

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50009672 (AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 467 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc. US Patent | Assay Description Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive control (100% inhibit... | US Patent US8895245 (2014) BindingDB Entry DOI: 10.7270/Q2WW7GBK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50009672 (AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 263 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc. US Patent | Assay Description Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive control (100% inhibit... | US Patent US8895245 (2014) BindingDB Entry DOI: 10.7270/Q2WW7GBK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM227458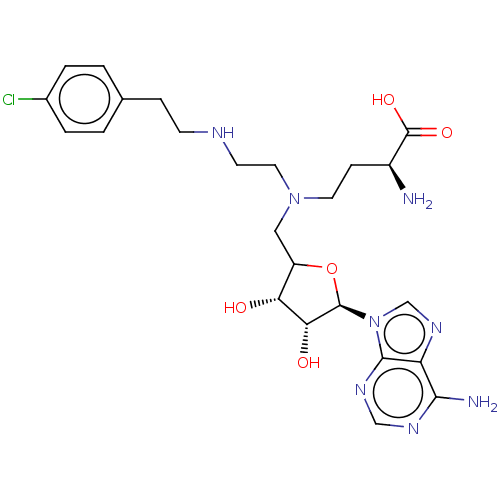 (US9333217, 75) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.18E+3 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc. US Patent | Assay Description Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive control (100% inhibit... | US Patent US9333217 (2016) BindingDB Entry DOI: 10.7270/Q2XP73TJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM227458 (US9333217, 75) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc. US Patent | Assay Description Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive control (100% inhibit... | US Patent US9333217 (2016) BindingDB Entry DOI: 10.7270/Q2XP73TJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM227458 (US9333217, 75) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc. US Patent | Assay Description Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive control (100% inhibit... | US Patent US9333217 (2016) BindingDB Entry DOI: 10.7270/Q2XP73TJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM227458 (US9333217, 75) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.56E+3 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc. US Patent | Assay Description Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive control (100% inhibit... | US Patent US9333217 (2016) BindingDB Entry DOI: 10.7270/Q2XP73TJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM227458 (US9333217, 75) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.95E+3 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc. US Patent | Assay Description Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive control (100% inhibit... | US Patent US9333217 (2016) BindingDB Entry DOI: 10.7270/Q2XP73TJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50009672 (AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 283 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc. US Patent | Assay Description Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive control (100% inhibit... | US Patent US8895245 (2014) BindingDB Entry DOI: 10.7270/Q2WW7GBK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50009672 (AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 380 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc. US Patent | Assay Description Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive control (100% inhibit... | US Patent US8895245 (2014) BindingDB Entry DOI: 10.7270/Q2WW7GBK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50009672 (AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc. US Patent | Assay Description Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive control (100% inhibit... | US Patent US8895245 (2014) BindingDB Entry DOI: 10.7270/Q2WW7GBK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM139690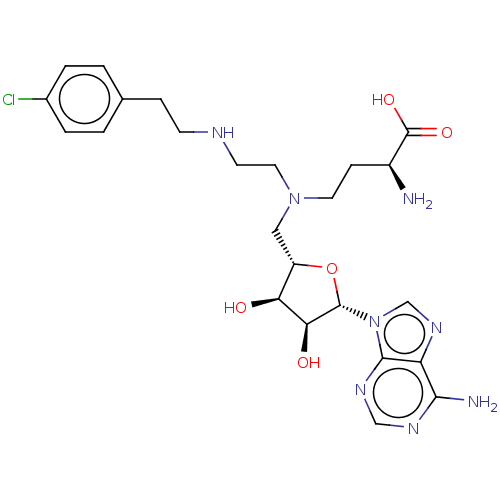 (US8895245, 75) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8.95E+3 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc. US Patent | Assay Description Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive control (100% inhibit... | US Patent US8895245 (2014) BindingDB Entry DOI: 10.7270/Q2WW7GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM139690 (US8895245, 75) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc. US Patent | Assay Description Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive control (100% inhibit... | US Patent US8895245 (2014) BindingDB Entry DOI: 10.7270/Q2WW7GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM139690 (US8895245, 75) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc. US Patent | Assay Description Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive control (100% inhibit... | US Patent US8895245 (2014) BindingDB Entry DOI: 10.7270/Q2WW7GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM139690 (US8895245, 75) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7.18E+3 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc. US Patent | Assay Description Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive control (100% inhibit... | US Patent US8895245 (2014) BindingDB Entry DOI: 10.7270/Q2WW7GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM139690 (US8895245, 75) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7.56E+3 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc. US Patent | Assay Description Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive control (100% inhibit... | US Patent US8895245 (2014) BindingDB Entry DOI: 10.7270/Q2WW7GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50009672 (AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 467 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc. US Patent | Assay Description S-Adenosyl-L-homocysteine (SAH) was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive c... | US Patent US9175331 (2015) BindingDB Entry DOI: 10.7270/Q2ZW1JQP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM190225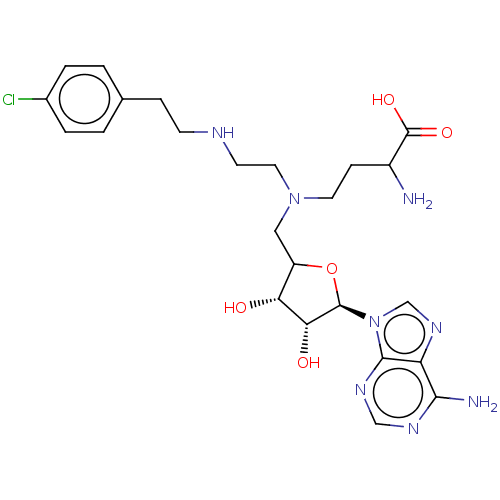 (US9175331, 75) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8.95E+3 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc. US Patent | Assay Description Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL , was plated in a 384 well microtiter plate. Positive control (100% inhib... | US Patent US9175331 (2015) BindingDB Entry DOI: 10.7270/Q2ZW1JQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50009672 (AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 467 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc. US Patent | Assay Description S-Adenosyl-L-homocysteine (SAH) was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive c... | US Patent US9333217 (2016) BindingDB Entry DOI: 10.7270/Q2XP73TJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50009672 (AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 263 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc. US Patent | Assay Description S-Adenosyl-L-homocysteine (SAH) was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive c... | US Patent US9333217 (2016) BindingDB Entry DOI: 10.7270/Q2XP73TJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50009672 (AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 283 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc. US Patent | Assay Description S-Adenosyl-L-homocysteine (SAH) was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive c... | US Patent US9333217 (2016) BindingDB Entry DOI: 10.7270/Q2XP73TJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50009672 (AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 380 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc. US Patent | Assay Description S-Adenosyl-L-homocysteine (SAH) was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive c... | US Patent US9333217 (2016) BindingDB Entry DOI: 10.7270/Q2XP73TJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50009672 (AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc. US Patent | Assay Description S-Adenosyl-L-homocysteine (SAH) was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive c... | US Patent US9333217 (2016) BindingDB Entry DOI: 10.7270/Q2XP73TJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM227711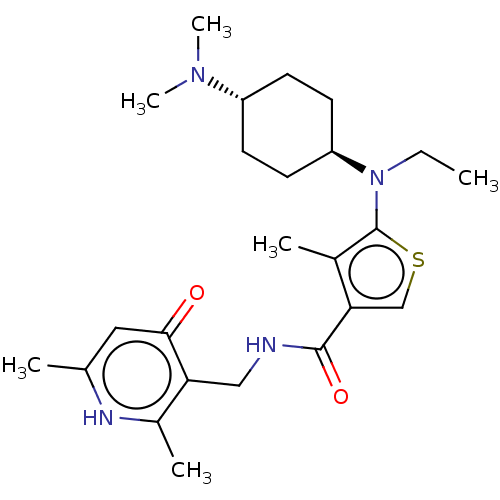 (US9556157, 2 | n-((2,6-dimethyl-4-oxo-1,4-dihydrop...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
GlaxoSmithKline Intellectual Property (No.2) Limited US Patent | Assay Description Compounds contained herein were evaluated for their ability to inhibit the methyltransferase activity of EZH2 within the PRC2 complex. Human PRC2 com... | US Patent US9556157 (2017) BindingDB Entry DOI: 10.7270/Q2MP558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM227713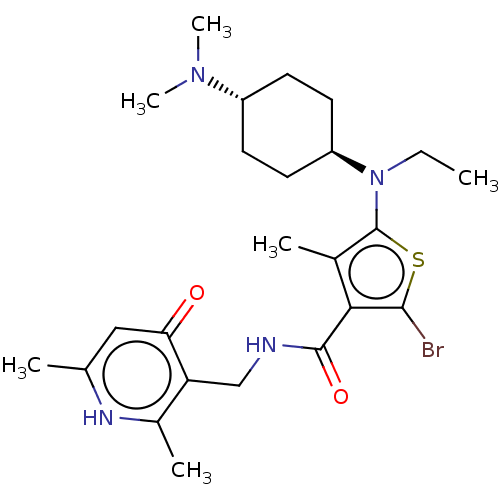 (2-bromo-n-((2,6-dimethyl-4-oxo-1,4-dihydropyridin-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 160 | n/a | n/a | n/a | n/a | 8.0 | 25 |
GlaxoSmithKline Intellectual Property (No.2) Limited US Patent | Assay Description Compounds contained herein were evaluated for their ability to inhibit the methyltransferase activity of EZH2 within the PRC2 complex. Human PRC2 com... | US Patent US9556157 (2017) BindingDB Entry DOI: 10.7270/Q2MP558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM227712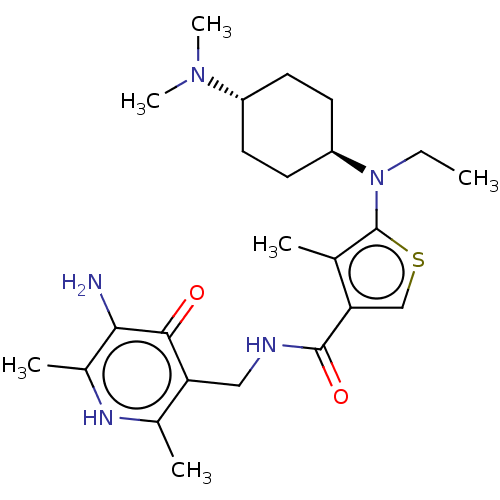 (US9556157, 3 | n-((5-amino-2,6-dimethyl-4-oxo-1,4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 32 | n/a | n/a | n/a | n/a | 8.0 | 25 |
GlaxoSmithKline Intellectual Property (No.2) Limited US Patent | Assay Description Compounds contained herein were evaluated for their ability to inhibit the methyltransferase activity of EZH2 within the PRC2 complex. Human PRC2 com... | US Patent US9556157 (2017) BindingDB Entry DOI: 10.7270/Q2MP558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM227711 (US9556157, 2 | n-((2,6-dimethyl-4-oxo-1,4-dihydrop...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 32 | n/a | n/a | n/a | n/a | 8.0 | 25 |
GlaxoSmithKline Intellectual Property (No.2) Limited US Patent | Assay Description Compounds contained herein were evaluated for their ability to inhibit the methyltransferase activity of EZH2 within the PRC2 complex. Human PRC2 com... | US Patent US9556157 (2017) BindingDB Entry DOI: 10.7270/Q2MP558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM227710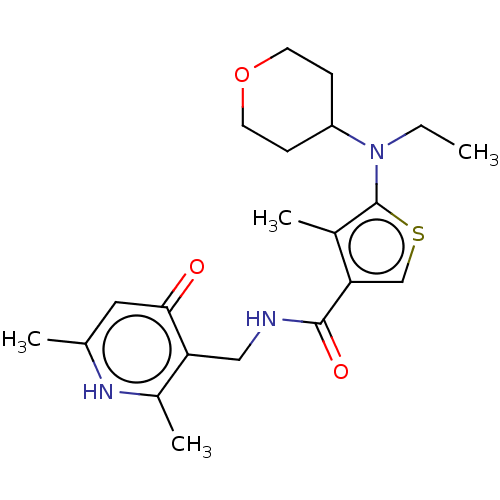 ( N-((2,6-dimethyl-4-oxo-1,4-dihydropyridin-3-yl)me...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 316 | n/a | n/a | n/a | n/a | 8.0 | 25 |
GlaxoSmithKline Intellectual Property (No.2) Limited US Patent | Assay Description Compounds contained herein were evaluated for their ability to inhibit the methyltransferase activity of EZH2 within the PRC2 complex. Human PRC2 com... | US Patent US9556157 (2017) BindingDB Entry DOI: 10.7270/Q2MP558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM227712 (US9556157, 3 | n-((5-amino-2,6-dimethyl-4-oxo-1,4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 8.0 | 25 |
GlaxoSmithKline Intellectual Property (No.2) Limited US Patent | Assay Description Compounds contained herein were evaluated for their ability to inhibit the methyltransferase activity of EZH2 within the PRC2 complex. Human PRC2 com... | US Patent US9556157 (2017) BindingDB Entry DOI: 10.7270/Q2MP558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||