| Show InChI InChI=1S/C23H31N9O2S/c1-14-17(13-30-3-5-32(6-4-30)22(33)15(2)24)35-19-18(14)28-20(16-11-26-23(25)27-12-16)29-21(19)31-7-9-34-10-8-31/h11-12,15H,3-10,13,24H2,1-2H3,(H2,25,26,27)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K subunit p110beta assessed as formation of phosphatidylinositide-3-phosphate product formation after 30 mins by fluorescence polari... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50358208

(CHEMBL1921986)Show SMILES C[C@@H](O)C(=O)N1CCN(Cc2sc3c(nc(nc3c2C)-c2cnc(N)nc2)N2CCOCC2)CC1 |r| Show InChI InChI=1S/C23H30N8O3S/c1-14-17(13-29-3-5-31(6-4-29)22(33)15(2)32)35-19-18(14)27-20(16-11-25-23(24)26-12-16)28-21(19)30-7-9-34-10-8-30/h11-12,15,32H,3-10,13H2,1-2H3,(H2,24,25,26)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K subunit p110gamma assessed as formation of phosphatidylinositide-3-phosphate product formation after 30 mins by fluorescence polar... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50358204

(CHEMBL1922094)Show SMILES C[C@H](O)C(=O)N1CCN(Cc2sc3c(nc(nc3c2C)-c2cnc(N)nc2)N2CCOCC2)CC1 |r| Show InChI InChI=1S/C23H30N8O3S/c1-14-17(13-29-3-5-31(6-4-29)22(33)15(2)32)35-19-18(14)27-20(16-11-25-23(24)26-12-16)28-21(19)30-7-9-34-10-8-30/h11-12,15,32H,3-10,13H2,1-2H3,(H2,24,25,26)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PIK3 gamma-mediated Akt phosphorylation at Ser473 in human PC3 cells by ELISA |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50358204

(CHEMBL1922094)Show SMILES C[C@H](O)C(=O)N1CCN(Cc2sc3c(nc(nc3c2C)-c2cnc(N)nc2)N2CCOCC2)CC1 |r| Show InChI InChI=1S/C23H30N8O3S/c1-14-17(13-29-3-5-31(6-4-29)22(33)15(2)32)35-19-18(14)27-20(16-11-25-23(24)26-12-16)28-21(19)30-7-9-34-10-8-30/h11-12,15,32H,3-10,13H2,1-2H3,(H2,24,25,26)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PIK3 gamma-mediated Akt phosphorylation at Ser473 in human PC3 cells by ELISA |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50358206

(CHEMBL1922091)Show SMILES C[C@H](N)C(=O)N1CCN(Cc2sc3c(nc(nc3c2C)-c2cnc(N)nc2)N2CCOCC2)CC1 |r| Show InChI InChI=1S/C23H31N9O2S/c1-14-17(13-30-3-5-32(6-4-30)22(33)15(2)24)35-19-18(14)28-20(16-11-26-23(25)27-12-16)29-21(19)31-7-9-34-10-8-31/h11-12,15H,3-10,13,24H2,1-2H3,(H2,25,26,27)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K subunit p110gamma assessed as formation of phosphatidylinositide-3-phosphate product formation after 30 mins by fluorescence polar... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50358207

(CHEMBL1922092)Show SMILES Cc1c(CN2CCN(CC2)C(=O)C(C)(C)N)sc2c(nc(nc12)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C24H33N9O2S/c1-15-17(14-31-4-6-33(7-5-31)22(34)24(2,3)26)36-19-18(15)29-20(16-12-27-23(25)28-13-16)30-21(19)32-8-10-35-11-9-32/h12-13H,4-11,14,26H2,1-3H3,(H2,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K subunit p110beta assessed as formation of phosphatidylinositide-3-phosphate product formation after 30 mins by fluorescence polari... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50358209

(CHEMBL1922095)Show SMILES Cc1c(CN2CCN(CC2)C(=O)C(C)(C)O)sc2c(nc(nc12)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C24H32N8O3S/c1-15-17(14-30-4-6-32(7-5-30)22(33)24(2,3)34)36-19-18(15)28-20(16-12-26-23(25)27-13-16)29-21(19)31-8-10-35-11-9-31/h12-13,34H,4-11,14H2,1-3H3,(H2,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K subunit p110beta assessed as formation of phosphatidylinositide-3-phosphate product formation after 30 mins by fluorescence polari... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50358204

(CHEMBL1922094)Show SMILES C[C@H](O)C(=O)N1CCN(Cc2sc3c(nc(nc3c2C)-c2cnc(N)nc2)N2CCOCC2)CC1 |r| Show InChI InChI=1S/C23H30N8O3S/c1-14-17(13-29-3-5-31(6-4-29)22(33)15(2)32)35-19-18(14)27-20(16-11-25-23(24)26-12-16)28-21(19)30-7-9-34-10-8-30/h11-12,15,32H,3-10,13H2,1-2H3,(H2,24,25,26)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 134 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of Syk |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50358208

(CHEMBL1921986)Show SMILES C[C@@H](O)C(=O)N1CCN(Cc2sc3c(nc(nc3c2C)-c2cnc(N)nc2)N2CCOCC2)CC1 |r| Show InChI InChI=1S/C23H30N8O3S/c1-14-17(13-29-3-5-31(6-4-29)22(33)15(2)32)35-19-18(14)27-20(16-11-25-23(24)26-12-16)28-21(19)30-7-9-34-10-8-30/h11-12,15,32H,3-10,13H2,1-2H3,(H2,24,25,26)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K subunit p110beta assessed as formation of phosphatidylinositide-3-phosphate product formation after 30 mins by fluorescence polari... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 9
(Homo sapiens (Human)) | BDBM50358204

(CHEMBL1922094)Show SMILES C[C@H](O)C(=O)N1CCN(Cc2sc3c(nc(nc3c2C)-c2cnc(N)nc2)N2CCOCC2)CC1 |r| Show InChI InChI=1S/C23H30N8O3S/c1-14-17(13-29-3-5-31(6-4-29)22(33)15(2)32)35-19-18(14)27-20(16-11-25-23(24)26-12-16)28-21(19)30-7-9-34-10-8-30/h11-12,15,32H,3-10,13H2,1-2H3,(H2,24,25,26)/t15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 232 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of Mlk1 |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fgr
(Homo sapiens (Human)) | BDBM50358204

(CHEMBL1922094)Show SMILES C[C@H](O)C(=O)N1CCN(Cc2sc3c(nc(nc3c2C)-c2cnc(N)nc2)N2CCOCC2)CC1 |r| Show InChI InChI=1S/C23H30N8O3S/c1-14-17(13-29-3-5-31(6-4-29)22(33)15(2)32)35-19-18(14)27-20(16-11-25-23(24)26-12-16)28-21(19)30-7-9-34-10-8-30/h11-12,15,32H,3-10,13H2,1-2H3,(H2,24,25,26)/t15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 697 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of Fgr |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM50358204

(CHEMBL1922094)Show SMILES C[C@H](O)C(=O)N1CCN(Cc2sc3c(nc(nc3c2C)-c2cnc(N)nc2)N2CCOCC2)CC1 |r| Show InChI InChI=1S/C23H30N8O3S/c1-14-17(13-29-3-5-31(6-4-29)22(33)15(2)32)35-19-18(14)27-20(16-11-25-23(24)26-12-16)28-21(19)30-7-9-34-10-8-30/h11-12,15,32H,3-10,13H2,1-2H3,(H2,24,25,26)/t15-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 794 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PIKKC2beta by scintillation proximity assay |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit alpha
(Homo sapiens (Human)) | BDBM50358204

(CHEMBL1922094)Show SMILES C[C@H](O)C(=O)N1CCN(Cc2sc3c(nc(nc3c2C)-c2cnc(N)nc2)N2CCOCC2)CC1 |r| Show InChI InChI=1S/C23H30N8O3S/c1-14-17(13-29-3-5-31(6-4-29)22(33)15(2)32)35-19-18(14)27-20(16-11-25-23(24)26-12-16)28-21(19)30-7-9-34-10-8-30/h11-12,15,32H,3-10,13H2,1-2H3,(H2,24,25,26)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PIKKC2alpha |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50358204

(CHEMBL1922094)Show SMILES C[C@H](O)C(=O)N1CCN(Cc2sc3c(nc(nc3c2C)-c2cnc(N)nc2)N2CCOCC2)CC1 |r| Show InChI InChI=1S/C23H30N8O3S/c1-14-17(13-29-3-5-31(6-4-29)22(33)15(2)32)35-19-18(14)27-20(16-11-25-23(24)26-12-16)28-21(19)30-7-9-34-10-8-30/h11-12,15,32H,3-10,13H2,1-2H3,(H2,24,25,26)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of VPS34 by scintillation proximity assay |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-kinase alpha
(Homo sapiens (Human)) | BDBM50358204

(CHEMBL1922094)Show SMILES C[C@H](O)C(=O)N1CCN(Cc2sc3c(nc(nc3c2C)-c2cnc(N)nc2)N2CCOCC2)CC1 |r| Show InChI InChI=1S/C23H30N8O3S/c1-14-17(13-29-3-5-31(6-4-29)22(33)15(2)32)35-19-18(14)27-20(16-11-25-23(24)26-12-16)28-21(19)30-7-9-34-10-8-30/h11-12,15,32H,3-10,13H2,1-2H3,(H2,24,25,26)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI4Kalpha |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-kinase beta
(Homo sapiens (Human)) | BDBM50358204

(CHEMBL1922094)Show SMILES C[C@H](O)C(=O)N1CCN(Cc2sc3c(nc(nc3c2C)-c2cnc(N)nc2)N2CCOCC2)CC1 |r| Show InChI InChI=1S/C23H30N8O3S/c1-14-17(13-29-3-5-31(6-4-29)22(33)15(2)32)35-19-18(14)27-20(16-11-25-23(24)26-12-16)28-21(19)30-7-9-34-10-8-30/h11-12,15,32H,3-10,13H2,1-2H3,(H2,24,25,26)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI4Kbeta |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data