

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50499430 (CHEMBL3740472) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate after 2 mins by LC-MS/MS analysis in presence of NADPH regeneration syste... | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50499447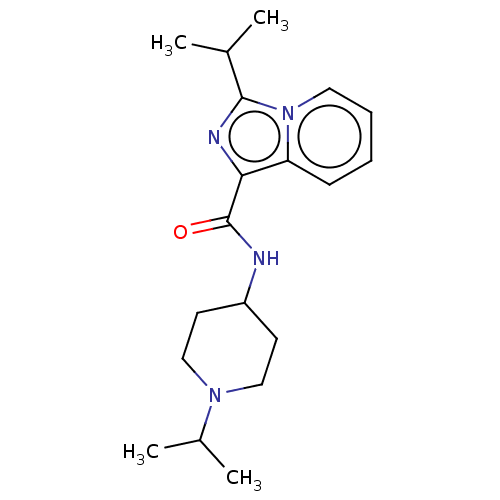 (CHEMBL3739521) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate after 2 mins by LC-MS/MS analysis in presence of NADPH regeneration syste... | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50499430 (CHEMBL3740472) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Inhibition of human ERG channel expressed in HEK293 cells after 5 mins by patch clamp assay | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50499445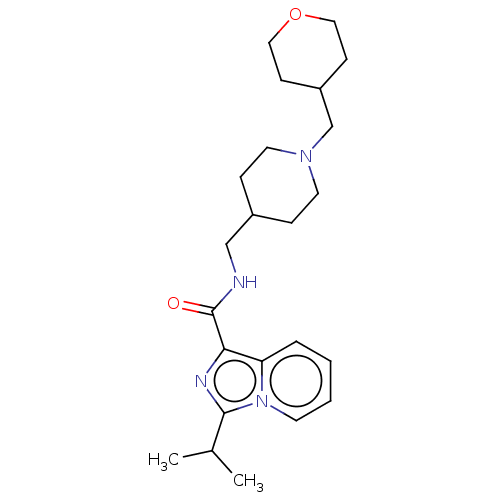 (CHEMBL3741608) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate after 12 mins by LC-MS/MS analysis in presence of NADPH regenerati... | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50499450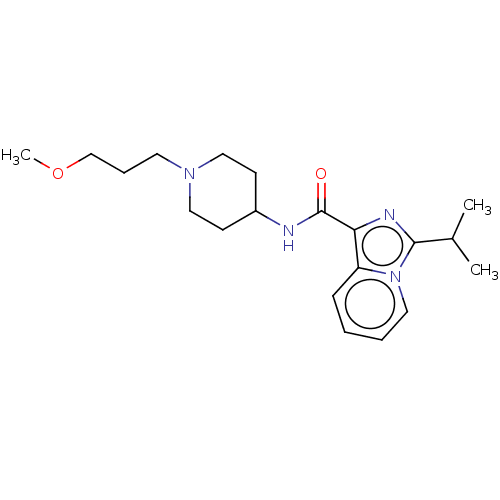 (CHEMBL3741776) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate after 2 mins by LC-MS/MS analysis in presence of NADPH regeneration syste... | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50499428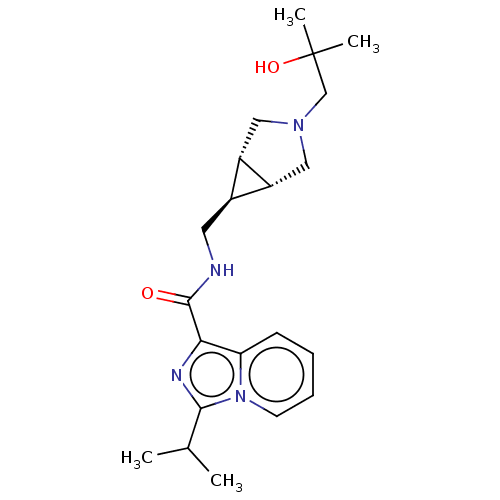 (CHEMBL3741377) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate after 2 mins by LC-MS/MS analysis in presence of NADPH regeneration syste... | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50499437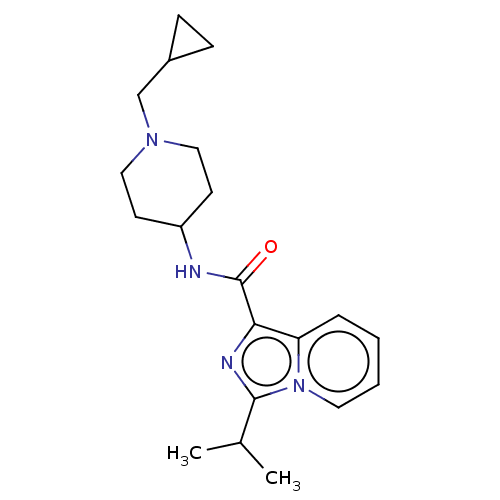 (CHEMBL3739470) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate after 2 mins by LC-MS/MS analysis in presence of NADPH regeneration syste... | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50499430 (CHEMBL3740472) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate after 12 mins by LC-MS/MS analysis in presence of NADPH regenerati... | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50499445 (CHEMBL3741608) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate after 2 mins by LC-MS/MS analysis in presence of NADPH regeneration syste... | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50499455 (CHEMBL3741821) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate after 2 mins by LC-MS/MS analysis in presence of NADPH regeneration syste... | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50499448 (CHEMBL3740908) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate after 2 mins by LC-MS/MS analysis in presence of NADPH regeneration syste... | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50499450 (CHEMBL3741776) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate after 12 mins by LC-MS/MS analysis in presence of NADPH regenerati... | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50499434 (CHEMBL3739647) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate after 2 mins by LC-MS/MS analysis in presence of NADPH regeneration syste... | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50499428 (CHEMBL3741377) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate after 12 mins by LC-MS/MS analysis in presence of NADPH regenerati... | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50499432 (CHEMBL3741595) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate after 2 mins by LC-MS/MS analysis in presence of NADPH regeneration syste... | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50499455 (CHEMBL3741821) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate after 12 mins by LC-MS/MS analysis in presence of NADPH regenerati... | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50499447 (CHEMBL3739521) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate after 12 mins by LC-MS/MS analysis in presence of NADPH regenerati... | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50499446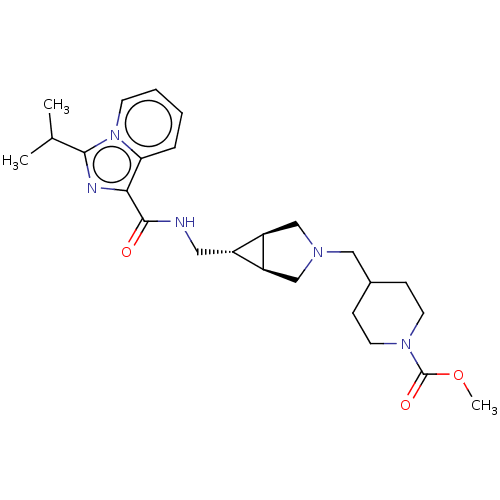 (CHEMBL3740669) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate after 2 mins by LC-MS/MS analysis in presence of NADPH regeneration syste... | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50499446 (CHEMBL3740669) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate after 12 mins by LC-MS/MS analysis in presence of NADPH regenerati... | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50499434 (CHEMBL3739647) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate after 12 mins by LC-MS/MS analysis in presence of NADPH regenerati... | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50499448 (CHEMBL3740908) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate after 12 mins by LC-MS/MS analysis in presence of NADPH regenerati... | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50499437 (CHEMBL3739470) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate after 12 mins by LC-MS/MS analysis in presence of NADPH regenerati... | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50499432 (CHEMBL3741595) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.34E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate after 12 mins by LC-MS/MS analysis in presence of NADPH regenerati... | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50499437 (CHEMBL3739470) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 49 | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Agonist activity at human 5HT4e receptor expressed in CHO cells assessed as cAMP level after 4 hrs by luciferase reporter gene assay | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50499438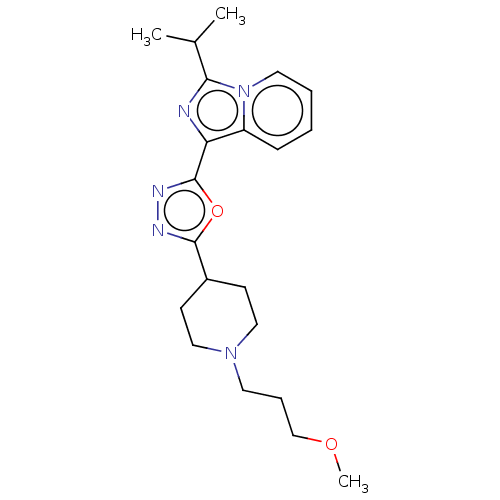 (CHEMBL3739467) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 736 | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Agonist activity at human 5HT4e receptor expressed in CHO cells assessed as cAMP level after 4 hrs by luciferase reporter gene assay | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50499439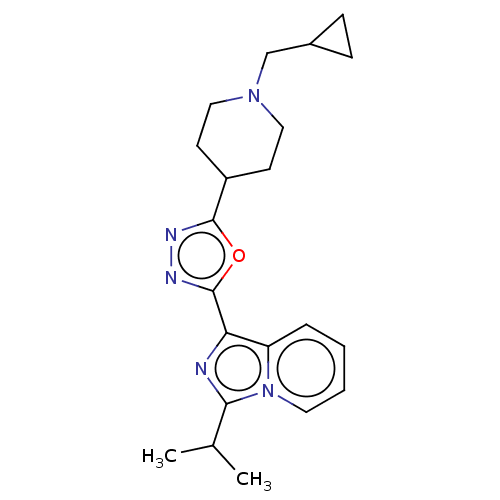 (CHEMBL3740000) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 857 | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Agonist activity at human 5HT4e receptor expressed in CHO cells assessed as cAMP level after 4 hrs by luciferase reporter gene assay | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50499440 (CHEMBL3741062) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 53 | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Agonist activity at human 5HT4e receptor expressed in CHO cells assessed as cAMP level after 4 hrs by luciferase reporter gene assay | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50499441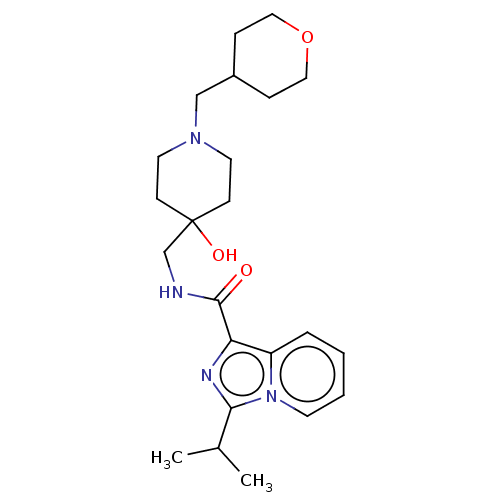 (CHEMBL3739459) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 57 | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Agonist activity at human 5HT4e receptor expressed in CHO cells assessed as cAMP level after 4 hrs by luciferase reporter gene assay | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50499442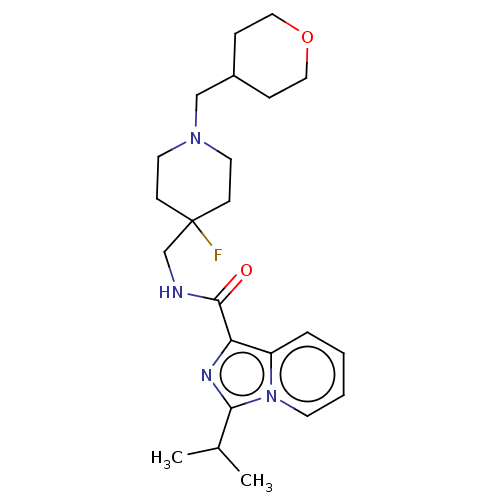 (CHEMBL3740278) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.36E+3 | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Agonist activity at human 5HT4e receptor expressed in CHO cells assessed as cAMP level after 4 hrs by luciferase reporter gene assay | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50499443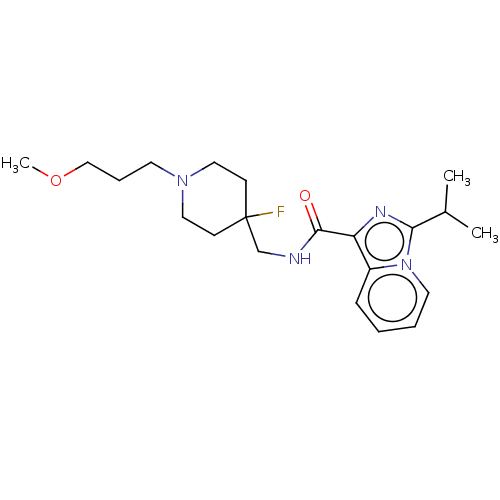 (CHEMBL3740864) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 633 | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Agonist activity at human 5HT4e receptor expressed in CHO cells assessed as cAMP level after 4 hrs by luciferase reporter gene assay | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50499444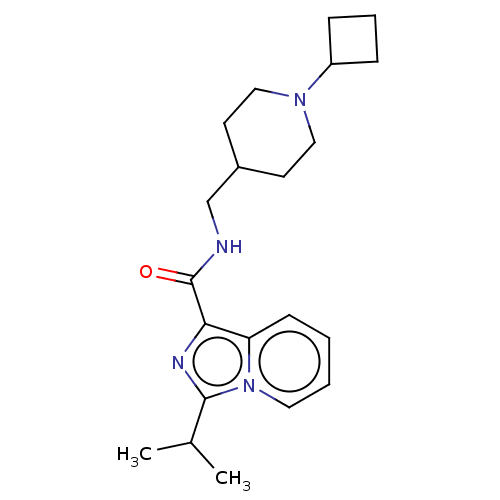 (CHEMBL3741733) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Agonist activity at human 5HT4e receptor expressed in CHO cells assessed as cAMP level after 4 hrs by luciferase reporter gene assay | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50499445 (CHEMBL3741608) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Agonist activity at human 5HT4e receptor expressed in CHO cells assessed as cAMP level after 4 hrs by luciferase reporter gene assay | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50499430 (CHEMBL3740472) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Agonist activity at human 5-HT4A receptor assessed as cAMP level after 4 hrs by luciferase reporter gene assay | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50499430 (CHEMBL3740472) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Agonist activity at human 5-HT4D receptor assessed as cAMP level after 4 hrs by luciferase reporter gene assay | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50436989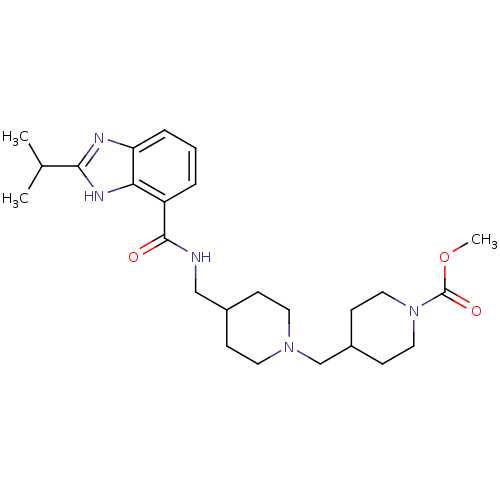 (CHEMBL2402904) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Agonist activity at 5-HT4 receptor in guinea pig colon | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (RAT) | BDBM84950 (CAS_183782 | NSC_183782 | RS 67333) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Agonist activity at 5-HT4 receptor in Sprague-Dawley rat oesophagus assessed as relaxation of carbachol precontracted oesophagus | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50122872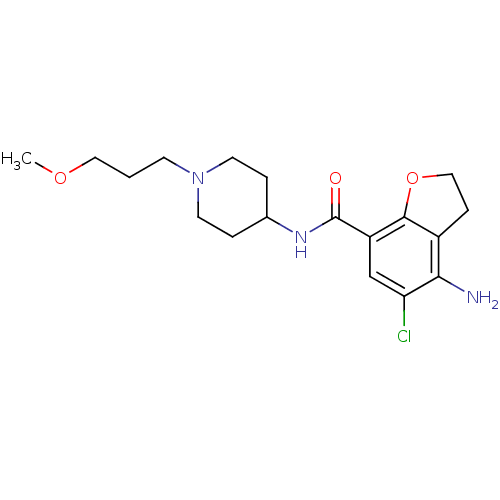 (4-Amino-5-chloro-2,3-dihydro-benzofuran-7-carboxyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Agonist activity at human 5HT4e receptor expressed in CHO cells assessed as cAMP level after 4 hrs by luciferase reporter gene assay | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (RAT) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Agonist activity at 5HT2b receptor in Wistar rat fundus measured for 90 secs | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM29526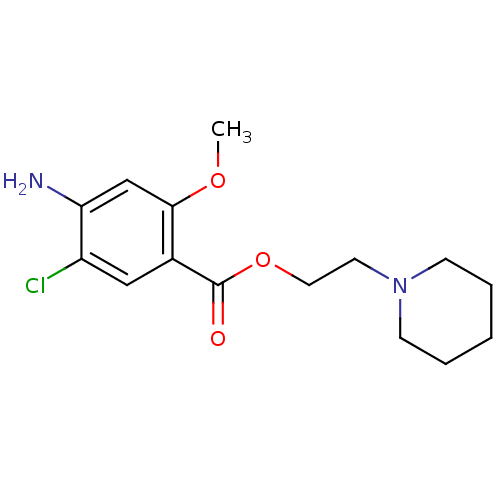 (2-Piperidinoethyl 4-amino-5-chloro-2-methoxybenzoa...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Agonist activity at 5-HT4 receptor in guinea pig ileum assessed as increase in response to electrical stimulation | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50499449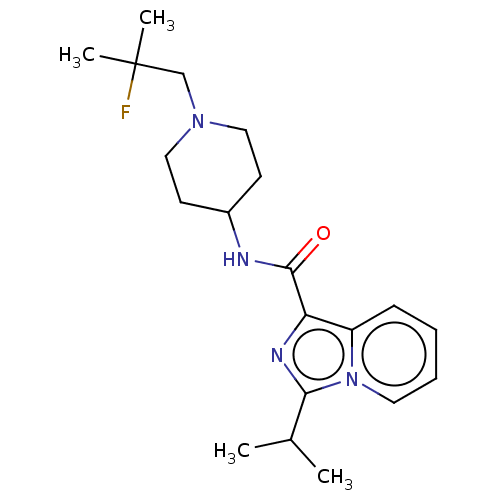 (CHEMBL3742086) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 132 | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Agonist activity at human 5HT4e receptor expressed in CHO cells assessed as cAMP level after 4 hrs by luciferase reporter gene assay | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50499450 (CHEMBL3741776) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Agonist activity at human 5HT4e receptor expressed in CHO cells assessed as cAMP level after 4 hrs by luciferase reporter gene assay | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50499447 (CHEMBL3739521) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Agonist activity at human 5HT4e receptor expressed in CHO cells assessed as cAMP level after 4 hrs by luciferase reporter gene assay | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50499451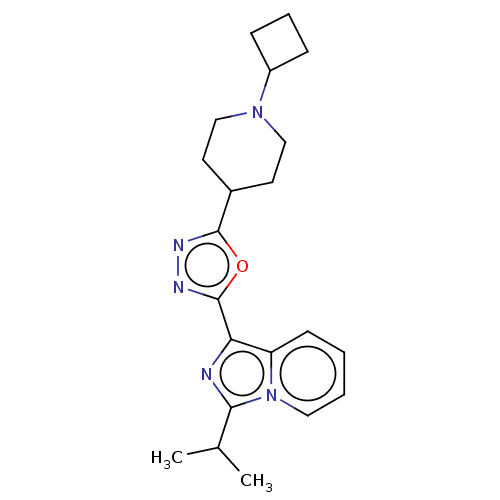 (CHEMBL3741199) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 840 | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Agonist activity at human 5HT4e receptor expressed in CHO cells assessed as cAMP level after 4 hrs by luciferase reporter gene assay | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50499452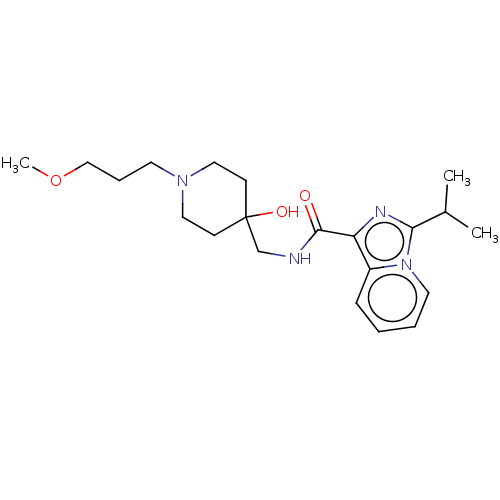 (CHEMBL3740030) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 316 | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Agonist activity at human 5HT4e receptor expressed in CHO cells assessed as cAMP level after 4 hrs by luciferase reporter gene assay | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50499453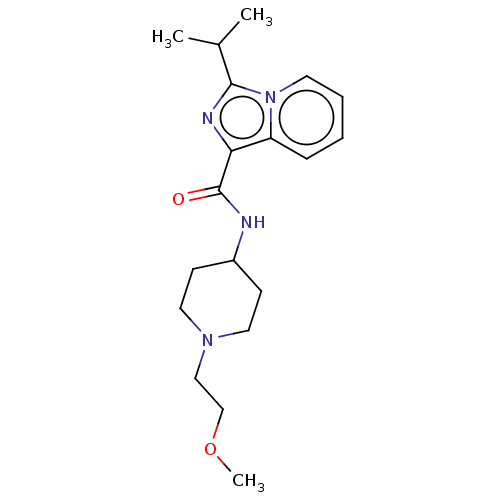 (CHEMBL3742190) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 144 | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Agonist activity at human 5HT4e receptor expressed in CHO cells assessed as cAMP level after 4 hrs by luciferase reporter gene assay | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50499454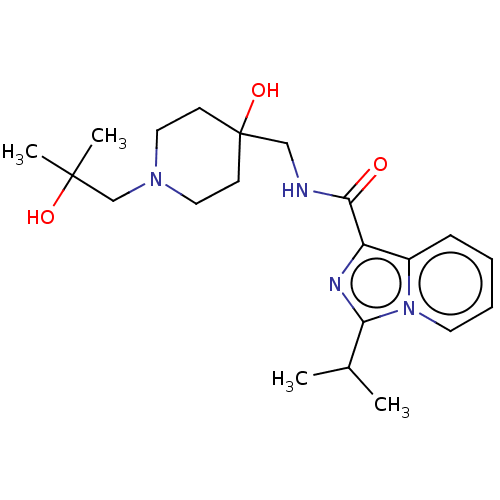 (CHEMBL3742145) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 72 | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Agonist activity at human 5HT4e receptor expressed in CHO cells assessed as cAMP level after 4 hrs by luciferase reporter gene assay | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50499456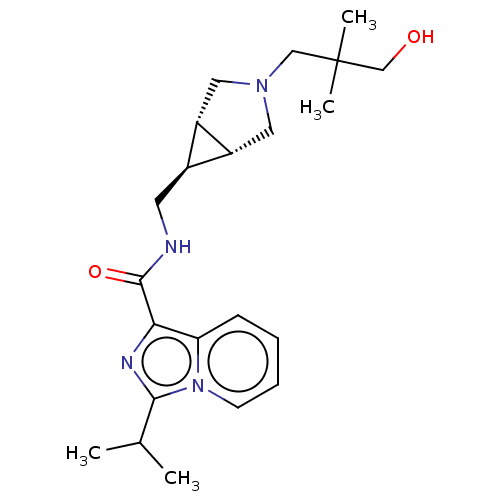 (CHEMBL3741548) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Agonist activity at human 5HT4e receptor expressed in CHO cells assessed as cAMP level after 4 hrs by luciferase reporter gene assay | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM84950 (CAS_183782 | NSC_183782 | RS 67333) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Agonist activity at human 5HT4e receptor expressed in CHO cells assessed as cAMP level after 4 hrs by luciferase reporter gene assay | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50499446 (CHEMBL3740669) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Agonist activity at human 5HT4e receptor expressed in CHO cells assessed as cAMP level after 4 hrs by luciferase reporter gene assay | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50499457 (CHEMBL3741787) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 9.70 | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Agonist activity at human 5HT4e receptor expressed in CHO cells assessed as cAMP level after 4 hrs by luciferase reporter gene assay | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 88 total ) | Next | Last >> |