 Found 2177 hits of ic50 data for polymerid = 2012
Found 2177 hits of ic50 data for polymerid = 2012 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Procathepsin L
(Homo sapiens (Human)) | BDBM50552672
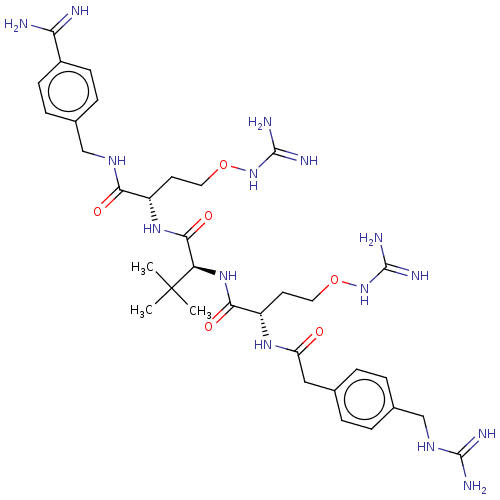
(CHEMBL4790628)Show SMILES CC(C)(C)[C@H](NC(=O)[C@H](CCONC(N)=N)NC(=O)Cc1ccc(CNC(N)=N)cc1)C(=O)N[C@@H](CCONC(N)=N)C(=O)NCc1ccc(cc1)C(N)=N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50602543
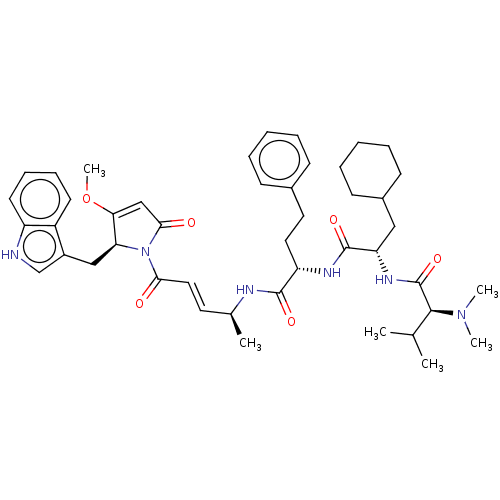
(CHEMBL4598338)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CCc1ccccc1)NC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50602544

(CHEMBL4594757)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50602542

(CHEMBL4594870)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1CCCCC1)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50602545

(CHEMBL4596647)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50033762

(Gallinamide A)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)\C=C\C(=O)N1[C@@H](C)C(OC)=CC1=O |r,c:38| Show InChI InChI=1S/C31H52N4O7/c1-12-20(6)28(34(9)10)31(40)42-25(16-19(4)5)30(39)33-23(15-18(2)3)29(38)32-21(7)13-14-26(36)35-22(8)24(41-11)17-27(35)37/h13-14,17-23,25,28H,12,15-16H2,1-11H3,(H,32,38)(H,33,39)/b14-13+/t20-,21-,22-,23-,25-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50505559

(CHEMBL4441303)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)\C=C\C(=O)N1[C@@H](CC(C)C)C(OC)=CC1=O |r,c:48| Show InChI InChI=1S/C40H62N4O7/c1-12-28(8)37(43(9)10)40(49)51-34(22-27(6)7)39(48)42-31(20-25(2)3)38(47)41-30(23-29-16-14-13-15-17-29)18-19-35(45)44-32(21-26(4)5)33(50-11)24-36(44)46/h13-19,24-28,30-32,34,37H,12,20-23H2,1-11H3,(H,41,47)(H,42,48)/b19-18+/t28-,30+,31-,32-,34-,37-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L using fluorogenic substrate cbz-FR-AMC monitored for 90 to 120 mins by spectrophotometry |
J Med Chem 62: 9026-9044 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00294
BindingDB Entry DOI: 10.7270/Q2H998GC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50121902

(CHEMBL287630 | [(S)-1-((2S,3R)-2-Benzenesulfonyl-4...)Show SMILES O=C(N[C@@H](Cc1ccccc1)C(=O)N[C@H]1[C@@H](NC1=O)S(=O)(=O)c1ccccc1)OCc1ccccc1 Show InChI InChI=1S/C26H25N3O6S/c30-23(28-22-24(31)29-25(22)36(33,34)20-14-8-3-9-15-20)21(16-18-10-4-1-5-11-18)27-26(32)35-17-19-12-6-2-7-13-19/h1-15,21-22,25H,16-17H2,(H,27,32)(H,28,30)(H,29,31)/t21-,22+,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
currently NAEJA Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human Cathepsin L |
Bioorg Med Chem Lett 13: 139-41 (2002)
BindingDB Entry DOI: 10.7270/Q27W6BJB |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50121907

(CHEMBL31788 | [(S)-1-((3R,4S)-2-Oxo-4-phenylsulfan...)Show SMILES O=C(N[C@@H](Cc1ccccc1)C(=O)N[C@H]1[C@@H](NC1=O)Sc1ccccc1)OCc1ccccc1 Show InChI InChI=1S/C26H25N3O4S/c30-23(28-22-24(31)29-25(22)34-20-14-8-3-9-15-20)21(16-18-10-4-1-5-11-18)27-26(32)33-17-19-12-6-2-7-13-19/h1-15,21-22,25H,16-17H2,(H,27,32)(H,28,30)(H,29,31)/t21-,22+,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
currently NAEJA Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human Cathepsin L |
Bioorg Med Chem Lett 13: 139-41 (2002)
BindingDB Entry DOI: 10.7270/Q27W6BJB |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50069985
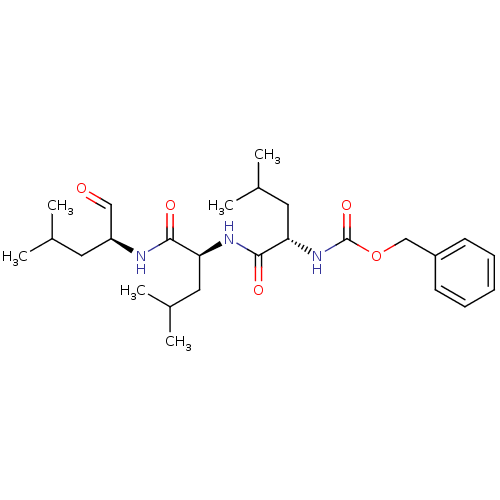
((S)-4-methyl-2-(3-phenyl-propionylamino)-pentanoic...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1)C=O |r| Show InChI InChI=1S/C26H41N3O5/c1-17(2)12-21(15-30)27-24(31)22(13-18(3)4)28-25(32)23(14-19(5)6)29-26(33)34-16-20-10-8-7-9-11-20/h7-11,15,17-19,21-23H,12-14,16H2,1-6H3,(H,27,31)(H,28,32)(H,29,33)/t21-,22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibition of dihydropteroate synthase from Escherichia coli. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19732

(2-Cyano-pyrimidine, 16b | 2-cyano-4-(cyclohexylami...)Show InChI InChI=1S/C21H25N5O2/c1-28-17-9-7-15(8-10-17)11-12-23-21(27)18-14-24-19(13-22)26-20(18)25-16-5-3-2-4-6-16/h7-10,14,16H,2-6,11-12H2,1H3,(H,23,27)(H,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Novartis Pharmaceuticals
| Assay Description
The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... |
J Med Chem 50: 591-4 (2007)
Article DOI: 10.1021/jm0613525
BindingDB Entry DOI: 10.7270/Q22805X5 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50229129
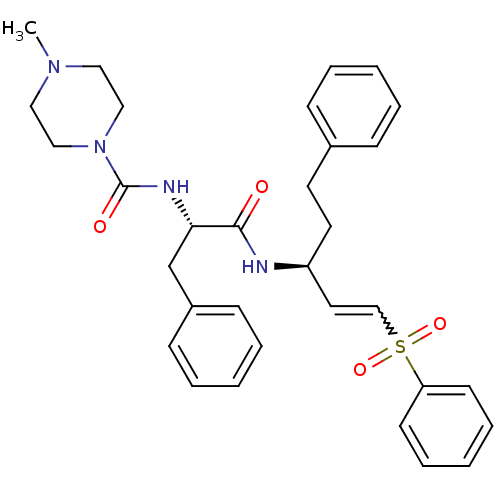
(4-Methyl-piperazine-1-carboxylic acid [(S)-1-((E)-...)Show SMILES CN1CCN(CC1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCc1ccccc1)C=CS(=O)(=O)c1ccccc1 |r,w:31.34| Show InChI InChI=1S/C32H38N4O4S/c1-35-20-22-36(23-21-35)32(38)34-30(25-27-13-7-3-8-14-27)31(37)33-28(18-17-26-11-5-2-6-12-26)19-24-41(39,40)29-15-9-4-10-16-29/h2-16,19,24,28,30H,17-18,20-23,25H2,1H3,(H,33,37)(H,34,38)/t28-,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University
Curated by ChEMBL
| Assay Description
Inhibition of human Cathepsin L using Cbz-Phe-Arg-AMC as substrate by fluorescence assay |
J Med Chem 63: 3298-3316 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02078
BindingDB Entry DOI: 10.7270/Q2PV6PQD |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM419133
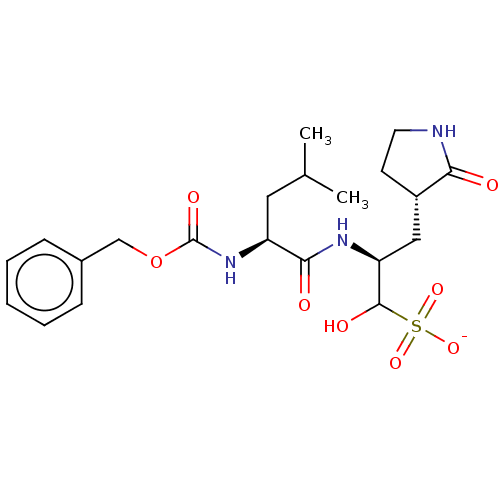
(BDBM429386 | GC376)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C(O)S([O-])(=O)=O Show InChI InChI=1S/C21H31N3O8S/c1-13(2)10-16(24-21(28)32-12-14-6-4-3-5-7-14)19(26)23-17(20(27)33(29,30)31)11-15-8-9-22-18(15)25/h3-7,13,15-17,20,27H,8-12H2,1-2H3,(H,22,25)(H,23,26)(H,24,28)(H,29,30,31)/p-1/t15?,16-,17-,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00247c
BindingDB Entry DOI: 10.7270/Q2HH6Q3R |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19733

(2-Cyano-pyrimidine, 16c | 2-cyano-4-(cyclohexylami...)Show InChI InChI=1S/C21H25N5O2/c1-28-17-9-5-6-15(12-17)10-11-23-21(27)18-14-24-19(13-22)26-20(18)25-16-7-3-2-4-8-16/h5-6,9,12,14,16H,2-4,7-8,10-11H2,1H3,(H,23,27)(H,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Novartis Pharmaceuticals
| Assay Description
The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... |
J Med Chem 50: 591-4 (2007)
Article DOI: 10.1021/jm0613525
BindingDB Entry DOI: 10.7270/Q22805X5 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19741
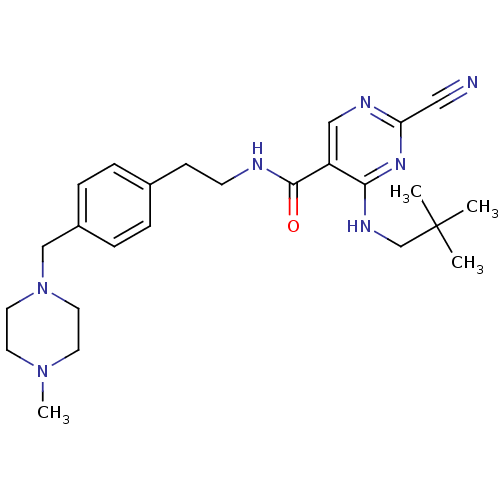
(2-Cyano-pyrimidine, 17f | 2-cyano-4-[(2,2-dimethyl...)Show SMILES CN1CCN(Cc2ccc(CCNC(=O)c3cnc(nc3NCC(C)(C)C)C#N)cc2)CC1 Show InChI InChI=1S/C25H35N7O/c1-25(2,3)18-29-23-21(16-28-22(15-26)30-23)24(33)27-10-9-19-5-7-20(8-6-19)17-32-13-11-31(4)12-14-32/h5-8,16H,9-14,17-18H2,1-4H3,(H,27,33)(H,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Novartis Pharmaceuticals
| Assay Description
The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... |
J Med Chem 50: 591-4 (2007)
Article DOI: 10.1021/jm0613525
BindingDB Entry DOI: 10.7270/Q22805X5 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19736
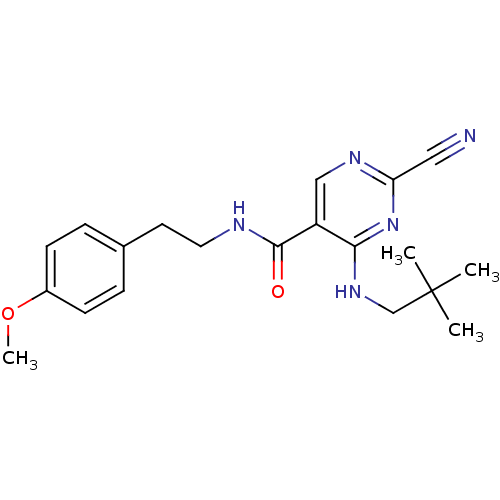
(2-Cyano-pyrimidine, 17a | 2-cyano-4-[(2,2-dimethyl...)Show SMILES COc1ccc(CCNC(=O)c2cnc(nc2NCC(C)(C)C)C#N)cc1 Show InChI InChI=1S/C20H25N5O2/c1-20(2,3)13-24-18-16(12-23-17(11-21)25-18)19(26)22-10-9-14-5-7-15(27-4)8-6-14/h5-8,12H,9-10,13H2,1-4H3,(H,22,26)(H,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Novartis Pharmaceuticals
| Assay Description
The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... |
J Med Chem 50: 591-4 (2007)
Article DOI: 10.1021/jm0613525
BindingDB Entry DOI: 10.7270/Q22805X5 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50250152
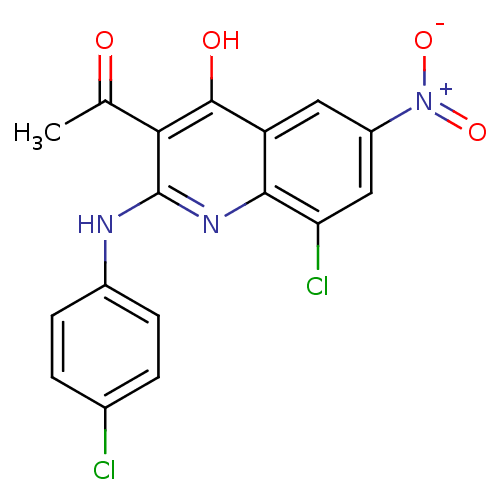
(3-acetyl-8-chloro-2-(4-chlorophenylamino)-6-nitroq...)Show SMILES CC(=O)c1c(Nc2ccc(Cl)cc2)nc2c(Cl)cc(cc2c1O)[N+]([O-])=O Show InChI InChI=1S/C17H11Cl2N3O4/c1-8(23)14-16(24)12-6-11(22(25)26)7-13(19)15(12)21-17(14)20-10-4-2-9(18)3-5-10/h2-7H,1H3,(H2,20,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.437 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin L after 30 mins by fluorometric end-point assay |
J Med Chem 52: 3093-7 (2009)
Article DOI: 10.1021/jm8014734
BindingDB Entry DOI: 10.7270/Q2KK9CQ4 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19735

(2-Cyano-pyrimidine, 16e | 2-cyano-4-(cyclohexylami...)Show SMILES CN1CCN(CC1)c1ccc(CCNC(=O)c2cnc(nc2NC2CCCCC2)C#N)cc1 Show InChI InChI=1S/C25H33N7O/c1-31-13-15-32(16-14-31)21-9-7-19(8-10-21)11-12-27-25(33)22-18-28-23(17-26)30-24(22)29-20-5-3-2-4-6-20/h7-10,18,20H,2-6,11-16H2,1H3,(H,27,33)(H,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Novartis Pharmaceuticals
| Assay Description
The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... |
J Med Chem 50: 591-4 (2007)
Article DOI: 10.1021/jm0613525
BindingDB Entry DOI: 10.7270/Q22805X5 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19743
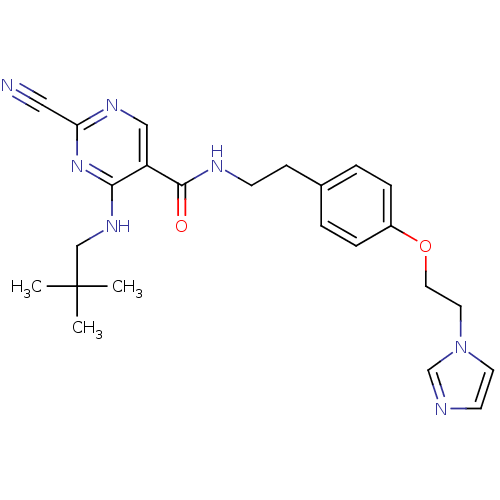
(2-Cyano-pyrimidine, 17h | 2-cyano-4-[(2,2-dimethyl...)Show SMILES CC(C)(C)CNc1nc(ncc1C(=O)NCCc1ccc(OCCn2ccnc2)cc1)C#N Show InChI InChI=1S/C24H29N7O2/c1-24(2,3)16-29-22-20(15-28-21(14-25)30-22)23(32)27-9-8-18-4-6-19(7-5-18)33-13-12-31-11-10-26-17-31/h4-7,10-11,15,17H,8-9,12-13,16H2,1-3H3,(H,27,32)(H,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Novartis Pharmaceuticals
| Assay Description
The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... |
J Med Chem 50: 591-4 (2007)
Article DOI: 10.1021/jm0613525
BindingDB Entry DOI: 10.7270/Q22805X5 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50349198

(CHEMBL1807649)Show SMILES CC(C)C[C@H](NC(=O)c1ccco1)C(=O)N[C@H]1CC[C@@H](C)N(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C23H30N4O6S/c1-15(2)13-18(26-23(30)20-7-6-12-33-20)22(29)25-17-10-9-16(3)27(14-19(17)28)34(31,32)21-8-4-5-11-24-21/h4-8,11-12,15-18H,9-10,13-14H2,1-3H3,(H,25,29)(H,26,30)/t16-,17+,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L using Z-LR-AMC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19745

(2-Cyano-pyrimidine, 17j | 2-cyano-4-[(2,2-dimethyl...)Show SMILES COc1ccc(CCNC(=O)c2cnc(nc2NCC(C)(C)C)C#N)cc1OCCn1ccnc1 Show InChI InChI=1S/C25H31N7O3/c1-25(2,3)16-30-23-19(15-29-22(14-26)31-23)24(33)28-8-7-18-5-6-20(34-4)21(13-18)35-12-11-32-10-9-27-17-32/h5-6,9-10,13,15,17H,7-8,11-12,16H2,1-4H3,(H,28,33)(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Novartis Pharmaceuticals
| Assay Description
The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... |
J Med Chem 50: 591-4 (2007)
Article DOI: 10.1021/jm0613525
BindingDB Entry DOI: 10.7270/Q22805X5 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM642834
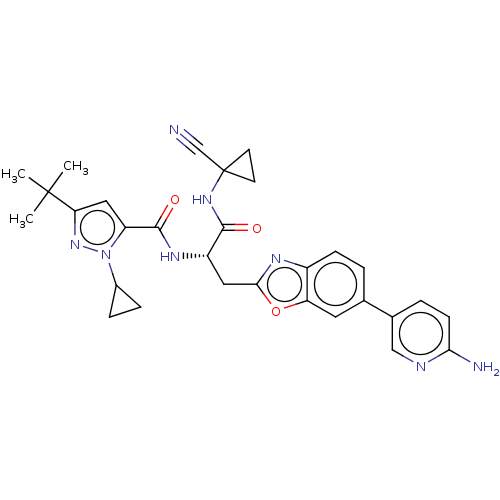
(US11858905, Compound 34)Show SMILES CC(C)(C)c1cc(C(=O)N[C@@H](Cc2nc3ccc(cc3o2)-c2ccc(N)nc2)C(=O)NC2(CC2)C#N)n(n1)C1CC1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50505555

(CHEMBL4447348)Show SMILES COC1=CC(=O)N([C@H]1CC(C)C)C(=O)\C=C\[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)OC(=O)[C@H](C(C)C)N(C)C |r,t:2| Show InChI InChI=1S/C39H60N4O7/c1-24(2)19-30(41-38(47)33(21-26(5)6)50-39(48)36(27(7)8)42(9)10)37(46)40-29(22-28-15-13-12-14-16-28)17-18-34(44)43-31(20-25(3)4)32(49-11)23-35(43)45/h12-18,23-27,29-31,33,36H,19-22H2,1-11H3,(H,40,46)(H,41,47)/b18-17+/t29-,30+,31+,33+,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.632 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L using fluorogenic substrate cbz-FR-AMC monitored for 90 to 120 mins by spectrophotometry |
J Med Chem 62: 9026-9044 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00294
BindingDB Entry DOI: 10.7270/Q2H998GC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19744
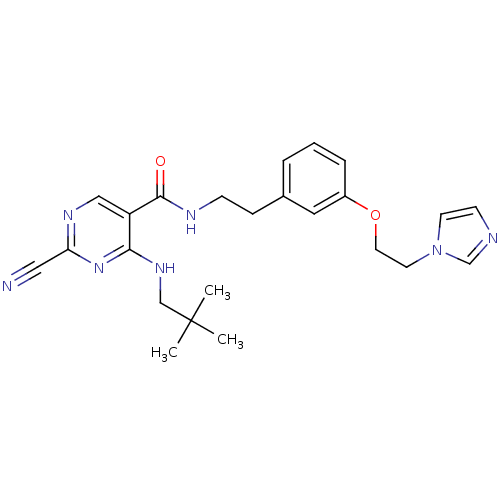
(2-Cyano-pyrimidine, 17i | 2-cyano-4-[(2,2-dimethyl...)Show SMILES CC(C)(C)CNc1nc(ncc1C(=O)NCCc1cccc(OCCn2ccnc2)c1)C#N Show InChI InChI=1S/C24H29N7O2/c1-24(2,3)16-29-22-20(15-28-21(14-25)30-22)23(32)27-8-7-18-5-4-6-19(13-18)33-12-11-31-10-9-26-17-31/h4-6,9-10,13,15,17H,7-8,11-12,16H2,1-3H3,(H,27,32)(H,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Novartis Pharmaceuticals
| Assay Description
The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... |
J Med Chem 50: 591-4 (2007)
Article DOI: 10.1021/jm0613525
BindingDB Entry DOI: 10.7270/Q22805X5 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50229129

(4-Methyl-piperazine-1-carboxylic acid [(S)-1-((E)-...)Show SMILES CN1CCN(CC1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCc1ccccc1)C=CS(=O)(=O)c1ccccc1 |r,w:31.34| Show InChI InChI=1S/C32H38N4O4S/c1-35-20-22-36(23-21-35)32(38)34-30(25-27-13-7-3-8-14-27)31(37)33-28(18-17-26-11-5-2-6-12-26)19-24-41(39,40)29-15-9-4-10-16-29/h2-16,19,24,28,30H,17-18,20-23,25H2,1H3,(H,33,37)(H,34,38)/t28-,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50461249

(CHEMBL1215628)Show SMILES CC(C)CN(NC(=O)c1ccc(CN2CCN(C)CC2)cc1)c1nc(ncc1Br)C#N Show InChI InChI=1S/C22H28BrN7O/c1-16(2)14-30(21-19(23)13-25-20(12-24)26-21)27-22(31)18-6-4-17(5-7-18)15-29-10-8-28(3)9-11-29/h4-7,13,16H,8-11,14-15H2,1-3H3,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L |
Bioorg Med Chem Lett 28: 1540-1544 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.069
BindingDB Entry DOI: 10.7270/Q2G44SW1 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50163831
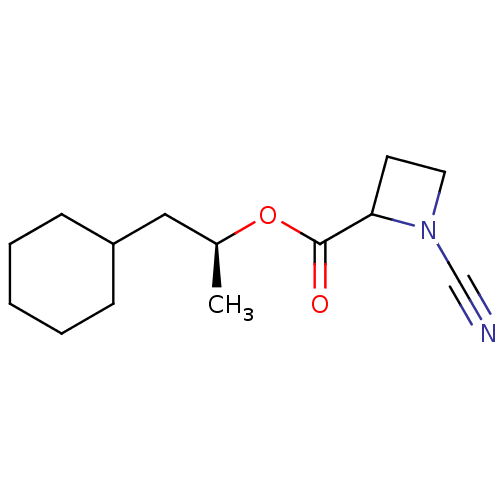
((2S)-1-cyclohexylpropan-2-yl 1-cyanoazetidine-2-ca...)Show InChI InChI=1S/C14H22N2O2/c1-11(9-12-5-3-2-4-6-12)18-14(17)13-7-8-16(13)10-15/h11-13H,2-9H2,1H3/t11-,13?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human cathepsin L using 5 uM Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 1815-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.033
BindingDB Entry DOI: 10.7270/Q2DR2V1G |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50067612
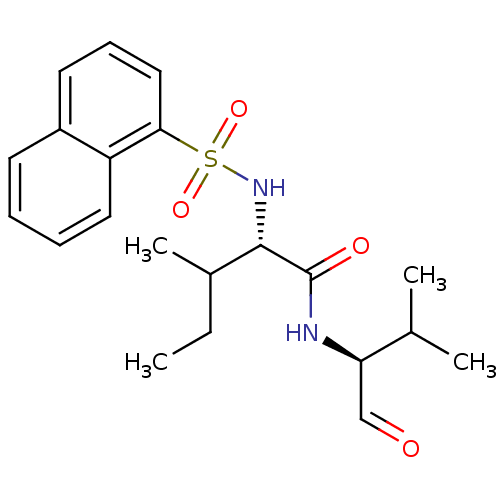
((S)-3-Methyl-2-(naphthalene-1-sulfonylamino)-penta...)Show SMILES CCC(C)[C@H](NS(=O)(=O)c1cccc2ccccc12)C(=O)N[C@H](C=O)C(C)C Show InChI InChI=1S/C21H28N2O4S/c1-5-15(4)20(21(25)22-18(13-24)14(2)3)23-28(26,27)19-12-8-10-16-9-6-7-11-17(16)19/h6-15,18,20,23H,5H2,1-4H3,(H,22,25)/t15?,18-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50084674
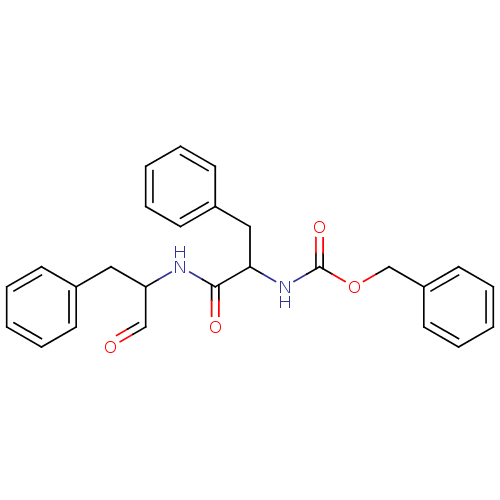
(CHEMBL115394 | [1-(1-Formyl-2-phenyl-ethylcarbamoy...)Show SMILES O=CC(Cc1ccccc1)NC(=O)C(Cc1ccccc1)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C26H26N2O4/c29-18-23(16-20-10-4-1-5-11-20)27-25(30)24(17-21-12-6-2-7-13-21)28-26(31)32-19-22-14-8-3-9-15-22/h1-15,18,23-24H,16-17,19H2,(H,27,30)(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin L, lysosomal cysteine protease |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50505557
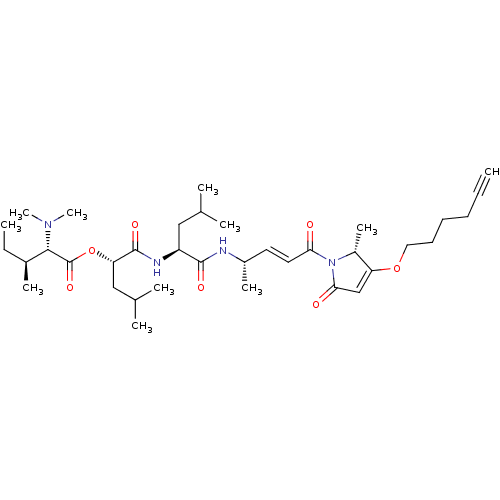
(CHEMBL4527930)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)\C=C\C(=O)N1[C@H](C)C(OCCCCC#C)=CC1=O |r,c:43| Show InChI InChI=1S/C36H58N4O7/c1-12-14-15-16-19-46-29-22-32(42)40(27(29)9)31(41)18-17-26(8)37-34(43)28(20-23(3)4)38-35(44)30(21-24(5)6)47-36(45)33(39(10)11)25(7)13-2/h1,17-18,22-28,30,33H,13-16,19-21H2,2-11H3,(H,37,43)(H,38,44)/b18-17+/t25-,26-,27+,28-,30-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L using fluorogenic substrate cbz-FR-AMC monitored for 90 to 120 mins by spectrophotometry |
J Med Chem 62: 9026-9044 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00294
BindingDB Entry DOI: 10.7270/Q2H998GC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM642843

(US11858905, Compound 43)Show SMILES CCNc1ccc(cn1)-c1ccc2nc(C[C@H](NC(=O)c3cc(nn3C3CC3)C3(C)CC3)C(=O)NC3(CC3)C#N)oc2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19740
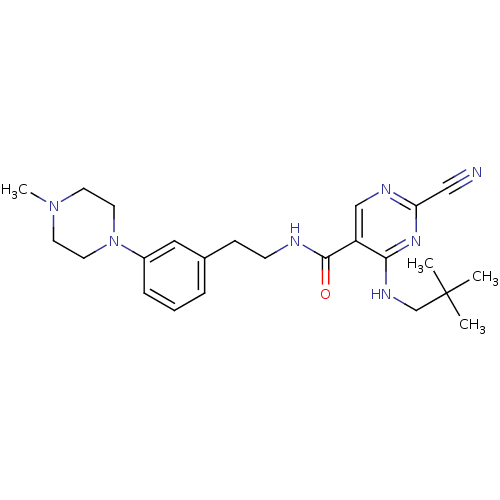
(2-Cyano-pyrimidine, 17e | 2-cyano-4-[(2,2-dimethyl...)Show SMILES CN1CCN(CC1)c1cccc(CCNC(=O)c2cnc(nc2NCC(C)(C)C)C#N)c1 Show InChI InChI=1S/C24H33N7O/c1-24(2,3)17-28-22-20(16-27-21(15-25)29-22)23(32)26-9-8-18-6-5-7-19(14-18)31-12-10-30(4)11-13-31/h5-7,14,16H,8-13,17H2,1-4H3,(H,26,32)(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Novartis Pharmaceuticals
| Assay Description
The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... |
J Med Chem 50: 591-4 (2007)
Article DOI: 10.1021/jm0613525
BindingDB Entry DOI: 10.7270/Q22805X5 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50084650
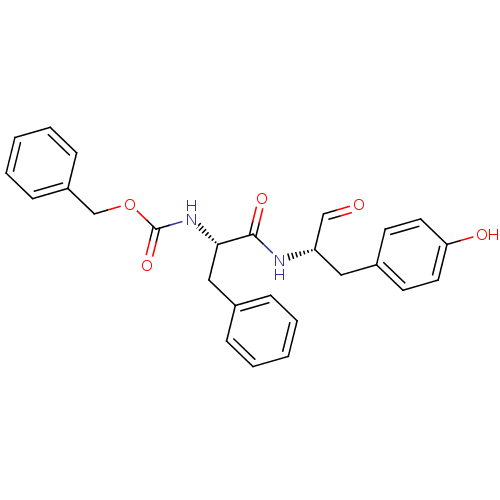
(CHEMBL177914 | {(S)-1-[(S)-1-Formyl-2-(4-hydroxy-p...)Show SMILES Oc1ccc(C[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)OCc2ccccc2)C=O)cc1 Show InChI InChI=1S/C26H26N2O5/c29-17-22(15-20-11-13-23(30)14-12-20)27-25(31)24(16-19-7-3-1-4-8-19)28-26(32)33-18-21-9-5-2-6-10-21/h1-14,17,22,24,30H,15-16,18H2,(H,27,31)(H,28,32)/t22-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin L, lysosomal cysteine protease |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM122528
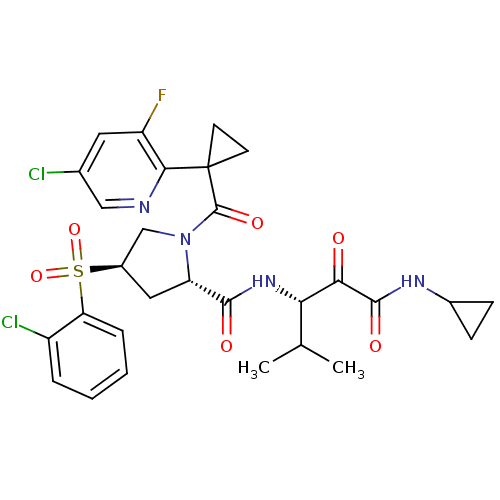
(US8729061, 58)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1C[C@H](CN1C(=O)C1(CC1)c1ncc(Cl)cc1F)S(=O)(=O)c1ccccc1Cl)C(=O)C(=O)NC1CC1 |r| Show InChI InChI=1S/C29H31Cl2FN4O6S/c1-15(2)23(24(37)27(39)34-17-7-8-17)35-26(38)21-12-18(43(41,42)22-6-4-3-5-19(22)31)14-36(21)28(40)29(9-10-29)25-20(32)11-16(30)13-33-25/h3-6,11,13,15,17-18,21,23H,7-10,12,14H2,1-2H3,(H,34,39)(H,35,38)/t18-,21+,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... |
US Patent US8729061 (2014)
BindingDB Entry DOI: 10.7270/Q2MS3RF6 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50067608
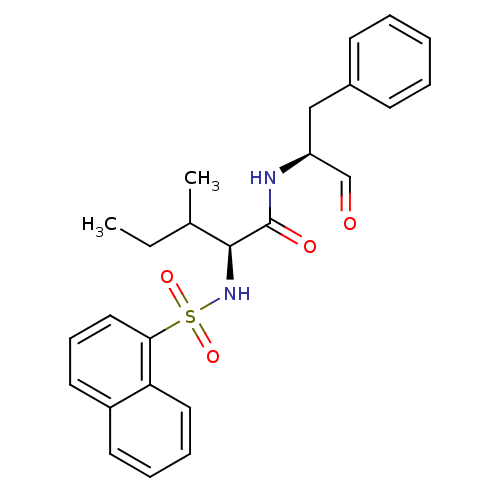
((S)-3-Methyl-2-(naphthalene-1-sulfonylamino)-penta...)Show SMILES CCC(C)[C@H](NS(=O)(=O)c1cccc2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C=O Show InChI InChI=1S/C25H28N2O4S/c1-3-18(2)24(25(29)26-21(17-28)16-19-10-5-4-6-11-19)27-32(30,31)23-15-9-13-20-12-7-8-14-22(20)23/h4-15,17-18,21,24,27H,3,16H2,1-2H3,(H,26,29)/t18?,21-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50067606
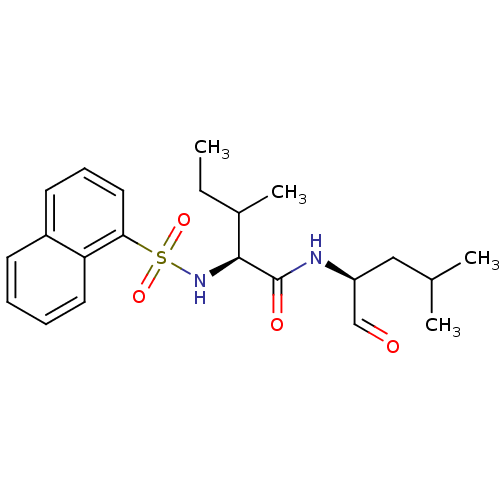
((S)-3-Methyl-2-(naphthalene-1-sulfonylamino)-penta...)Show SMILES CCC(C)[C@H](NS(=O)(=O)c1cccc2ccccc12)C(=O)N[C@@H](CC(C)C)C=O Show InChI InChI=1S/C22H30N2O4S/c1-5-16(4)21(22(26)23-18(14-25)13-15(2)3)24-29(27,28)20-12-8-10-17-9-6-7-11-19(17)20/h6-12,14-16,18,21,24H,5,13H2,1-4H3,(H,23,26)/t16?,18-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50067604

((S)-N-[(R)-1-Formyl-2-(1H-indol-3-yl)-ethyl]-3-met...)Show SMILES CC(C)[C@H](NS(=O)(=O)c1cccc2ccccc12)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C=O Show InChI InChI=1S/C26H27N3O4S/c1-17(2)25(29-34(32,33)24-13-7-9-18-8-3-4-11-22(18)24)26(31)28-20(16-30)14-19-15-27-23-12-6-5-10-21(19)23/h3-13,15-17,20,25,27,29H,14H2,1-2H3,(H,28,31)/t20-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM448319
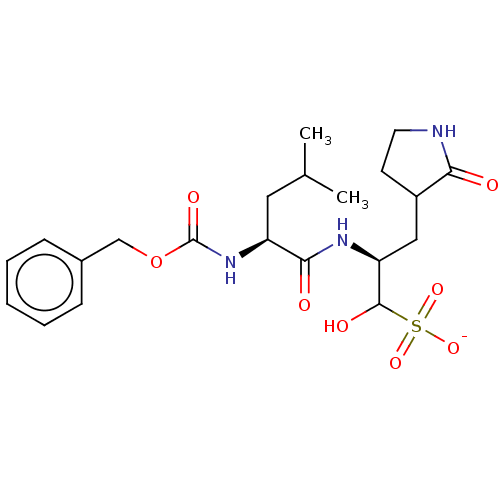
(GC-376 | GC376)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](CC1CCNC1=O)C(O)S([O-])(=O)=O Show InChI InChI=1S/C21H31N3O8S/c1-13(2)10-16(24-21(28)32-12-14-6-4-3-5-7-14)19(26)23-17(20(27)33(29,30)31)11-15-8-9-22-18(15)25/h3-7,13,15-17,20,27H,8-12H2,1-2H3,(H,22,25)(H,23,26)(H,24,28)(H,29,30,31)/p-1/t15?,16-,17-,20?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
FRET-based enzymatic assay. |
Citation and Details
Article DOI: 10.1021/jacs.1c08060
BindingDB Entry DOI: 10.7270/Q2BK1GHG |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM642846

(US11858905, Compound 46)Show SMILES COCCN1CCC(=CC1)c1ccc2nc(C[C@H](NC(=O)c3cc(nn3C3CC3)C3(C)CC3)C(=O)NC3(CC3)C#N)oc2c1 |r,c:7| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50349203
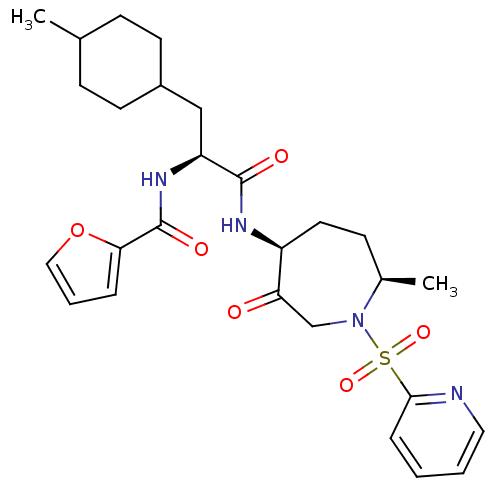
(CHEMBL1807698)Show SMILES CC1CCC(C[C@H](NC(=O)c2ccco2)C(=O)N[C@H]2CC[C@@H](C)N(CC2=O)S(=O)(=O)c2ccccn2)CC1 |r,wU:6.6,wD:21.22,18.18,(28.97,-10.04,;27.63,-10.81,;27.63,-12.35,;26.31,-13.13,;24.98,-12.36,;23.65,-13.13,;23.65,-14.69,;22.31,-15.43,;20.99,-14.64,;21.01,-13.11,;19.65,-15.4,;18.26,-14.75,;17.2,-15.88,;17.95,-17.22,;19.46,-16.92,;24.98,-15.47,;24.96,-17.01,;26.33,-14.72,;27.64,-15.51,;27.51,-17.06,;28.62,-18.12,;30.15,-17.9,;31,-19.19,;30.94,-16.59,;30.39,-15.15,;28.92,-14.67,;28.91,-13.13,;32.47,-16.72,;33.12,-18.11,;31.95,-18.17,;33.36,-15.46,;32.7,-14.06,;33.57,-12.8,;35.12,-12.92,;35.77,-14.33,;34.89,-15.59,;24.96,-10.82,;26.3,-10.04,)| Show InChI InChI=1S/C27H36N4O6S/c1-18-8-11-20(12-9-18)16-22(30-27(34)24-6-5-15-37-24)26(33)29-21-13-10-19(2)31(17-23(21)32)38(35,36)25-7-3-4-14-28-25/h3-7,14-15,18-22H,8-13,16-17H2,1-2H3,(H,29,33)(H,30,34)/t18?,19-,20?,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L using Z-LR-AMC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50505566

(CHEMBL4582558)Show SMILES COC1=CC(=O)N([C@@H]1Cc1ccccc1)C(=O)\C=C\[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)OC(=O)[C@H](Cc1ccccc1)N(C)C |r,t:2| Show InChI InChI=1S/C46H58N4O7/c1-31(2)25-37(48-45(54)41(26-32(3)4)57-46(55)39(49(5)6)29-35-21-15-10-16-22-35)44(53)47-36(27-33-17-11-8-12-18-33)23-24-42(51)50-38(40(56-7)30-43(50)52)28-34-19-13-9-14-20-34/h8-24,30-32,36-39,41H,25-29H2,1-7H3,(H,47,53)(H,48,54)/b24-23+/t36-,37+,38-,39+,41+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L using fluorogenic substrate cbz-FR-AMC monitored for 90 to 120 mins by spectrophotometry |
J Med Chem 62: 9026-9044 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00294
BindingDB Entry DOI: 10.7270/Q2H998GC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19731

(2-Cyano-pyrimidine, 16a | 2-cyano-4-(cyclohexylami...)Show InChI InChI=1S/C20H23N5O/c21-13-18-23-14-17(19(25-18)24-16-9-5-2-6-10-16)20(26)22-12-11-15-7-3-1-4-8-15/h1,3-4,7-8,14,16H,2,5-6,9-12H2,(H,22,26)(H,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Novartis Pharmaceuticals
| Assay Description
The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... |
J Med Chem 50: 591-4 (2007)
Article DOI: 10.1021/jm0613525
BindingDB Entry DOI: 10.7270/Q22805X5 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM642835

(US11858905, Compound 35)Show SMILES Cc1cc(ccn1)-c1ccc2nc(C[C@H](NC(=O)c3cc(nn3C3CC3)C3(C)CC3)C(=O)NCC#N)oc2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM429100
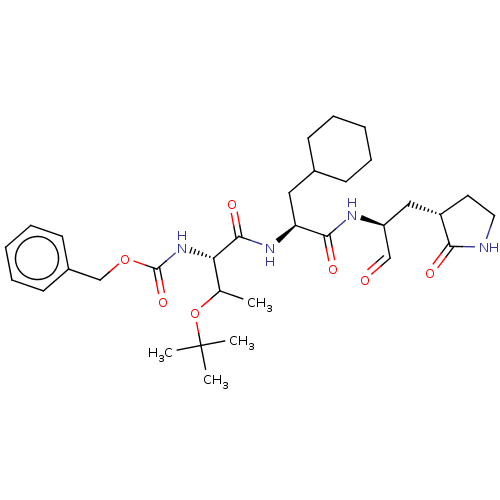
(MPI8 | jm5b01461, Compound 45)Show SMILES CC(OC(C)(C)C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C=O Show InChI InChI=1S/C32H48N4O7/c1-21(43-32(2,3)4)27(36-31(41)42-20-23-13-9-6-10-14-23)30(40)35-26(17-22-11-7-5-8-12-22)29(39)34-25(19-37)18-24-15-16-33-28(24)38/h6,9-10,13-14,19,21-22,24-27H,5,7-8,11-12,15-18,20H2,1-4H3,(H,33,38)(H,34,39)(H,35,40)(H,36,41)/t21?,24-,25-,26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
FRET-based enzymatic assay. |
Citation and Details
Article DOI: 10.1021/jacs.1c08060
BindingDB Entry DOI: 10.7270/Q2BK1GHG |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50349197

(CHEMBL1807647)Show SMILES C[C@@H]1CC[C@H](NC(=O)[C@H](CC2CCCCC2)NC(=O)c2ccco2)C(=O)CN1S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H34N4O6S/c1-18-12-13-20(22(31)17-30(18)37(34,35)24-11-5-6-14-27-24)28-25(32)21(16-19-8-3-2-4-9-19)29-26(33)23-10-7-15-36-23/h5-7,10-11,14-15,18-21H,2-4,8-9,12-13,16-17H2,1H3,(H,28,32)(H,29,33)/t18-,20+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L using Z-LR-AMC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM442762
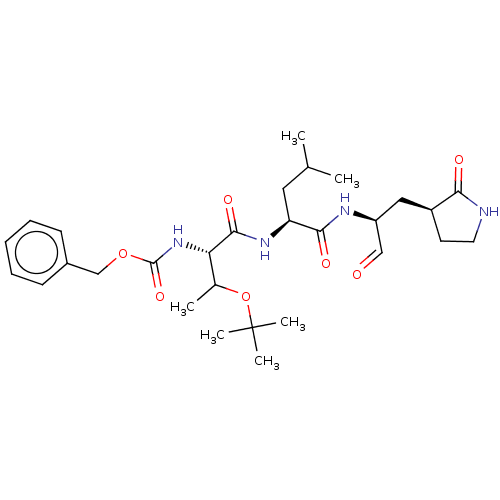
(MPI6)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)OC(C)(C)C)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C=O Show InChI InChI=1S/C29H44N4O7/c1-18(2)14-23(26(36)31-22(16-34)15-21-12-13-30-25(21)35)32-27(37)24(19(3)40-29(4,5)6)33-28(38)39-17-20-10-8-7-9-11-20/h7-11,16,18-19,21-24H,12-15,17H2,1-6H3,(H,30,35)(H,31,36)(H,32,37)(H,33,38)/t19?,21-,22-,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibition of dihydropteroate synthase from Escherichia coli. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19737
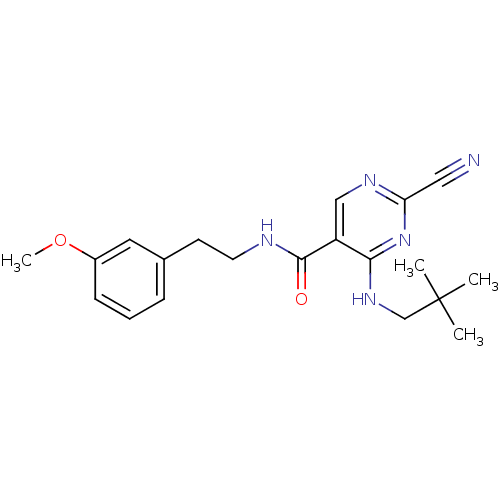
(2-Cyano-pyrimidine, 17b | 2-cyano-4-[(2,2-dimethyl...)Show SMILES COc1cccc(CCNC(=O)c2cnc(nc2NCC(C)(C)C)C#N)c1 Show InChI InChI=1S/C20H25N5O2/c1-20(2,3)13-24-18-16(12-23-17(11-21)25-18)19(26)22-9-8-14-6-5-7-15(10-14)27-4/h5-7,10,12H,8-9,13H2,1-4H3,(H,22,26)(H,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Novartis Pharmaceuticals
| Assay Description
The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... |
J Med Chem 50: 591-4 (2007)
Article DOI: 10.1021/jm0613525
BindingDB Entry DOI: 10.7270/Q22805X5 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50414644
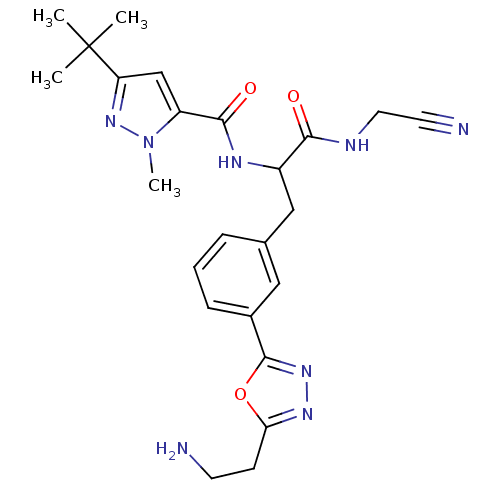
(CHEMBL555122)Show SMILES Cn1nc(cc1C(=O)NC(Cc1cccc(c1)-c1nnc(CCN)o1)C(=O)NCC#N)C(C)(C)C Show InChI InChI=1S/C24H30N8O3/c1-24(2,3)19-14-18(32(4)31-19)22(34)28-17(21(33)27-11-10-26)13-15-6-5-7-16(12-15)23-30-29-20(35-23)8-9-25/h5-7,12,14,17H,8-9,11,13,25H2,1-4H3,(H,27,33)(H,28,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50505560
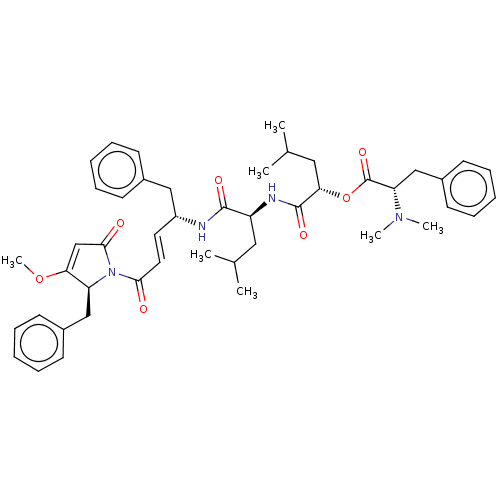
(CHEMBL4471760)Show SMILES COC1=CC(=O)N([C@H]1Cc1ccccc1)C(=O)\C=C\[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)OC(=O)[C@H](Cc1ccccc1)N(C)C |r,t:2| Show InChI InChI=1S/C46H58N4O7/c1-31(2)25-37(48-45(54)41(26-32(3)4)57-46(55)39(49(5)6)29-35-21-15-10-16-22-35)44(53)47-36(27-33-17-11-8-12-18-33)23-24-42(51)50-38(40(56-7)30-43(50)52)28-34-19-13-9-14-20-34/h8-24,30-32,36-39,41H,25-29H2,1-7H3,(H,47,53)(H,48,54)/b24-23+/t36-,37+,38+,39+,41+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L using fluorogenic substrate cbz-FR-AMC monitored for 90 to 120 mins by spectrophotometry |
J Med Chem 62: 9026-9044 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00294
BindingDB Entry DOI: 10.7270/Q2H998GC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM642829
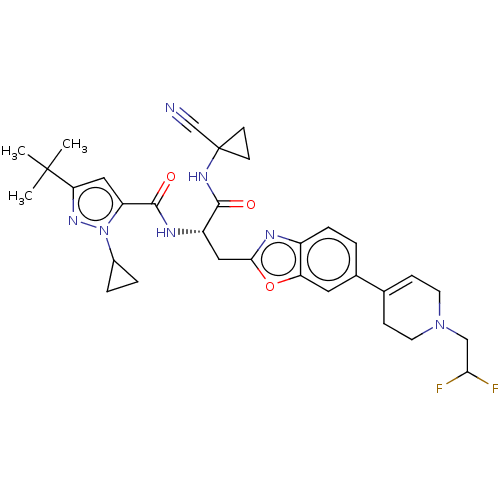
(US11858905, Compound 29)Show SMILES CC(C)(C)c1cc(C(=O)N[C@@H](Cc2nc3ccc(cc3o2)C2=CCN(CC(F)F)CC2)C(=O)NC2(CC2)C#N)n(n1)C1CC1 |r,t:23| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data