

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50389383 (CHEMBL2064464) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.318 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex using acetylthiocholine substrate prein... | Eur J Med Chem 55: 23-31 (2012) Article DOI: 10.1016/j.ejmech.2012.06.051 BindingDB Entry DOI: 10.7270/Q2HH6M4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50389383 (CHEMBL2064464) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex using acetylthiocholine substrate preincubated fo... | Eur J Med Chem 55: 23-31 (2012) Article DOI: 10.1016/j.ejmech.2012.06.051 BindingDB Entry DOI: 10.7270/Q2HH6M4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50389382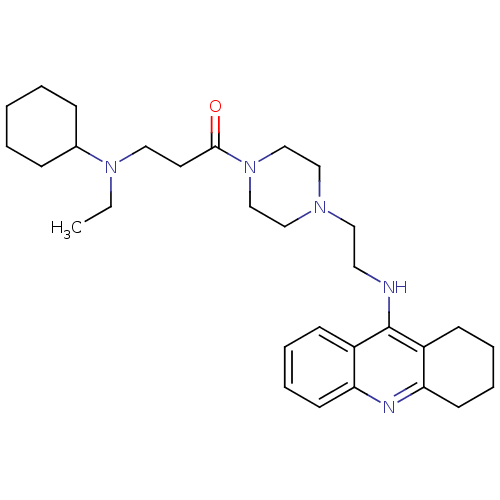 (CHEMBL2064465) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex using acetylthiocholine substrate preincubated fo... | Eur J Med Chem 55: 23-31 (2012) Article DOI: 10.1016/j.ejmech.2012.06.051 BindingDB Entry DOI: 10.7270/Q2HH6M4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50389382 (CHEMBL2064465) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex using acetylthiocholine substrate prein... | Eur J Med Chem 55: 23-31 (2012) Article DOI: 10.1016/j.ejmech.2012.06.051 BindingDB Entry DOI: 10.7270/Q2HH6M4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50389380 (CHEMBL2064468) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex using acetylthiocholine substrate preincubated fo... | Eur J Med Chem 55: 23-31 (2012) Article DOI: 10.1016/j.ejmech.2012.06.051 BindingDB Entry DOI: 10.7270/Q2HH6M4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50389381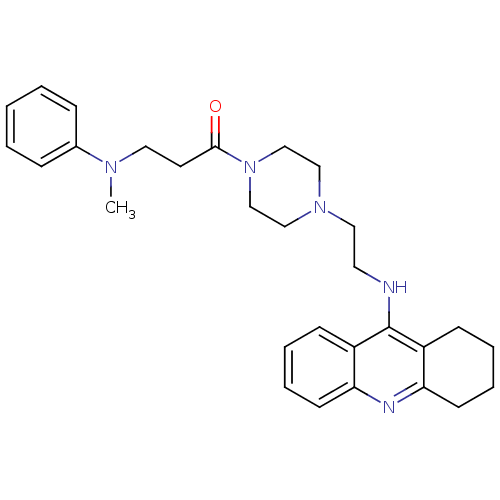 (CHEMBL2064466) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex using acetylthiocholine substrate preincubated fo... | Eur J Med Chem 55: 23-31 (2012) Article DOI: 10.1016/j.ejmech.2012.06.051 BindingDB Entry DOI: 10.7270/Q2HH6M4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50389384 (CHEMBL2064404) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex using acetylthiocholine substrate preincubated fo... | Eur J Med Chem 55: 23-31 (2012) Article DOI: 10.1016/j.ejmech.2012.06.051 BindingDB Entry DOI: 10.7270/Q2HH6M4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50389379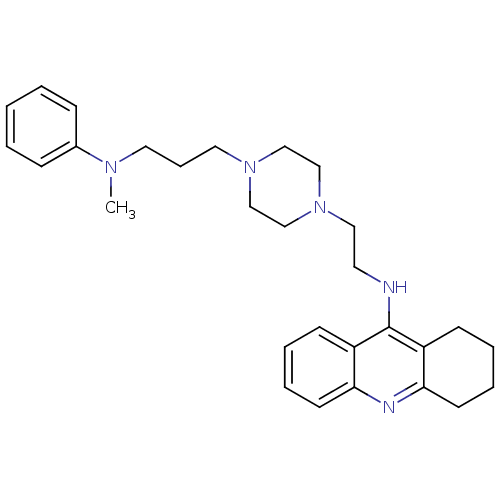 (CHEMBL2064470) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex using acetylthiocholine substrate prein... | Eur J Med Chem 55: 23-31 (2012) Article DOI: 10.1016/j.ejmech.2012.06.051 BindingDB Entry DOI: 10.7270/Q2HH6M4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50389380 (CHEMBL2064468) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 47.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex using acetylthiocholine substrate prein... | Eur J Med Chem 55: 23-31 (2012) Article DOI: 10.1016/j.ejmech.2012.06.051 BindingDB Entry DOI: 10.7270/Q2HH6M4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50389384 (CHEMBL2064404) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 76.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex using acetylthiocholine substrate prein... | Eur J Med Chem 55: 23-31 (2012) Article DOI: 10.1016/j.ejmech.2012.06.051 BindingDB Entry DOI: 10.7270/Q2HH6M4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50389379 (CHEMBL2064470) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex using acetylthiocholine substrate preincubated fo... | Eur J Med Chem 55: 23-31 (2012) Article DOI: 10.1016/j.ejmech.2012.06.051 BindingDB Entry DOI: 10.7270/Q2HH6M4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50389381 (CHEMBL2064466) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 85.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex using acetylthiocholine substrate prein... | Eur J Med Chem 55: 23-31 (2012) Article DOI: 10.1016/j.ejmech.2012.06.051 BindingDB Entry DOI: 10.7270/Q2HH6M4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex using acetylthiocholine substrate prein... | Eur J Med Chem 55: 23-31 (2012) Article DOI: 10.1016/j.ejmech.2012.06.051 BindingDB Entry DOI: 10.7270/Q2HH6M4Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 225 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex using acetylthiocholine substrate preincubated fo... | Eur J Med Chem 55: 23-31 (2012) Article DOI: 10.1016/j.ejmech.2012.06.051 BindingDB Entry DOI: 10.7270/Q2HH6M4Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50327939 (7-methoxytacrine | CHEMBL1256415) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex using acetylthiocholine substrate preincubated fo... | Eur J Med Chem 55: 23-31 (2012) Article DOI: 10.1016/j.ejmech.2012.06.051 BindingDB Entry DOI: 10.7270/Q2HH6M4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50327939 (7-methoxytacrine | CHEMBL1256415) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 6.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex using acetylthiocholine substrate prein... | Eur J Med Chem 55: 23-31 (2012) Article DOI: 10.1016/j.ejmech.2012.06.051 BindingDB Entry DOI: 10.7270/Q2HH6M4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50389385 (CHEMBL2064471) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.49 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine substrate preincubated for 5 mins before substrate addition by Ellman's method | Eur J Med Chem 55: 23-31 (2012) Article DOI: 10.1016/j.ejmech.2012.06.051 BindingDB Entry DOI: 10.7270/Q2HH6M4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50389382 (CHEMBL2064465) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.97 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine substrate preincubated for 5 mins before substrate addition by Ellman's method | Eur J Med Chem 55: 23-31 (2012) Article DOI: 10.1016/j.ejmech.2012.06.051 BindingDB Entry DOI: 10.7270/Q2HH6M4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50389383 (CHEMBL2064464) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14.9 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine substrate preincubated for 5 mins before substrate addition by Ellman's method | Eur J Med Chem 55: 23-31 (2012) Article DOI: 10.1016/j.ejmech.2012.06.051 BindingDB Entry DOI: 10.7270/Q2HH6M4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University Curated by ChEMBL | Assay Description Inhibition of human plasmatic BChE using butylthiocholne substrate preincubated for 5 mins before substrate addition by Ellman's method | Eur J Med Chem 55: 23-31 (2012) Article DOI: 10.1016/j.ejmech.2012.06.051 BindingDB Entry DOI: 10.7270/Q2HH6M4Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50389381 (CHEMBL2064466) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33.7 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University Curated by ChEMBL | Assay Description Inhibition of human plasmatic BChE using butylthiocholne substrate preincubated for 5 mins before substrate addition by Ellman's method | Eur J Med Chem 55: 23-31 (2012) Article DOI: 10.1016/j.ejmech.2012.06.051 BindingDB Entry DOI: 10.7270/Q2HH6M4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50389380 (CHEMBL2064468) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40.7 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine substrate preincubated for 5 mins before substrate addition by Ellman's method | Eur J Med Chem 55: 23-31 (2012) Article DOI: 10.1016/j.ejmech.2012.06.051 BindingDB Entry DOI: 10.7270/Q2HH6M4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50389382 (CHEMBL2064465) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41.2 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University Curated by ChEMBL | Assay Description Inhibition of human plasmatic BChE using butylthiocholne substrate preincubated for 5 mins before substrate addition by Ellman's method | Eur J Med Chem 55: 23-31 (2012) Article DOI: 10.1016/j.ejmech.2012.06.051 BindingDB Entry DOI: 10.7270/Q2HH6M4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50389384 (CHEMBL2064404) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51.7 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine substrate preincubated for 5 mins before substrate addition by Ellman's method | Eur J Med Chem 55: 23-31 (2012) Article DOI: 10.1016/j.ejmech.2012.06.051 BindingDB Entry DOI: 10.7270/Q2HH6M4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50389383 (CHEMBL2064464) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63.7 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University Curated by ChEMBL | Assay Description Inhibition of human plasmatic BChE using butylthiocholne substrate preincubated for 5 mins before substrate addition by Ellman's method | Eur J Med Chem 55: 23-31 (2012) Article DOI: 10.1016/j.ejmech.2012.06.051 BindingDB Entry DOI: 10.7270/Q2HH6M4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50389381 (CHEMBL2064466) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70.3 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine substrate preincubated for 5 mins before substrate addition by Ellman's method | Eur J Med Chem 55: 23-31 (2012) Article DOI: 10.1016/j.ejmech.2012.06.051 BindingDB Entry DOI: 10.7270/Q2HH6M4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50389384 (CHEMBL2064404) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 77.8 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University Curated by ChEMBL | Assay Description Inhibition of human plasmatic BChE using butylthiocholne substrate preincubated for 5 mins before substrate addition by Ellman's method | Eur J Med Chem 55: 23-31 (2012) Article DOI: 10.1016/j.ejmech.2012.06.051 BindingDB Entry DOI: 10.7270/Q2HH6M4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50389379 (CHEMBL2064470) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 84.8 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine substrate preincubated for 5 mins before substrate addition by Ellman's method | Eur J Med Chem 55: 23-31 (2012) Article DOI: 10.1016/j.ejmech.2012.06.051 BindingDB Entry DOI: 10.7270/Q2HH6M4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50389386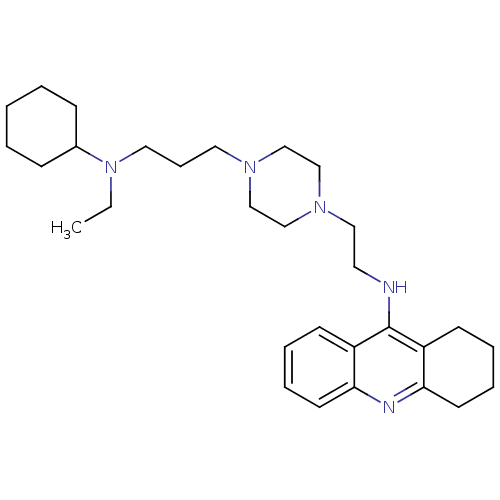 (CHEMBL2064469) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University Curated by ChEMBL | Assay Description Inhibition of human plasmatic BChE using butylthiocholne substrate preincubated for 5 mins before substrate addition by Ellman's method | Eur J Med Chem 55: 23-31 (2012) Article DOI: 10.1016/j.ejmech.2012.06.051 BindingDB Entry DOI: 10.7270/Q2HH6M4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50389380 (CHEMBL2064468) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 137 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University Curated by ChEMBL | Assay Description Inhibition of human plasmatic BChE using butylthiocholne substrate preincubated for 5 mins before substrate addition by Ellman's method | Eur J Med Chem 55: 23-31 (2012) Article DOI: 10.1016/j.ejmech.2012.06.051 BindingDB Entry DOI: 10.7270/Q2HH6M4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50389386 (CHEMBL2064469) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 194 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine substrate preincubated for 5 mins before substrate addition by Ellman's method | Eur J Med Chem 55: 23-31 (2012) Article DOI: 10.1016/j.ejmech.2012.06.051 BindingDB Entry DOI: 10.7270/Q2HH6M4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50389379 (CHEMBL2064470) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 211 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University Curated by ChEMBL | Assay Description Inhibition of human plasmatic BChE using butylthiocholne substrate preincubated for 5 mins before substrate addition by Ellman's method | Eur J Med Chem 55: 23-31 (2012) Article DOI: 10.1016/j.ejmech.2012.06.051 BindingDB Entry DOI: 10.7270/Q2HH6M4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine substrate preincubated for 5 mins before substrate addition by Ellman's method | Eur J Med Chem 55: 23-31 (2012) Article DOI: 10.1016/j.ejmech.2012.06.051 BindingDB Entry DOI: 10.7270/Q2HH6M4Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50389385 (CHEMBL2064471) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University Curated by ChEMBL | Assay Description Inhibition of human plasmatic BChE using butylthiocholne substrate preincubated for 5 mins before substrate addition by Ellman's method | Eur J Med Chem 55: 23-31 (2012) Article DOI: 10.1016/j.ejmech.2012.06.051 BindingDB Entry DOI: 10.7270/Q2HH6M4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50327939 (7-methoxytacrine | CHEMBL1256415) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine substrate preincubated for 5 mins before substrate addition by Ellman's method | Eur J Med Chem 55: 23-31 (2012) Article DOI: 10.1016/j.ejmech.2012.06.051 BindingDB Entry DOI: 10.7270/Q2HH6M4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50327939 (7-methoxytacrine | CHEMBL1256415) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University Curated by ChEMBL | Assay Description Inhibition of human plasmatic BChE using butylthiocholne substrate preincubated for 5 mins before substrate addition by Ellman's method | Eur J Med Chem 55: 23-31 (2012) Article DOI: 10.1016/j.ejmech.2012.06.051 BindingDB Entry DOI: 10.7270/Q2HH6M4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||