

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM335705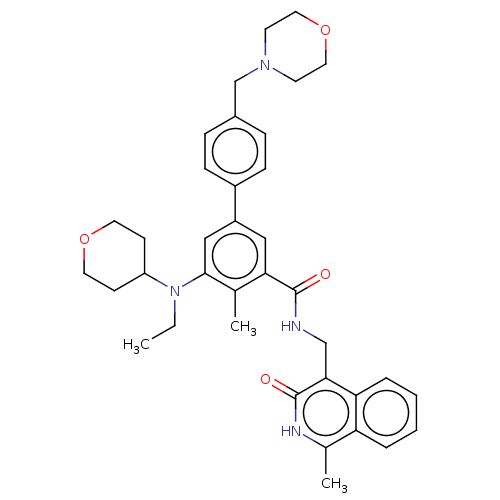 (5-(Ethyl(tetrahydro-2H-pyran-4- yl)amino)-4-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <50 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of hitone methyl transferases can be readily tested by assays known to those sk... | US Patent US9738630 (2017) BindingDB Entry DOI: 10.7270/Q23X88R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM335708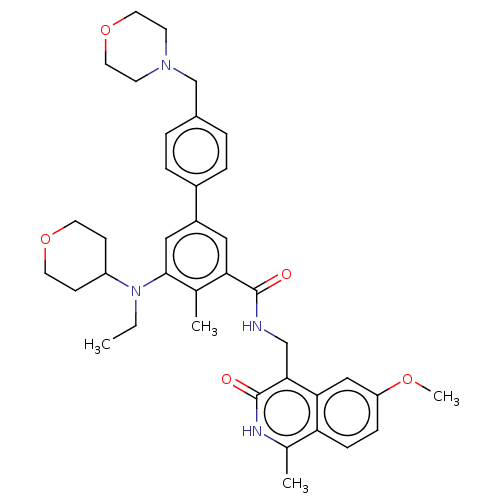 (5-(Ethyl(tetrahydro-2H-pyran-4- yl)amino)-N-((6-me...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <5.00E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of hitone methyl transferases can be readily tested by assays known to those sk... | US Patent US9738630 (2017) BindingDB Entry DOI: 10.7270/Q23X88R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM335707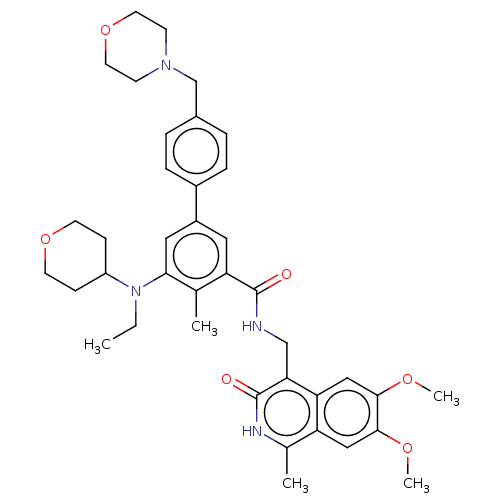 (N-((6,7-Dimethoxy-1-methyl-3- oxo-2,3-dihydroisoqu...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <5.00E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of hitone methyl transferases can be readily tested by assays known to those sk... | US Patent US9738630 (2017) BindingDB Entry DOI: 10.7270/Q23X88R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM335709 (5-(Ethyl(tetrahydro-2H-pyran-4- yl)amino)-N-((5-fl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <5.00E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of hitone methyl transferases can be readily tested by assays known to those sk... | US Patent US9738630 (2017) BindingDB Entry DOI: 10.7270/Q23X88R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM335711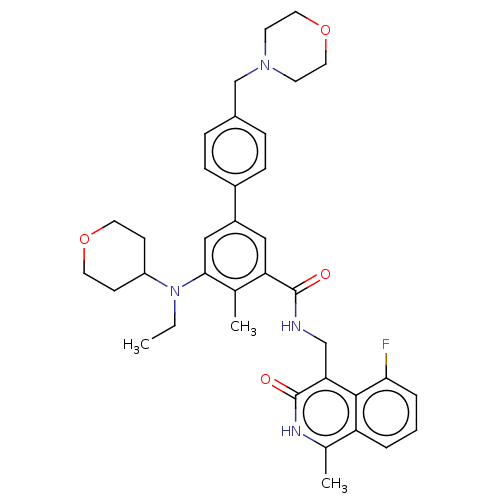 (5-(Ethyl(tetrahydro-2H-pyran-4- yl)amino)-N-((5-fl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <50 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of hitone methyl transferases can be readily tested by assays known to those sk... | US Patent US9738630 (2017) BindingDB Entry DOI: 10.7270/Q23X88R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM335712 (5-(Ethyl(tetrahydro-2H-pyran-4- yl)amino)-4-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <500 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of hitone methyl transferases can be readily tested by assays known to those sk... | US Patent US9738630 (2017) BindingDB Entry DOI: 10.7270/Q23X88R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM335713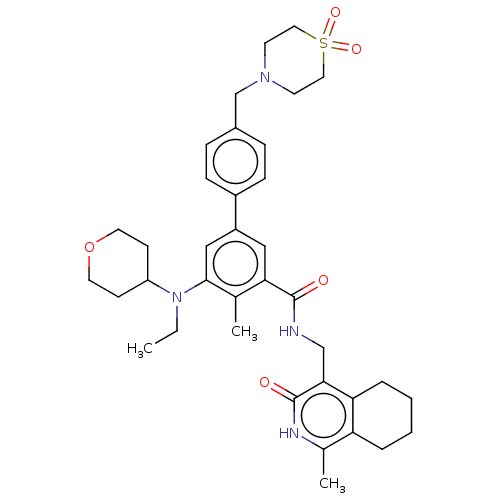 (4'-((1,1-Dioxidothiomorpholino) methyl)-5-(ethyl(t...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <500 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of hitone methyl transferases can be readily tested by assays known to those sk... | US Patent US9738630 (2017) BindingDB Entry DOI: 10.7270/Q23X88R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM335714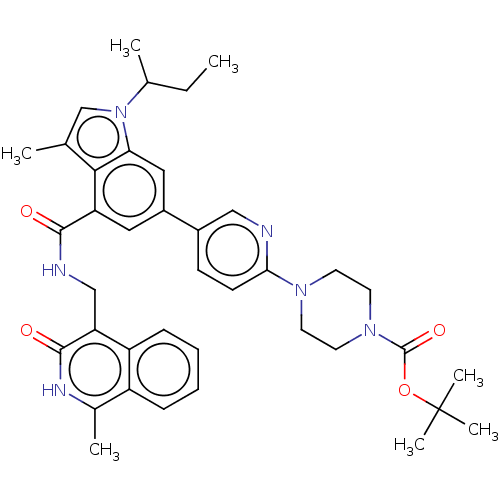 (US9738630, Example 9 | tert-Butyl 4-(5-(1-(sec-but...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <5.00E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of hitone methyl transferases can be readily tested by assays known to those sk... | US Patent US9738630 (2017) BindingDB Entry DOI: 10.7270/Q23X88R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM335715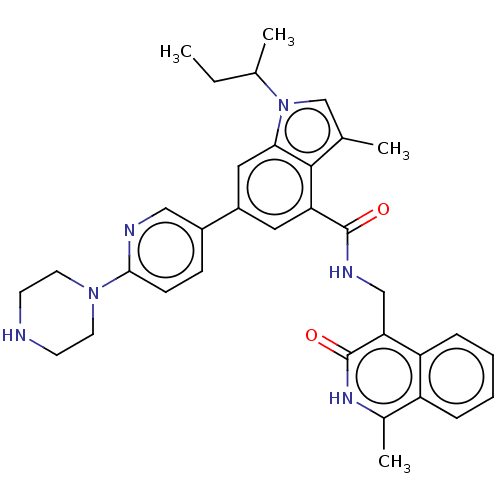 (1-(sec-Butyl)-3-methyl-N-((1- methyl-3-oxo-2,3- di...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <500 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of hitone methyl transferases can be readily tested by assays known to those sk... | US Patent US9738630 (2017) BindingDB Entry DOI: 10.7270/Q23X88R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM335716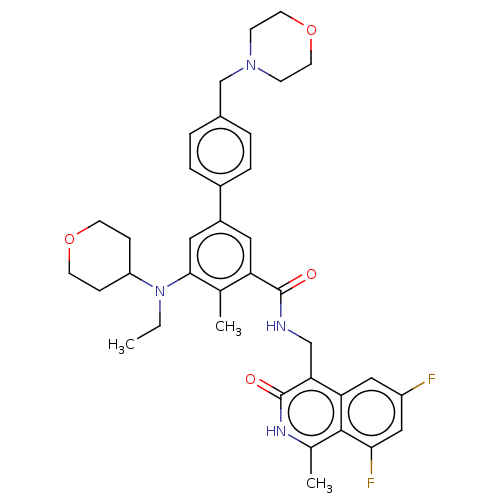 (N-((6,8-Difluoro-1-methyl-3-oxo- 2,3-dihydroisoqui...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <5.00E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of hitone methyl transferases can be readily tested by assays known to those sk... | US Patent US9738630 (2017) BindingDB Entry DOI: 10.7270/Q23X88R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM335717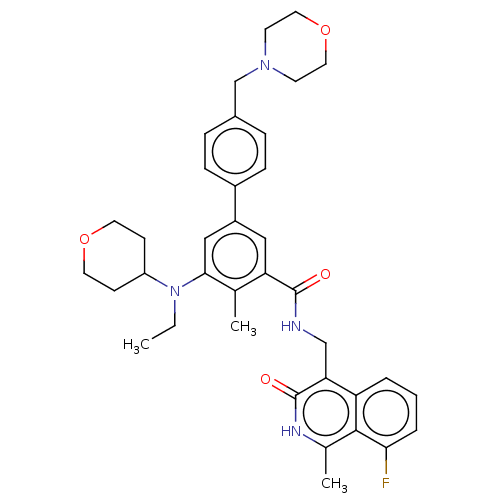 (5-(Ethyl(tetrahydro-2H-pyran-4- yl)amino)-N-((8-fl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <5.00E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of hitone methyl transferases can be readily tested by assays known to those sk... | US Patent US9738630 (2017) BindingDB Entry DOI: 10.7270/Q23X88R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM335706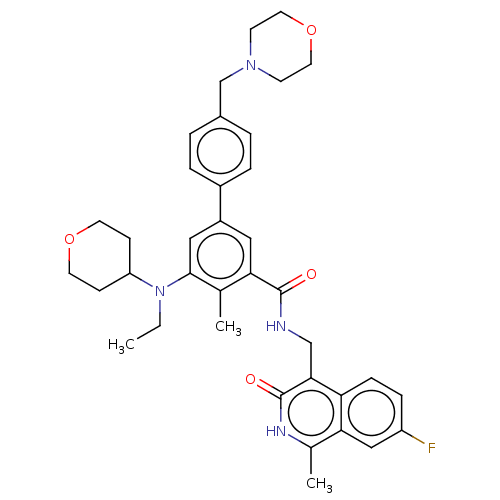 (5-(Ethyl(tetrahydro-2H-pyran-4- yl)amino)-N-((7-fl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <500 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of hitone methyl transferases can be readily tested by assays known to those sk... | US Patent US9738630 (2017) BindingDB Entry DOI: 10.7270/Q23X88R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM335719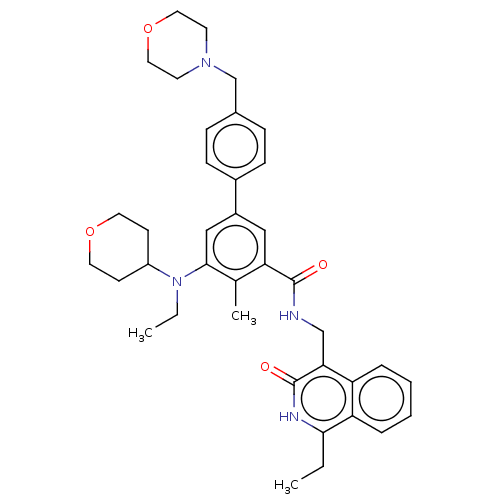 (5-(Ethyl(tetrahydro-2H-pyran-4- yl)amino)-N-((1-et...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <500 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of hitone methyl transferases can be readily tested by assays known to those sk... | US Patent US9738630 (2017) BindingDB Entry DOI: 10.7270/Q23X88R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM335720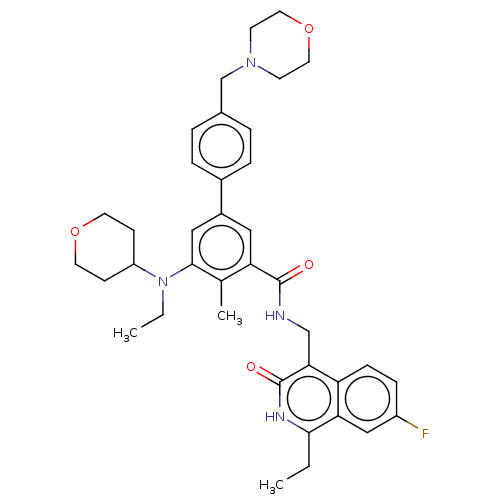 (5-(Ethyl(tetrahydro-2H-pyran-4- yl)amino)-N-((1-et...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <50 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of hitone methyl transferases can be readily tested by assays known to those sk... | US Patent US9738630 (2017) BindingDB Entry DOI: 10.7270/Q23X88R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM335722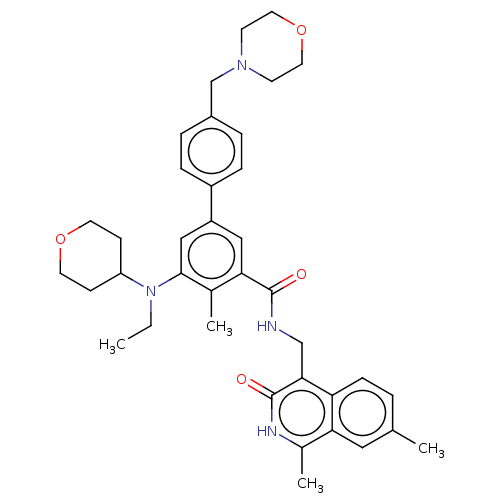 (N-((1,7-Dimethyl-3-oxo-2,3- dihydroisoquinolin-4-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <50 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of hitone methyl transferases can be readily tested by assays known to those sk... | US Patent US9738630 (2017) BindingDB Entry DOI: 10.7270/Q23X88R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM335723 (2,2-Dimethyl-N-((1-methyl-3- oxo-2,3-dihydroisoqui...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <5.00E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of hitone methyl transferases can be readily tested by assays known to those sk... | US Patent US9738630 (2017) BindingDB Entry DOI: 10.7270/Q23X88R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM335706 (5-(Ethyl(tetrahydro-2H-pyran-4- yl)amino)-N-((7-fl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <50 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of hitone methyl transferases can be readily tested by assays known to those sk... | US Patent US9738630 (2017) BindingDB Entry DOI: 10.7270/Q23X88R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||