

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cycloartenol synthase (Arabidopsis thaliana) | BDBM50433360 (CHEMBL2375380) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians-Universit£t M£nchen Curated by ChEMBL | Assay Description Inhibition of Arabidopsis thaliana cycloartenol synthase expressed in Saccharomyces cerevisiae SMY8[pSM60.21] using [14C]-(3S)-2,3-oxidosqualene as s... | Eur J Med Chem 63: 758-64 (2013) Article DOI: 10.1016/j.ejmech.2013.03.002 BindingDB Entry DOI: 10.7270/Q2FB54B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cycloartenol synthase (Arabidopsis thaliana) | BDBM50433362 (CHEMBL2377451) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians-Universit£t M£nchen Curated by ChEMBL | Assay Description Inhibition of Arabidopsis thaliana cycloartenol synthase expressed in Saccharomyces cerevisiae SMY8[pSM60.21] using [14C]-(3S)-2,3-oxidosqualene as s... | Eur J Med Chem 63: 758-64 (2013) Article DOI: 10.1016/j.ejmech.2013.03.002 BindingDB Entry DOI: 10.7270/Q2FB54B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cycloartenol synthase (Arabidopsis thaliana) | BDBM50433361 (CHEMBL2377452) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians-Universit£t M£nchen Curated by ChEMBL | Assay Description Inhibition of Arabidopsis thaliana cycloartenol synthase expressed in Saccharomyces cerevisiae SMY8[pSM60.21] using [14C]-(3S)-2,3-oxidosqualene as s... | Eur J Med Chem 63: 758-64 (2013) Article DOI: 10.1016/j.ejmech.2013.03.002 BindingDB Entry DOI: 10.7270/Q2FB54B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cycloartenol synthase (Arabidopsis thaliana) | BDBM50433363 (CHEMBL2377449) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians-Universit£t M£nchen Curated by ChEMBL | Assay Description Inhibition of Arabidopsis thaliana cycloartenol synthase expressed in Saccharomyces cerevisiae SMY8[pSM60.21] using [14C]-(3S)-2,3-oxidosqualene as s... | Eur J Med Chem 63: 758-64 (2013) Article DOI: 10.1016/j.ejmech.2013.03.002 BindingDB Entry DOI: 10.7270/Q2FB54B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Pneumocystis carinii) | BDBM50433363 (CHEMBL2377449) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians-Universit£t M£nchen Curated by ChEMBL | Assay Description Inhibition of Pneumocystis carinii lanosterol synthase expressed in Saccharomyces cerevisiae SMY8[pBJ4.21] using [14C]-(3S)-2,3-oxidosqualene as subs... | Eur J Med Chem 63: 758-64 (2013) Article DOI: 10.1016/j.ejmech.2013.03.002 BindingDB Entry DOI: 10.7270/Q2FB54B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Homo sapiens (Human)) | BDBM50433363 (CHEMBL2377449) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians-Universit£t M£nchen Curated by ChEMBL | Assay Description Inhibition of human lanosterol synthase expressed in Saccharomyces cerevisiae SMY8[pSOB1.1] using [14C]-(3S)-2,3-oxidosqualene as substrate | Eur J Med Chem 63: 758-64 (2013) Article DOI: 10.1016/j.ejmech.2013.03.002 BindingDB Entry DOI: 10.7270/Q2FB54B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase ERG7 (Saccharomyces cerevisiae) | BDBM50433362 (CHEMBL2377451) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians-Universit£t M£nchen Curated by ChEMBL | Assay Description Inhibition of wild type yeast lanosterol synthase expressed in Saccharomyces cerevisiae SMY8[pSM61.21] using [14C]-(3S)-2,3-oxidosqualene as substrat... | Eur J Med Chem 63: 758-64 (2013) Article DOI: 10.1016/j.ejmech.2013.03.002 BindingDB Entry DOI: 10.7270/Q2FB54B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cycloartenol synthase (Arabidopsis thaliana) | BDBM50433364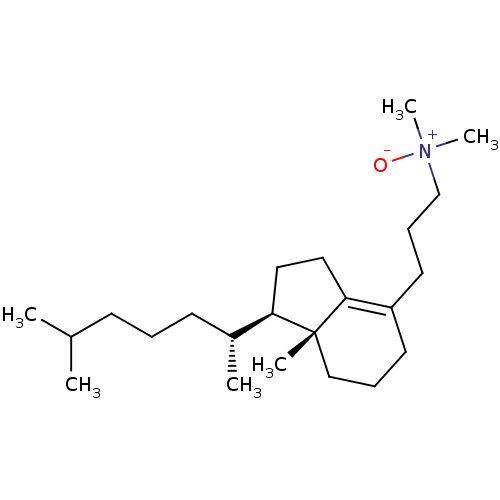 (CHEMBL2377453) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians-Universit£t M£nchen Curated by ChEMBL | Assay Description Inhibition of Arabidopsis thaliana cycloartenol synthase expressed in Saccharomyces cerevisiae SMY8[pSM60.21] using [14C]-(3S)-2,3-oxidosqualene as s... | Eur J Med Chem 63: 758-64 (2013) Article DOI: 10.1016/j.ejmech.2013.03.002 BindingDB Entry DOI: 10.7270/Q2FB54B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase ERG7 (Saccharomyces cerevisiae) | BDBM50433363 (CHEMBL2377449) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians-Universit£t M£nchen Curated by ChEMBL | Assay Description Inhibition of wild type yeast lanosterol synthase expressed in Saccharomyces cerevisiae SMY8[pSM61.21] using [14C]-(3S)-2,3-oxidosqualene as substrat... | Eur J Med Chem 63: 758-64 (2013) Article DOI: 10.1016/j.ejmech.2013.03.002 BindingDB Entry DOI: 10.7270/Q2FB54B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase ERG7 (Saccharomyces cerevisiae) | BDBM50433361 (CHEMBL2377452) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians-Universit£t M£nchen Curated by ChEMBL | Assay Description Inhibition of wild type yeast lanosterol synthase expressed in Saccharomyces cerevisiae SMY8[pSM61.21] using [14C]-(3S)-2,3-oxidosqualene as substrat... | Eur J Med Chem 63: 758-64 (2013) Article DOI: 10.1016/j.ejmech.2013.03.002 BindingDB Entry DOI: 10.7270/Q2FB54B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase ERG7 (Saccharomyces cerevisiae) | BDBM50433364 (CHEMBL2377453) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians-Universit£t M£nchen Curated by ChEMBL | Assay Description Inhibition of wild type yeast lanosterol synthase expressed in Saccharomyces cerevisiae SMY8[pSM61.21] using [14C]-(3S)-2,3-oxidosqualene as substrat... | Eur J Med Chem 63: 758-64 (2013) Article DOI: 10.1016/j.ejmech.2013.03.002 BindingDB Entry DOI: 10.7270/Q2FB54B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase ERG7 (Saccharomyces cerevisiae) | BDBM50433360 (CHEMBL2375380) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians-Universit£t M£nchen Curated by ChEMBL | Assay Description Inhibition of wild type yeast lanosterol synthase expressed in Saccharomyces cerevisiae SMY8[pSM61.21] using [14C]-(3S)-2,3-oxidosqualene as substrat... | Eur J Med Chem 63: 758-64 (2013) Article DOI: 10.1016/j.ejmech.2013.03.002 BindingDB Entry DOI: 10.7270/Q2FB54B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Homo sapiens (Human)) | BDBM50433362 (CHEMBL2377451) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians-Universit£t M£nchen Curated by ChEMBL | Assay Description Inhibition of human lanosterol synthase expressed in Saccharomyces cerevisiae SMY8[pSOB1.1] using [14C]-(3S)-2,3-oxidosqualene as substrate | Eur J Med Chem 63: 758-64 (2013) Article DOI: 10.1016/j.ejmech.2013.03.002 BindingDB Entry DOI: 10.7270/Q2FB54B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Pneumocystis carinii) | BDBM50433360 (CHEMBL2375380) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians-Universit£t M£nchen Curated by ChEMBL | Assay Description Inhibition of Pneumocystis carinii lanosterol synthase expressed in Saccharomyces cerevisiae SMY8[pBJ4.21] using [14C]-(3S)-2,3-oxidosqualene as subs... | Eur J Med Chem 63: 758-64 (2013) Article DOI: 10.1016/j.ejmech.2013.03.002 BindingDB Entry DOI: 10.7270/Q2FB54B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Homo sapiens (Human)) | BDBM50433361 (CHEMBL2377452) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians-Universit£t M£nchen Curated by ChEMBL | Assay Description Inhibition of human lanosterol synthase expressed in Saccharomyces cerevisiae SMY8[pSOB1.1] using [14C]-(3S)-2,3-oxidosqualene as substrate | Eur J Med Chem 63: 758-64 (2013) Article DOI: 10.1016/j.ejmech.2013.03.002 BindingDB Entry DOI: 10.7270/Q2FB54B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Homo sapiens (Human)) | BDBM50433360 (CHEMBL2375380) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians-Universit£t M£nchen Curated by ChEMBL | Assay Description Inhibition of human lanosterol synthase expressed in Saccharomyces cerevisiae SMY8[pSOB1.1] using [14C]-(3S)-2,3-oxidosqualene as substrate | Eur J Med Chem 63: 758-64 (2013) Article DOI: 10.1016/j.ejmech.2013.03.002 BindingDB Entry DOI: 10.7270/Q2FB54B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Pneumocystis carinii) | BDBM50433362 (CHEMBL2377451) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians-Universit£t M£nchen Curated by ChEMBL | Assay Description Inhibition of Pneumocystis carinii lanosterol synthase expressed in Saccharomyces cerevisiae SMY8[pBJ4.21] using [14C]-(3S)-2,3-oxidosqualene as subs... | Eur J Med Chem 63: 758-64 (2013) Article DOI: 10.1016/j.ejmech.2013.03.002 BindingDB Entry DOI: 10.7270/Q2FB54B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Pneumocystis carinii) | BDBM50433364 (CHEMBL2377453) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians-Universit£t M£nchen Curated by ChEMBL | Assay Description Inhibition of Pneumocystis carinii lanosterol synthase expressed in Saccharomyces cerevisiae SMY8[pBJ4.21] using [14C]-(3S)-2,3-oxidosqualene as subs... | Eur J Med Chem 63: 758-64 (2013) Article DOI: 10.1016/j.ejmech.2013.03.002 BindingDB Entry DOI: 10.7270/Q2FB54B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Pneumocystis carinii) | BDBM50433361 (CHEMBL2377452) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians-Universit£t M£nchen Curated by ChEMBL | Assay Description Inhibition of Pneumocystis carinii lanosterol synthase expressed in Saccharomyces cerevisiae SMY8[pBJ4.21] using [14C]-(3S)-2,3-oxidosqualene as subs... | Eur J Med Chem 63: 758-64 (2013) Article DOI: 10.1016/j.ejmech.2013.03.002 BindingDB Entry DOI: 10.7270/Q2FB54B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Homo sapiens (Human)) | BDBM50433364 (CHEMBL2377453) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians-Universit£t M£nchen Curated by ChEMBL | Assay Description Inhibition of human lanosterol synthase expressed in Saccharomyces cerevisiae SMY8[pSOB1.1] using [14C]-(3S)-2,3-oxidosqualene as substrate | Eur J Med Chem 63: 758-64 (2013) Article DOI: 10.1016/j.ejmech.2013.03.002 BindingDB Entry DOI: 10.7270/Q2FB54B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Homo sapiens (Human)) | BDBM50433365 (CHEMBL2377450) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians-Universit£t M£nchen Curated by ChEMBL | Assay Description Inhibition of human lanosterol synthase expressed in Saccharomyces cerevisiae SMY8[pSOB1.1] using [14C]-(3S)-2,3-oxidosqualene as substrate | Eur J Med Chem 63: 758-64 (2013) Article DOI: 10.1016/j.ejmech.2013.03.002 BindingDB Entry DOI: 10.7270/Q2FB54B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cycloartenol synthase (Arabidopsis thaliana) | BDBM50433365 (CHEMBL2377450) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians-Universit£t M£nchen Curated by ChEMBL | Assay Description Inhibition of Arabidopsis thaliana cycloartenol synthase expressed in Saccharomyces cerevisiae SMY8[pSM60.21] using [14C]-(3S)-2,3-oxidosqualene as s... | Eur J Med Chem 63: 758-64 (2013) Article DOI: 10.1016/j.ejmech.2013.03.002 BindingDB Entry DOI: 10.7270/Q2FB54B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Pneumocystis carinii) | BDBM50433365 (CHEMBL2377450) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians-Universit£t M£nchen Curated by ChEMBL | Assay Description Inhibition of Pneumocystis carinii lanosterol synthase expressed in Saccharomyces cerevisiae SMY8[pBJ4.21] using [14C]-(3S)-2,3-oxidosqualene as subs... | Eur J Med Chem 63: 758-64 (2013) Article DOI: 10.1016/j.ejmech.2013.03.002 BindingDB Entry DOI: 10.7270/Q2FB54B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase ERG7 (Saccharomyces cerevisiae) | BDBM50433365 (CHEMBL2377450) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians-Universit£t M£nchen Curated by ChEMBL | Assay Description Inhibition of wild type yeast lanosterol synthase expressed in Saccharomyces cerevisiae SMY8[pSM61.21] using [14C]-(3S)-2,3-oxidosqualene as substrat... | Eur J Med Chem 63: 758-64 (2013) Article DOI: 10.1016/j.ejmech.2013.03.002 BindingDB Entry DOI: 10.7270/Q2FB54B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||