 Found 33 hits Enz. Inhib. hit(s) with all data for entry = 50040455
Found 33 hits Enz. Inhib. hit(s) with all data for entry = 50040455 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50394447

(CHEMBL2159662)Show SMILES CC(=O)c1ccccc1NC(=O)c1ccc(OCCCCC[n+]2cccc3ccccc23)cc1 Show InChI InChI=1S/C29H28N2O3/c1-22(32)26-12-4-5-13-27(26)30-29(33)24-15-17-25(18-16-24)34-21-8-2-7-19-31-20-9-11-23-10-3-6-14-28(23)31/h3-6,9-18,20H,2,7-8,19,21H2,1H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine as substrate |
Bioorg Med Chem 20: 6739-50 (2012)
Article DOI: 10.1016/j.bmc.2012.09.016
BindingDB Entry DOI: 10.7270/Q2ZW1N1Z |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE using acetylthiocholine as substrate |
Bioorg Med Chem 20: 6739-50 (2012)
Article DOI: 10.1016/j.bmc.2012.09.016
BindingDB Entry DOI: 10.7270/Q2ZW1N1Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50394448

(CHEMBL2159661)Show SMILES CC(=O)c1ccccc1NC(=O)c1ccc(OCCCCCC[n+]2ccc3ccccc3c2)cc1 Show InChI InChI=1S/C30H30N2O3/c1-23(33)28-12-6-7-13-29(28)31-30(34)25-14-16-27(17-15-25)35-21-9-3-2-8-19-32-20-18-24-10-4-5-11-26(24)22-32/h4-7,10-18,20,22H,2-3,8-9,19,21H2,1H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine as substrate |
Bioorg Med Chem 20: 6739-50 (2012)
Article DOI: 10.1016/j.bmc.2012.09.016
BindingDB Entry DOI: 10.7270/Q2ZW1N1Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine as substrate |
Bioorg Med Chem 20: 6739-50 (2012)
Article DOI: 10.1016/j.bmc.2012.09.016
BindingDB Entry DOI: 10.7270/Q2ZW1N1Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50394449
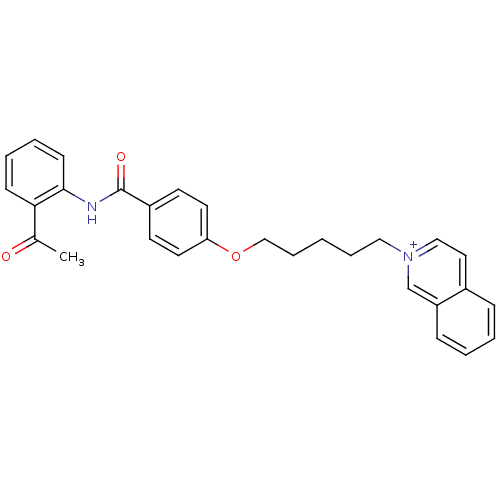
(CHEMBL2159660)Show SMILES CC(=O)c1ccccc1NC(=O)c1ccc(OCCCCC[n+]2ccc3ccccc3c2)cc1 Show InChI InChI=1S/C29H28N2O3/c1-22(32)27-11-5-6-12-28(27)30-29(33)24-13-15-26(16-14-24)34-20-8-2-7-18-31-19-17-23-9-3-4-10-25(23)21-31/h3-6,9-17,19,21H,2,7-8,18,20H2,1H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine as substrate |
Bioorg Med Chem 20: 6739-50 (2012)
Article DOI: 10.1016/j.bmc.2012.09.016
BindingDB Entry DOI: 10.7270/Q2ZW1N1Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50394450
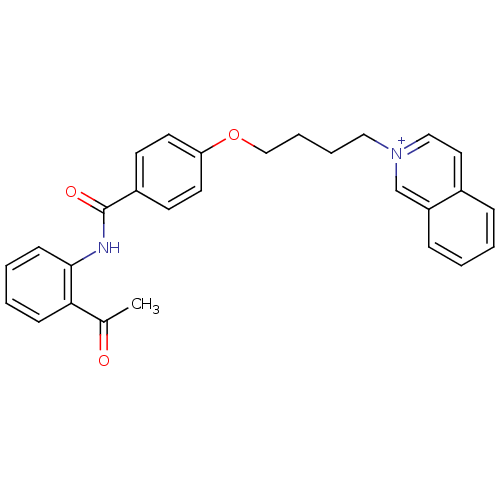
(CHEMBL2159659)Show SMILES CC(=O)c1ccccc1NC(=O)c1ccc(OCCCC[n+]2ccc3ccccc3c2)cc1 Show InChI InChI=1S/C28H26N2O3/c1-21(31)26-10-4-5-11-27(26)29-28(32)23-12-14-25(15-13-23)33-19-7-6-17-30-18-16-22-8-2-3-9-24(22)20-30/h2-5,8-16,18,20H,6-7,17,19H2,1H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine as substrate |
Bioorg Med Chem 20: 6739-50 (2012)
Article DOI: 10.1016/j.bmc.2012.09.016
BindingDB Entry DOI: 10.7270/Q2ZW1N1Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50394445
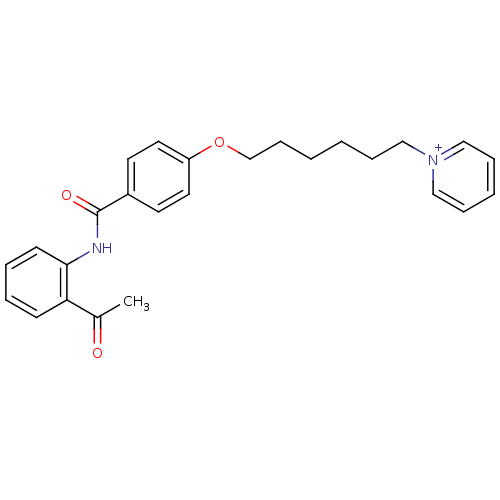
(CHEMBL2159664)Show SMILES CC(=O)c1ccccc1NC(=O)c1ccc(OCCCCCC[n+]2ccccc2)cc1 Show InChI InChI=1S/C26H28N2O3/c1-21(29)24-11-5-6-12-25(24)27-26(30)22-13-15-23(16-14-22)31-20-10-3-2-7-17-28-18-8-4-9-19-28/h4-6,8-9,11-16,18-19H,2-3,7,10,17,20H2,1H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine as substrate |
Bioorg Med Chem 20: 6739-50 (2012)
Article DOI: 10.1016/j.bmc.2012.09.016
BindingDB Entry DOI: 10.7270/Q2ZW1N1Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50394451
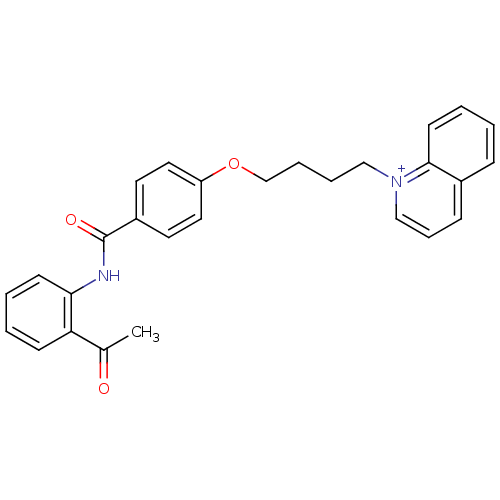
(CHEMBL2159658)Show SMILES CC(=O)c1ccccc1NC(=O)c1ccc(OCCCC[n+]2cccc3ccccc23)cc1 Show InChI InChI=1S/C28H26N2O3/c1-21(31)25-11-3-4-12-26(25)29-28(32)23-14-16-24(17-15-23)33-20-7-6-18-30-19-8-10-22-9-2-5-13-27(22)30/h2-5,8-17,19H,6-7,18,20H2,1H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine as substrate |
Bioorg Med Chem 20: 6739-50 (2012)
Article DOI: 10.1016/j.bmc.2012.09.016
BindingDB Entry DOI: 10.7270/Q2ZW1N1Z |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50394447

(CHEMBL2159662)Show SMILES CC(=O)c1ccccc1NC(=O)c1ccc(OCCCCC[n+]2cccc3ccccc23)cc1 Show InChI InChI=1S/C29H28N2O3/c1-22(32)26-12-4-5-13-27(26)30-29(33)24-15-17-25(18-16-24)34-21-8-2-7-19-31-20-9-11-23-10-3-6-14-28(23)31/h3-6,9-18,20H,2,7-8,19,21H2,1H3/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE using acetylthiocholine as substrate |
Bioorg Med Chem 20: 6739-50 (2012)
Article DOI: 10.1016/j.bmc.2012.09.016
BindingDB Entry DOI: 10.7270/Q2ZW1N1Z |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50394450

(CHEMBL2159659)Show SMILES CC(=O)c1ccccc1NC(=O)c1ccc(OCCCC[n+]2ccc3ccccc3c2)cc1 Show InChI InChI=1S/C28H26N2O3/c1-21(31)26-10-4-5-11-27(26)29-28(32)23-12-14-25(15-13-23)33-19-7-6-17-30-18-16-22-8-2-3-9-24(22)20-30/h2-5,8-16,18,20H,6-7,17,19H2,1H3/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE using acetylthiocholine as substrate |
Bioorg Med Chem 20: 6739-50 (2012)
Article DOI: 10.1016/j.bmc.2012.09.016
BindingDB Entry DOI: 10.7270/Q2ZW1N1Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10404

((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...)Show SMILES COc1ccc2CN(C)CC[C@@]34C=C[C@H](O)C[C@@H]3Oc1c24 |r,c:12| Show InChI InChI=1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine as substrate |
Bioorg Med Chem 20: 6739-50 (2012)
Article DOI: 10.1016/j.bmc.2012.09.016
BindingDB Entry DOI: 10.7270/Q2ZW1N1Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM50394445

(CHEMBL2159664)Show SMILES CC(=O)c1ccccc1NC(=O)c1ccc(OCCCCCC[n+]2ccccc2)cc1 Show InChI InChI=1S/C26H28N2O3/c1-21(29)24-11-5-6-12-25(24)27-26(30)22-13-15-23(16-14-22)31-20-10-3-2-7-17-28-18-8-4-9-19-28/h4-6,8-9,11-16,18-19H,2-3,7,10,17,20H2,1H3/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE using acetylthiocholine as substrate |
Bioorg Med Chem 20: 6739-50 (2012)
Article DOI: 10.1016/j.bmc.2012.09.016
BindingDB Entry DOI: 10.7270/Q2ZW1N1Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50394446

(CHEMBL2159663)Show SMILES CC(=O)c1ccccc1NC(=O)c1ccc(OCCCCC[n+]2ccccc2)cc1 Show InChI InChI=1S/C25H26N2O3/c1-20(28)23-10-4-5-11-24(23)26-25(29)21-12-14-22(15-13-21)30-19-9-3-8-18-27-16-6-2-7-17-27/h2,4-7,10-17H,3,8-9,18-19H2,1H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine as substrate |
Bioorg Med Chem 20: 6739-50 (2012)
Article DOI: 10.1016/j.bmc.2012.09.016
BindingDB Entry DOI: 10.7270/Q2ZW1N1Z |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50394449

(CHEMBL2159660)Show SMILES CC(=O)c1ccccc1NC(=O)c1ccc(OCCCCC[n+]2ccc3ccccc3c2)cc1 Show InChI InChI=1S/C29H28N2O3/c1-22(32)27-11-5-6-12-28(27)30-29(33)24-13-15-26(16-14-24)34-20-8-2-7-18-31-19-17-23-9-3-4-10-25(23)21-31/h3-6,9-17,19,21H,2,7-8,18,20H2,1H3/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE using acetylthiocholine as substrate |
Bioorg Med Chem 20: 6739-50 (2012)
Article DOI: 10.1016/j.bmc.2012.09.016
BindingDB Entry DOI: 10.7270/Q2ZW1N1Z |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50394446

(CHEMBL2159663)Show SMILES CC(=O)c1ccccc1NC(=O)c1ccc(OCCCCC[n+]2ccccc2)cc1 Show InChI InChI=1S/C25H26N2O3/c1-20(28)23-10-4-5-11-24(23)26-25(29)21-12-14-22(15-13-21)30-19-9-3-8-18-27-16-6-2-7-17-27/h2,4-7,10-17H,3,8-9,18-19H2,1H3/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE using acetylthiocholine as substrate |
Bioorg Med Chem 20: 6739-50 (2012)
Article DOI: 10.1016/j.bmc.2012.09.016
BindingDB Entry DOI: 10.7270/Q2ZW1N1Z |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50394452

(CHEMBL2159657)Show SMILES O=C(NCCc1c[nH]c2ccccc12)c1ccc(OCCCC[n+]2cccc3ccccc23)cc1 Show InChI InChI=1S/C30H29N3O2/c34-30(31-18-17-25-22-32-28-11-3-2-10-27(25)28)24-13-15-26(16-14-24)35-21-6-5-19-33-20-7-9-23-8-1-4-12-29(23)33/h1-4,7-16,20,22,32H,5-6,17-19,21H2/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE using acetylthiocholine as substrate |
Bioorg Med Chem 20: 6739-50 (2012)
Article DOI: 10.1016/j.bmc.2012.09.016
BindingDB Entry DOI: 10.7270/Q2ZW1N1Z |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50394448

(CHEMBL2159661)Show SMILES CC(=O)c1ccccc1NC(=O)c1ccc(OCCCCCC[n+]2ccc3ccccc3c2)cc1 Show InChI InChI=1S/C30H30N2O3/c1-23(33)28-12-6-7-13-29(28)31-30(34)25-14-16-27(17-15-25)35-21-9-3-2-8-19-32-20-18-24-10-4-5-11-26(24)22-32/h4-7,10-18,20,22H,2-3,8-9,19,21H2,1H3/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE using acetylthiocholine as substrate |
Bioorg Med Chem 20: 6739-50 (2012)
Article DOI: 10.1016/j.bmc.2012.09.016
BindingDB Entry DOI: 10.7270/Q2ZW1N1Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50394452

(CHEMBL2159657)Show SMILES O=C(NCCc1c[nH]c2ccccc12)c1ccc(OCCCC[n+]2cccc3ccccc23)cc1 Show InChI InChI=1S/C30H29N3O2/c34-30(31-18-17-25-22-32-28-11-3-2-10-27(25)28)24-13-15-26(16-14-24)35-21-6-5-19-33-20-7-9-23-8-1-4-12-29(23)33/h1-4,7-16,20,22,32H,5-6,17-19,21H2/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine as substrate |
Bioorg Med Chem 20: 6739-50 (2012)
Article DOI: 10.1016/j.bmc.2012.09.016
BindingDB Entry DOI: 10.7270/Q2ZW1N1Z |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50394451

(CHEMBL2159658)Show SMILES CC(=O)c1ccccc1NC(=O)c1ccc(OCCCC[n+]2cccc3ccccc23)cc1 Show InChI InChI=1S/C28H26N2O3/c1-21(31)25-11-3-4-12-26(25)29-28(32)23-14-16-24(17-15-23)33-20-7-6-18-30-19-8-10-22-9-2-5-13-27(22)30/h2-5,8-17,19H,6-7,18,20H2,1H3/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 166 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE using acetylthiocholine as substrate |
Bioorg Med Chem 20: 6739-50 (2012)
Article DOI: 10.1016/j.bmc.2012.09.016
BindingDB Entry DOI: 10.7270/Q2ZW1N1Z |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM10404

((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...)Show SMILES COc1ccc2CN(C)CC[C@@]34C=C[C@H](O)C[C@@H]3Oc1c24 |r,c:12| Show InChI InChI=1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 192 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE using acetylthiocholine as substrate |
Bioorg Med Chem 20: 6739-50 (2012)
Article DOI: 10.1016/j.bmc.2012.09.016
BindingDB Entry DOI: 10.7270/Q2ZW1N1Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50394453

(CHEMBL2159656)Show SMILES C[N+](C)=c1ccn(CCCCOc2ccc(cc2)C(=O)NCCc2c[nH]c3ccccc23)cc1 |(-4.34,.4,;-4.32,-1.14,;-5.64,-1.92,;-2.97,-1.89,;-2.96,-3.44,;-1.61,-4.2,;-.29,-3.41,;1.08,-4.18,;2.4,-3.39,;3.74,-4.15,;5.07,-3.36,;6.41,-4.12,;7.74,-3.33,;7.73,-1.79,;9.05,-1.01,;10.39,-1.76,;10.41,-3.3,;9.08,-4.09,;11.77,-1.04,;11.75,.49,;13.1,-1.8,;13.12,-3.34,;14.47,-4.09,;14.49,-5.64,;13.26,-6.55,;13.74,-8.02,;15.3,-7.99,;16.34,-9.13,;17.83,-8.79,;18.29,-7.32,;17.25,-6.19,;15.75,-6.52,;-.31,-1.87,;-1.65,-1.11,)| Show InChI InChI=1S/C28H32N4O2/c1-31(2)24-14-18-32(19-15-24)17-5-6-20-34-25-11-9-22(10-12-25)28(33)29-16-13-23-21-30-27-8-4-3-7-26(23)27/h3-4,7-12,14-15,18-19,21,30H,5-6,13,16-17,20H2,1-2H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 198 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine as substrate |
Bioorg Med Chem 20: 6739-50 (2012)
Article DOI: 10.1016/j.bmc.2012.09.016
BindingDB Entry DOI: 10.7270/Q2ZW1N1Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50394458

(CHEMBL2158019)Show SMILES O=C(NCCc1c[nH]c2ccccc12)c1ccc(OCCCCn2c3ccccc3c3ccccc23)cc1 Show InChI InChI=1S/C33H31N3O2/c37-33(34-20-19-25-23-35-30-12-4-1-9-27(25)30)24-15-17-26(18-16-24)38-22-8-7-21-36-31-13-5-2-10-28(31)29-11-3-6-14-32(29)36/h1-6,9-18,23,35H,7-8,19-22H2,(H,34,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine as substrate |
Bioorg Med Chem 20: 6739-50 (2012)
Article DOI: 10.1016/j.bmc.2012.09.016
BindingDB Entry DOI: 10.7270/Q2ZW1N1Z |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50394453

(CHEMBL2159656)Show SMILES C[N+](C)=c1ccn(CCCCOc2ccc(cc2)C(=O)NCCc2c[nH]c3ccccc23)cc1 |(-4.34,.4,;-4.32,-1.14,;-5.64,-1.92,;-2.97,-1.89,;-2.96,-3.44,;-1.61,-4.2,;-.29,-3.41,;1.08,-4.18,;2.4,-3.39,;3.74,-4.15,;5.07,-3.36,;6.41,-4.12,;7.74,-3.33,;7.73,-1.79,;9.05,-1.01,;10.39,-1.76,;10.41,-3.3,;9.08,-4.09,;11.77,-1.04,;11.75,.49,;13.1,-1.8,;13.12,-3.34,;14.47,-4.09,;14.49,-5.64,;13.26,-6.55,;13.74,-8.02,;15.3,-7.99,;16.34,-9.13,;17.83,-8.79,;18.29,-7.32,;17.25,-6.19,;15.75,-6.52,;-.31,-1.87,;-1.65,-1.11,)| Show InChI InChI=1S/C28H32N4O2/c1-31(2)24-14-18-32(19-15-24)17-5-6-20-34-25-11-9-22(10-12-25)28(33)29-16-13-23-21-30-27-8-4-3-7-26(23)27/h3-4,7-12,14-15,18-19,21,30H,5-6,13,16-17,20H2,1-2H3/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE using acetylthiocholine as substrate |
Bioorg Med Chem 20: 6739-50 (2012)
Article DOI: 10.1016/j.bmc.2012.09.016
BindingDB Entry DOI: 10.7270/Q2ZW1N1Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50394456

(CHEMBL2159653)Show SMILES O=C(NCCc1c[nH]c2ccccc12)c1ccc(OCCCCOc2cccc(c2)C(=O)c2ccccc2)cc1 Show InChI InChI=1S/C34H32N2O4/c37-33(25-9-2-1-3-10-25)27-11-8-12-30(23-27)40-22-7-6-21-39-29-17-15-26(16-18-29)34(38)35-20-19-28-24-36-32-14-5-4-13-31(28)32/h1-5,8-18,23-24,36H,6-7,19-22H2,(H,35,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine as substrate |
Bioorg Med Chem 20: 6739-50 (2012)
Article DOI: 10.1016/j.bmc.2012.09.016
BindingDB Entry DOI: 10.7270/Q2ZW1N1Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50394455

(CHEMBL2159654)Show SMILES O=C(NCCc1c[nH]c2ccccc12)c1ccc(OCCCCOc2ccc(cc2)C(=O)c2ccccc2)cc1 Show InChI InChI=1S/C34H32N2O4/c37-33(25-8-2-1-3-9-25)26-12-16-29(17-13-26)39-22-6-7-23-40-30-18-14-27(15-19-30)34(38)35-21-20-28-24-36-32-11-5-4-10-31(28)32/h1-5,8-19,24,36H,6-7,20-23H2,(H,35,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine as substrate |
Bioorg Med Chem 20: 6739-50 (2012)
Article DOI: 10.1016/j.bmc.2012.09.016
BindingDB Entry DOI: 10.7270/Q2ZW1N1Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50394454
(CHEMBL2159655)Show SMILES O=C(NCCc1c[nH]c2ccccc12)c1ccc(OCCCCOc2ccc3-c4ccccc4C(=O)c3c2)cc1 Show InChI InChI=1S/C34H30N2O4/c37-33-30-9-2-1-8-28(30)29-16-15-26(21-31(29)33)40-20-6-5-19-39-25-13-11-23(12-14-25)34(38)35-18-17-24-22-36-32-10-4-3-7-27(24)32/h1-4,7-16,21-22,36H,5-6,17-20H2,(H,35,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine as substrate |
Bioorg Med Chem 20: 6739-50 (2012)
Article DOI: 10.1016/j.bmc.2012.09.016
BindingDB Entry DOI: 10.7270/Q2ZW1N1Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50394457

(CHEMBL2159652)Show SMILES O=C(NCCc1c[nH]c2ccccc12)c1ccc(OCCCCOc2cccc3[nH]c4ccccc4c23)cc1 Show InChI InChI=1S/C33H31N3O3/c37-33(34-19-18-24-22-35-28-10-3-1-8-26(24)28)23-14-16-25(17-15-23)38-20-5-6-21-39-31-13-7-12-30-32(31)27-9-2-4-11-29(27)36-30/h1-4,7-17,22,35-36H,5-6,18-21H2,(H,34,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine as substrate |
Bioorg Med Chem 20: 6739-50 (2012)
Article DOI: 10.1016/j.bmc.2012.09.016
BindingDB Entry DOI: 10.7270/Q2ZW1N1Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50394459

(CHEMBL2159651)Show SMILES O=C(NCCc1c[nH]c2ccccc12)c1ccc(OCCCCn2ccc3ccccc23)cc1 Show InChI InChI=1S/C29H29N3O2/c33-29(30-17-15-24-21-31-27-9-3-2-8-26(24)27)23-11-13-25(14-12-23)34-20-6-5-18-32-19-16-22-7-1-4-10-28(22)32/h1-4,7-14,16,19,21,31H,5-6,15,17-18,20H2,(H,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine as substrate |
Bioorg Med Chem 20: 6739-50 (2012)
Article DOI: 10.1016/j.bmc.2012.09.016
BindingDB Entry DOI: 10.7270/Q2ZW1N1Z |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50394446

(CHEMBL2159663)Show SMILES CC(=O)c1ccccc1NC(=O)c1ccc(OCCCCC[n+]2ccccc2)cc1 Show InChI InChI=1S/C25H26N2O3/c1-20(28)23-10-4-5-11-24(23)26-25(29)21-12-14-22(15-13-21)30-19-9-3-8-18-27-16-6-2-7-17-27/h2,4-7,10-17H,3,8-9,18-19H2,1H3/p+1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of beta-secretase using rhodamine-EVNLDAEFK-quencher as substrate after 60 mins by fluorescence resonance energy transfer assay |
Bioorg Med Chem 20: 6739-50 (2012)
Article DOI: 10.1016/j.bmc.2012.09.016
BindingDB Entry DOI: 10.7270/Q2ZW1N1Z |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50067040
(((E,E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-hept...)Show SMILES COc1cc(C=CC(=O)CC(=O)C=Cc2ccc(O)c(OC)c2)ccc1O |w:12.11,5.4| Show InChI InChI=1S/C21H20O6/c1-26-20-11-14(5-9-18(20)24)3-7-16(22)13-17(23)8-4-15-6-10-19(25)21(12-15)27-2/h3-12,24-25H,13H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of beta-secretase using rhodamine-EVNLDAEFK-quencher as substrate after 60 mins by fluorescence resonance energy transfer assay |
Bioorg Med Chem 20: 6739-50 (2012)
Article DOI: 10.1016/j.bmc.2012.09.016
BindingDB Entry DOI: 10.7270/Q2ZW1N1Z |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50394447

(CHEMBL2159662)Show SMILES CC(=O)c1ccccc1NC(=O)c1ccc(OCCCCC[n+]2cccc3ccccc23)cc1 Show InChI InChI=1S/C29H28N2O3/c1-22(32)26-12-4-5-13-27(26)30-29(33)24-15-17-25(18-16-24)34-21-8-2-7-19-31-20-9-11-23-10-3-6-14-28(23)31/h3-6,9-18,20H,2,7-8,19,21H2,1H3/p+1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of beta-secretase using rhodamine-EVNLDAEFK-quencher as substrate after 60 mins by fluorescence resonance energy transfer assay |
Bioorg Med Chem 20: 6739-50 (2012)
Article DOI: 10.1016/j.bmc.2012.09.016
BindingDB Entry DOI: 10.7270/Q2ZW1N1Z |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50394449

(CHEMBL2159660)Show SMILES CC(=O)c1ccccc1NC(=O)c1ccc(OCCCCC[n+]2ccc3ccccc3c2)cc1 Show InChI InChI=1S/C29H28N2O3/c1-22(32)27-11-5-6-12-28(27)30-29(33)24-13-15-26(16-14-24)34-20-8-2-7-18-31-19-17-23-9-3-4-10-25(23)21-31/h3-6,9-17,19,21H,2,7-8,18,20H2,1H3/p+1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of beta-secretase using rhodamine-EVNLDAEFK-quencher as substrate after 60 mins by fluorescence resonance energy transfer assay |
Bioorg Med Chem 20: 6739-50 (2012)
Article DOI: 10.1016/j.bmc.2012.09.016
BindingDB Entry DOI: 10.7270/Q2ZW1N1Z |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50394448

(CHEMBL2159661)Show SMILES CC(=O)c1ccccc1NC(=O)c1ccc(OCCCCCC[n+]2ccc3ccccc3c2)cc1 Show InChI InChI=1S/C30H30N2O3/c1-23(33)28-12-6-7-13-29(28)31-30(34)25-14-16-27(17-15-25)35-21-9-3-2-8-19-32-20-18-24-10-4-5-11-26(24)22-32/h4-7,10-18,20,22H,2-3,8-9,19,21H2,1H3/p+1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of beta-secretase using rhodamine-EVNLDAEFK-quencher as substrate after 60 mins by fluorescence resonance energy transfer assay |
Bioorg Med Chem 20: 6739-50 (2012)
Article DOI: 10.1016/j.bmc.2012.09.016
BindingDB Entry DOI: 10.7270/Q2ZW1N1Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data