 Found 5470 hits of ki data for polymerid = 10163,2212
Found 5470 hits of ki data for polymerid = 10163,2212 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50304170
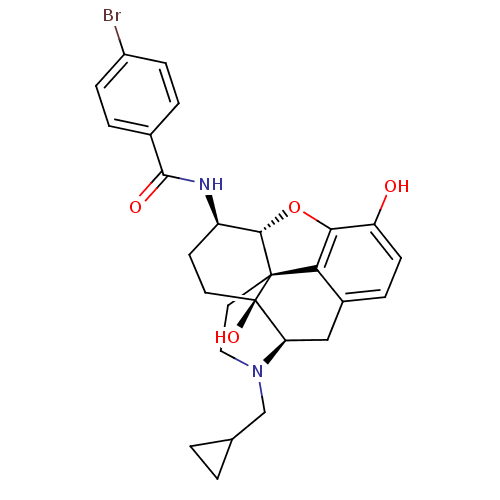
(17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)[C@@H](CC[C@@]35O)NC(=O)c1ccc(Br)cc1 |r| Show InChI InChI=1S/C27H29BrN2O4/c28-18-6-3-16(4-7-18)25(32)29-19-9-10-27(33)21-13-17-5-8-20(31)23-22(17)26(27,24(19)34-23)11-12-30(21)14-15-1-2-15/h3-8,15,19,21,24,31,33H,1-2,9-14H2,(H,29,32)/t19-,21-,24+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at kappa opioid receptor expressed in HEK293 cells assessed as inhibition of compound 16-induced [35S]GTPgammaS binding |
Bioorg Med Chem 17: 6671-81 (2009)
Article DOI: 10.1016/j.bmc.2009.07.069
BindingDB Entry DOI: 10.7270/Q2PK0H37 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM224024

(BDBM50241435 | Dynorphin A (1-13) | YGGFLRRXRPKLK)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#8])=O Show InChI InChI=1S/C75H126N24O15/c1-7-45(6)61(70(111)94-53(25-17-35-87-75(83)84)71(112)99-36-18-26-58(99)69(110)93-50(21-11-13-31-76)64(105)96-56(38-44(4)5)67(108)95-54(72(113)114)22-12-14-32-77)98-65(106)52(24-16-34-86-74(81)82)91-63(104)51(23-15-33-85-73(79)80)92-66(107)55(37-43(2)3)97-68(109)57(40-46-19-9-8-10-20-46)90-60(102)42-88-59(101)41-89-62(103)49(78)39-47-27-29-48(100)30-28-47/h8-10,19-20,27-30,43-45,49-58,61,100H,7,11-18,21-26,31-42,76-78H2,1-6H3,(H,88,101)(H,89,103)(H,90,102)(H,91,104)(H,92,107)(H,93,110)(H,94,111)(H,95,108)(H,96,105)(H,97,109)(H,98,106)(H,113,114)(H4,79,80,85)(H4,81,82,86)(H4,83,84,87)/t45-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,61-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| PubMed
| 0.000800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Binding affinity towards Wild-type kappa opioid receptor expressed in HEK cells |
J Med Chem 43: 1251-2 (2001)
BindingDB Entry DOI: 10.7270/Q270824F |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50366764

(CHEMBL1790045 | MCL-117)Show SMILES Oc1ccc2C[C@@H]3[C@@H]4CCCC[C@]4(CCN3CC#C)c2c1 Show InChI InChI=1S/C19H23NO/c1-2-10-20-11-9-19-8-4-3-5-16(19)18(20)12-14-6-7-15(21)13-17(14)19/h1,6-7,13,16,18,21H,3-5,8-12H2/t16-,18+,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cells |
J Med Chem 49: 256-62 (2006)
Article DOI: 10.1021/jm050577x
BindingDB Entry DOI: 10.7270/Q2HQ40QF |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50378578

(CHEMBL1627119)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@H](COP([O-])(=O)OP([O-])(=O)OP([O-])([O-])=S)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H16N5O13P3S/c11-10-13-7-4(8(18)14-10)12-2-15(7)9-6(17)5(16)3(26-9)1-25-29(19,20)27-30(21,22)28-31(23,24)32/h2-3,5-6,9,16-17H,1H2,(H,19,20)(H,21,22)(H2,23,24,32)(H3,11,13,14,18)/p-4/t3-,5-,6-,9-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at kappa opioid receptor expressed in HEK293 cells assessed as inhibition of compound 16-induced [35S]GTPgammaS binding |
Bioorg Med Chem 17: 6671-81 (2009)
Article DOI: 10.1016/j.bmc.2009.07.069
BindingDB Entry DOI: 10.7270/Q2PK0H37 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50102711
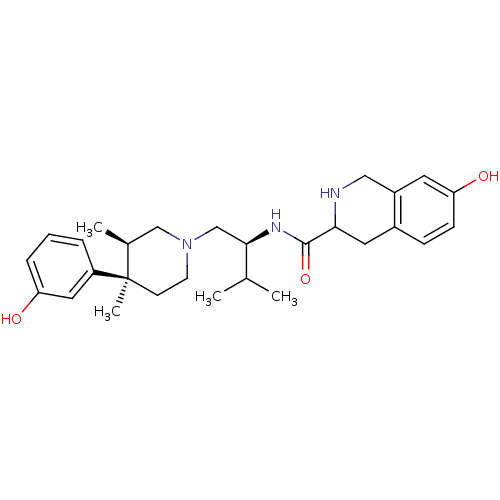
(7-Hydroxy-1,2,3,4-tetrahydro-isoquinoline-3-carbox...)Show SMILES CC(C)[C@@H](CN1CC[C@](C)([C@@H](C)C1)c1cccc(O)c1)NC(=O)C1Cc2ccc(O)cc2CN1 Show InChI InChI=1S/C28H39N3O3/c1-18(2)26(30-27(34)25-13-20-8-9-24(33)12-21(20)15-29-25)17-31-11-10-28(4,19(3)16-31)22-6-5-7-23(32)14-22/h5-9,12,14,18-19,25-26,29,32-33H,10-11,13,15-17H2,1-4H3,(H,30,34)/t19-,25?,26+,28+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Antagonist activity on agonist (U50,488) stimulated [35S]GTP-gamma-S, binding in cloned opioid receptor kappa 1 |
J Med Chem 44: 2687-90 (2001)
BindingDB Entry DOI: 10.7270/Q25T3M58 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50130563

((3R)-7-Hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphe...)Show SMILES CC(C)[C@@H](CN1CC[C@](C)([C@@H](C)C1)c1cccc(O)c1)NC(=O)[C@H]1Cc2ccc(O)cc2CN1 |r| Show InChI InChI=1S/C28H39N3O3/c1-18(2)26(30-27(34)25-13-20-8-9-24(33)12-21(20)15-29-25)17-31-11-10-28(4,19(3)16-31)22-6-5-7-23(32)14-22/h5-9,12,14,18-19,25-26,29,32-33H,10-11,13,15-17H2,1-4H3,(H,30,34)/t19-,25+,26+,28+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology
Curated by ChEMBL
| Assay Description
Antagonist activity at kappa opioid receptor (unknown origin) assessed as stimulation of [35S]GTPgammaS |
Eur J Med Chem 141: 632-647 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.012
BindingDB Entry DOI: 10.7270/Q2V98BQ2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50001714
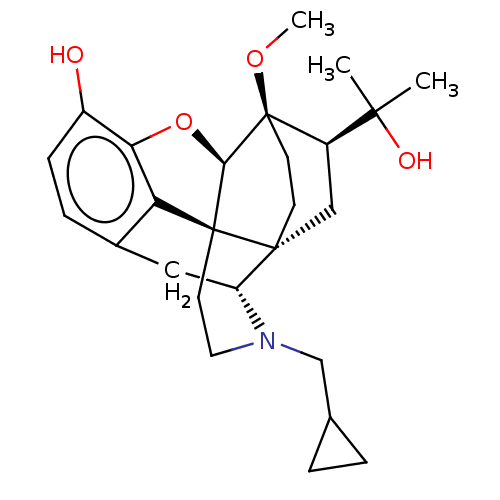
(2-[3-cyclopropylmethyl-11-hydroxy-15-methoxy-(1S,2...)Show SMILES CO[C@]12CC[C@@]3(C[C@@H]1C(C)(C)O)[C@H]1Cc4ccc(O)c5O[C@@H]2[C@]3(CCN1CC1CC1)c45 |THB:8:7:22.21:4.3| Show InChI InChI=1S/C26H35NO4/c1-23(2,29)18-13-24-8-9-26(18,30-3)22-25(24)10-11-27(14-15-4-5-15)19(24)12-16-6-7-17(28)21(31-22)20(16)25/h6-7,15,18-19,22,28-29H,4-5,8-14H2,1-3H3/t18-,19-,22-,24-,25+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 45: 330-4 (1994)
Article DOI: 10.1016/j.bioorg.2015.02.008
BindingDB Entry DOI: 10.7270/Q21Z42X1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM199410
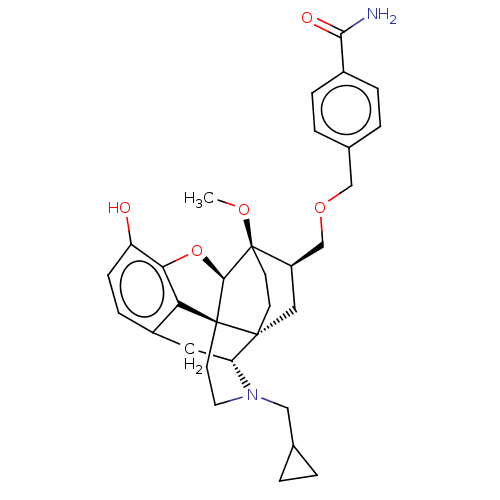
(US9221831, 75)Show SMILES CO[C@]12CC[C@@]3(C[C@@H]1COCc1ccc(cc1)C(N)=O)[C@H]1Cc4ccc(O)c5O[C@@H]2[C@]3(CCN1CC1CC1)c45 |r,TLB:8:7:30.29:4.3| Show InChI InChI=1S/C32H38N2O5/c1-37-32-11-10-30(15-23(32)18-38-17-20-4-6-21(7-5-20)28(33)36)25-14-22-8-9-24(35)27-26(22)31(30,29(32)39-27)12-13-34(25)16-19-2-3-19/h4-9,19,23,25,29,35H,2-3,10-18H2,1H3,(H2,33,36)/t23-,25-,29-,30-,31+,32-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Purdue Pharma, L.P.
US Patent
| Assay Description
Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb... |
US Patent US9221831 (2015)
BindingDB Entry DOI: 10.7270/Q2T72G8M |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50304175
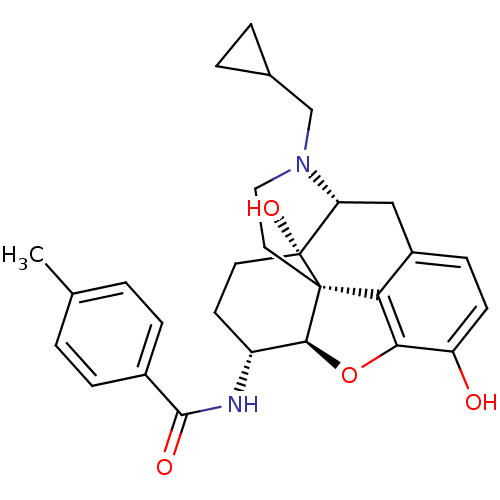
(17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...)Show SMILES Cc1ccc(cc1)C(=O)N[C@@H]1CC[C@@]2(O)[C@H]3Cc4ccc(O)c5O[C@@H]1[C@]2(CCN3CC1CC1)c45 |r| Show InChI InChI=1S/C28H32N2O4/c1-16-2-6-18(7-3-16)26(32)29-20-10-11-28(33)22-14-19-8-9-21(31)24-23(19)27(28,25(20)34-24)12-13-30(22)15-17-4-5-17/h2-3,6-9,17,20,22,25,31,33H,4-5,10-15H2,1H3,(H,29,32)/t20-,22-,25+,27+,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at kappa opioid receptor expressed in HEK293 cells assessed as inhibition of compound 16-induced [35S]GTPgammaS binding |
Bioorg Med Chem 17: 6671-81 (2009)
Article DOI: 10.1016/j.bmc.2009.07.069
BindingDB Entry DOI: 10.7270/Q2PK0H37 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50430590
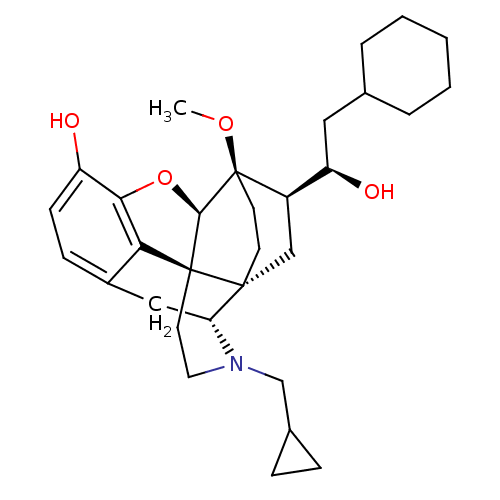
(CHEMBL2338742)Show SMILES CO[C@]12CC[C@@]3(C[C@@H]1[C@H](O)CC1CCCCC1)[C@H]1Cc4ccc(O)c5O[C@@H]2[C@]3(CCN1CC1CC1)c45 |r,THB:8:7:27.26:4.3| Show InChI InChI=1S/C31H43NO4/c1-35-31-12-11-29(17-22(31)24(34)15-19-5-3-2-4-6-19)25-16-21-9-10-23(33)27-26(21)30(29,28(31)36-27)13-14-32(25)18-20-7-8-20/h9-10,19-20,22,24-25,28,33-34H,2-8,11-18H2,1H3/t22-,24-,25-,28-,29-,30+,31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human KOR expressed in CHO cells |
J Med Chem 56: 3207-16 (2013)
Article DOI: 10.1021/jm301543e
BindingDB Entry DOI: 10.7270/Q28G8N2Q |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50577958
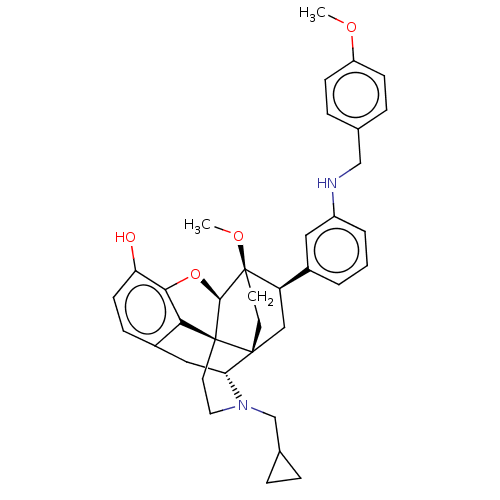
(CHEMBL4859512)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@H](C1)c1cccc(NCc2ccc(OC)cc2)c1)ccc3O |r,THB:10:9:5.4.6:17| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membrane measured after 30 mins by liquid scintillation counting m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01082
BindingDB Entry DOI: 10.7270/Q2NZ8CG1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50240938
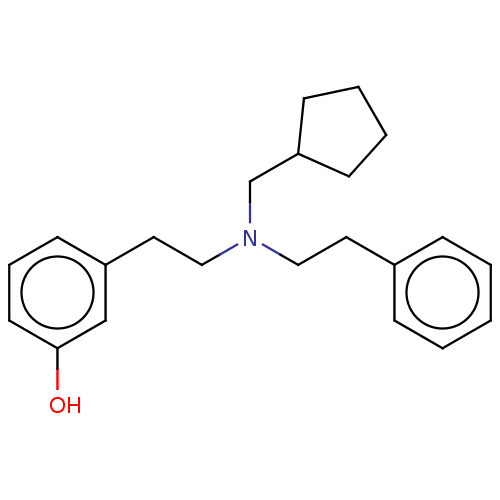
(CHEMBL4071862)Show InChI InChI=1S/C22H29NO/c24-22-12-6-11-20(17-22)14-16-23(18-21-9-4-5-10-21)15-13-19-7-2-1-3-8-19/h1-3,6-8,11-12,17,21,24H,4-5,9-10,13-16,18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membranes after 60 mins by liquid scintillation counting method |
J Med Chem 60: 7579-7590 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00981
BindingDB Entry DOI: 10.7270/Q21C202C |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50325534
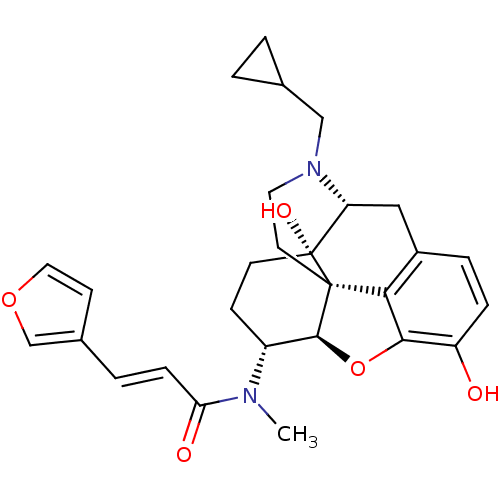
(CHEMBL267495 | nalfurafine)Show SMILES CN([C@@H]1CC[C@@]2(O)[C@H]3Cc4ccc(O)c5O[C@@H]1[C@]2(CCN3CC1CC1)c45)C(=O)\C=C\c1ccoc1 Show InChI InChI=1S/C28H32N2O5/c1-29(23(32)7-4-18-9-13-34-16-18)20-8-10-28(33)22-14-19-5-6-21(31)25-24(19)27(28,26(20)35-25)11-12-30(22)15-17-2-3-17/h4-7,9,13,16-17,20,22,26,31,33H,2-3,8,10-12,14-15H2,1H3/b7-4+/t20-,22-,26+,27+,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128527
BindingDB Entry DOI: 10.7270/Q2HT2TDM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50430589
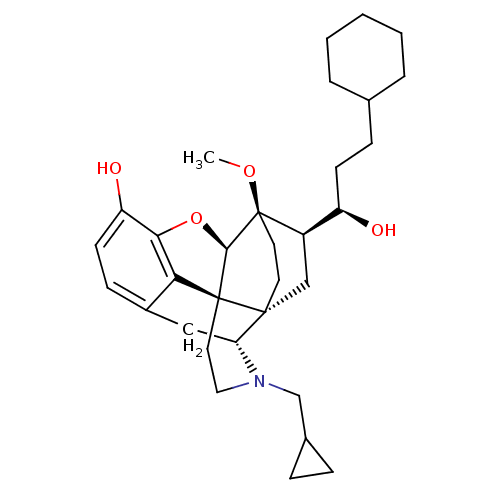
(CHEMBL2338716)Show SMILES CO[C@]12CC[C@@]3(C[C@@H]1[C@H](O)CCC1CCCCC1)[C@H]1Cc4ccc(O)c5O[C@@H]2[C@]3(CCN1CC1CC1)c45 |r,THB:8:7:28.27:4.3| Show InChI InChI=1S/C32H45NO4/c1-36-32-14-13-30(18-23(32)24(34)11-9-20-5-3-2-4-6-20)26-17-22-10-12-25(35)28-27(22)31(30,29(32)37-28)15-16-33(26)19-21-7-8-21/h10,12,20-21,23-24,26,29,34-35H,2-9,11,13-19H2,1H3/t23-,24-,26-,29-,30-,31+,32-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human KOR expressed in CHO cells |
J Med Chem 56: 3207-16 (2013)
Article DOI: 10.1021/jm301543e
BindingDB Entry DOI: 10.7270/Q28G8N2Q |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM82551

(C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)c1[nH]c2[C@@H]4Oc6c7c(C[C@H]8N(CC9CC9)CC[C@@]47[C@@]8(O)Cc2c1C[C@@]35O)ccc6O |r| Show InChI InChI=1S/C40H43N3O6/c44-25-7-5-21-13-27-39(46)15-23-24-16-40(47)28-14-22-6-8-26(45)34-30(22)38(40,10-12-43(28)18-20-3-4-20)36(49-34)32(24)41-31(23)35-37(39,29(21)33(25)48-35)9-11-42(27)17-19-1-2-19/h5-8,19-20,27-28,35-36,41,44-47H,1-4,9-18H2/t27-,28-,35+,36+,37+,38+,39-,40-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 45: 330-4 (1994)
Article DOI: 10.1016/j.bioorg.2015.02.008
BindingDB Entry DOI: 10.7270/Q21Z42X1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50430588
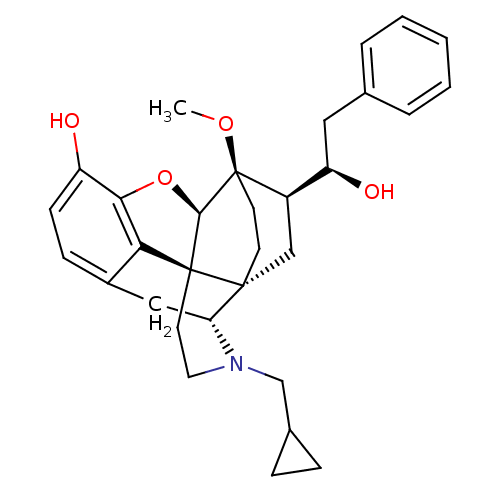
(CHEMBL2338717)Show SMILES CO[C@]12CC[C@@]3(C[C@@H]1[C@H](O)Cc1ccccc1)[C@H]1Cc4ccc(O)c5O[C@@H]2[C@]3(CCN1CC1CC1)c45 |r,THB:8:7:27.26:4.3| Show InChI InChI=1S/C31H37NO4/c1-35-31-12-11-29(17-22(31)24(34)15-19-5-3-2-4-6-19)25-16-21-9-10-23(33)27-26(21)30(29,28(31)36-27)13-14-32(25)18-20-7-8-20/h2-6,9-10,20,22,24-25,28,33-34H,7-8,11-18H2,1H3/t22-,24-,25-,28-,29-,30+,31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human KOR expressed in CHO cells |
J Med Chem 56: 3207-16 (2013)
Article DOI: 10.1021/jm301543e
BindingDB Entry DOI: 10.7270/Q28G8N2Q |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50001714

(2-[3-cyclopropylmethyl-11-hydroxy-15-methoxy-(1S,2...)Show SMILES CO[C@]12CC[C@@]3(C[C@@H]1C(C)(C)O)[C@H]1Cc4ccc(O)c5O[C@@H]2[C@]3(CCN1CC1CC1)c45 |THB:8:7:22.21:4.3| Show InChI InChI=1S/C26H35NO4/c1-23(2,29)18-13-24-8-9-26(18,30-3)22-25(24)10-11-27(14-15-4-5-15)19(24)12-16-6-7-17(28)21(31-22)20(16)25/h6-7,15,18-19,22,28-29H,4-5,8-14H2,1-3H3/t18-,19-,22-,24-,25+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oslo
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned kappa opioid receptor expressed in HEK293 cells by radioligand displacement assay |
J Med Chem 57: 5464-9 (2014)
Article DOI: 10.1021/jm500503k
BindingDB Entry DOI: 10.7270/Q2RB764T |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM199350
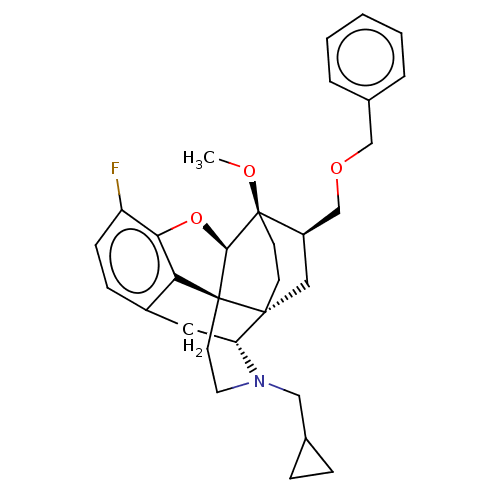
(US9221831, 15)Show SMILES CO[C@]12CC[C@@]3(C[C@@H]1COCc1ccccc1)[C@H]1Cc4ccc(F)c5O[C@@H]2[C@]3(CCN1CC1CC1)c45 |r,TLB:8:7:27.26:4.3| Show InChI InChI=1S/C31H36FNO3/c1-34-31-12-11-29(16-23(31)19-35-18-21-5-3-2-4-6-21)25-15-22-9-10-24(32)27-26(22)30(29,28(31)36-27)13-14-33(25)17-20-7-8-20/h2-6,9-10,20,23,25,28H,7-8,11-19H2,1H3/t23-,25-,28-,29-,30+,31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Purdue Pharma, L.P.
US Patent
| Assay Description
Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb... |
US Patent US9221831 (2015)
BindingDB Entry DOI: 10.7270/Q2T72G8M |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50430615
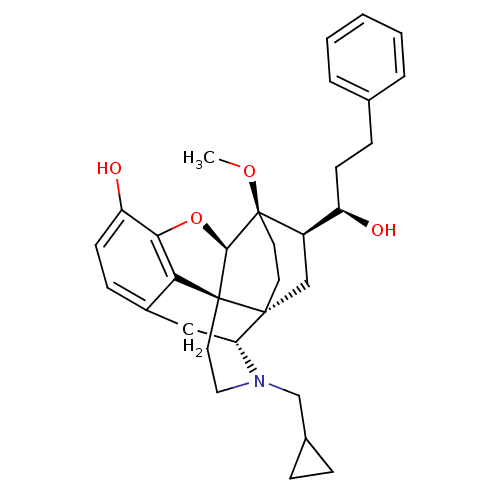
(CHEMBL2338718)Show SMILES CO[C@]12CC[C@@]3(C[C@@H]1[C@H](O)CCc1ccccc1)[C@H]1Cc4ccc(O)c5O[C@@H]2[C@]3(CCN1CC1CC1)c45 |r,THB:8:7:28.27:4.3| Show InChI InChI=1S/C32H39NO4/c1-36-32-14-13-30(18-23(32)24(34)11-9-20-5-3-2-4-6-20)26-17-22-10-12-25(35)28-27(22)31(30,29(32)37-28)15-16-33(26)19-21-7-8-21/h2-6,10,12,21,23-24,26,29,34-35H,7-9,11,13-19H2,1H3/t23-,24-,26-,29-,30-,31+,32-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human KOR expressed in CHO cells |
J Med Chem 56: 3207-16 (2013)
Article DOI: 10.1021/jm301543e
BindingDB Entry DOI: 10.7270/Q28G8N2Q |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM86549
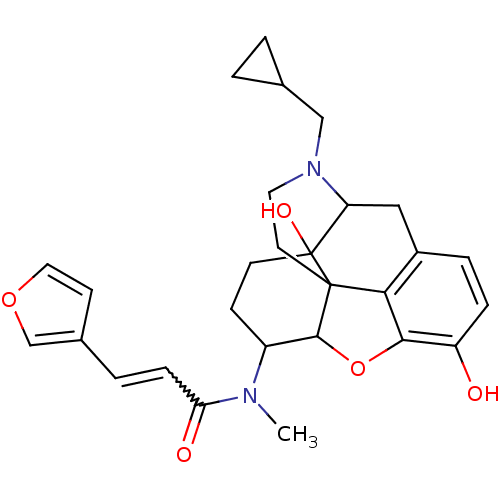
(CAS_213055 | NSC_213055 | TRK-820)Show SMILES CN(C1CCC2(O)C3Cc4ccc(O)c5OC1C2(CCN3CC1CC1)c45)C(=O)C=Cc1ccoc1 |w:28.33,TLB:4:5:8.9.25:18.19.20,6:5:8.9.25:18.19.20| Show InChI InChI=1S/C28H32N2O5/c1-29(23(32)7-4-18-9-13-34-16-18)20-8-10-28(33)22-14-19-5-6-21(31)25-24(19)27(28,26(20)35-25)11-12-30(22)15-17-2-3-17/h4-7,9,13,16-17,20,22,26,31,33H,2-3,8,10-12,14-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 312: 220-30 (2005)
Article DOI: 10.1124/jpet.104.073668
BindingDB Entry DOI: 10.7270/Q26T0K6G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM364547
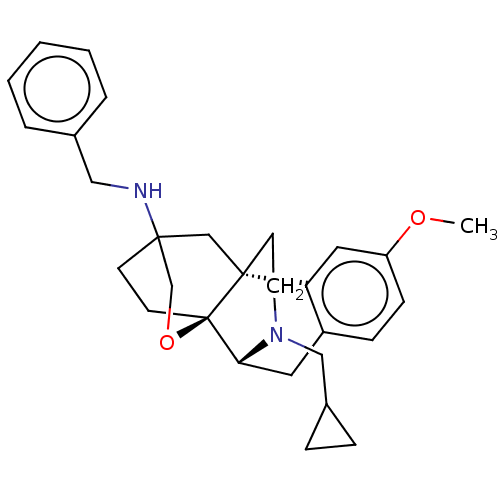
((4bR,6S,8aS,9R)-N-benzyl-11- (cyclopropylmethyl)-3...)Show SMILES COc1ccc2C[C@H]3N(CC4CC4)CC[C@@]4(CC5(CC[C@@]34OC5)NCc3ccccc3)c2c1 |r,TLB:9:8:20:31.5.6| Show InChI InChI=1S/C29H36N2O2/c1-32-24-10-9-23-15-26-29-12-11-27(20-33-29,30-17-21-5-3-2-4-6-21)19-28(29,25(23)16-24)13-14-31(26)18-22-7-8-22/h2-6,9-10,16,22,26,30H,7-8,11-15,17-20H2,1H3/t26-,27?,28-,29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF
| Assay Description
Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb... |
J Med Chem 50: 4255-9 (2007)
BindingDB Entry DOI: 10.7270/Q2222X2R |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM82551

(C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)c1[nH]c2[C@@H]4Oc6c7c(C[C@H]8N(CC9CC9)CC[C@@]47[C@@]8(O)Cc2c1C[C@@]35O)ccc6O |r| Show InChI InChI=1S/C40H43N3O6/c44-25-7-5-21-13-27-39(46)15-23-24-16-40(47)28-14-22-6-8-26(45)34-30(22)38(40,10-12-43(28)18-20-3-4-20)36(49-34)32(24)41-31(23)35-37(39,29(21)33(25)48-35)9-11-42(27)17-19-1-2-19/h5-8,19-20,27-28,35-36,41,44-47H,1-4,9-18H2/t27-,28-,35+,36+,37+,38+,39-,40-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute
Curated by ChEMBL
| Assay Description
Activity at human cloned kappa opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 19: 203-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.134
BindingDB Entry DOI: 10.7270/Q2RX9D1K |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50596292

(CHEMBL5185211)Show SMILES [H][C@]12Oc3c4c(C[C@]5([H])C(CC[C@H]1N(C)C(=O)\C=C\c1ccoc1)[C@]24CCN5CC1CC1)ccc3O |r,THB:28:27:9:4.5.6| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0272 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128527
BindingDB Entry DOI: 10.7270/Q2HT2TDM |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM83436
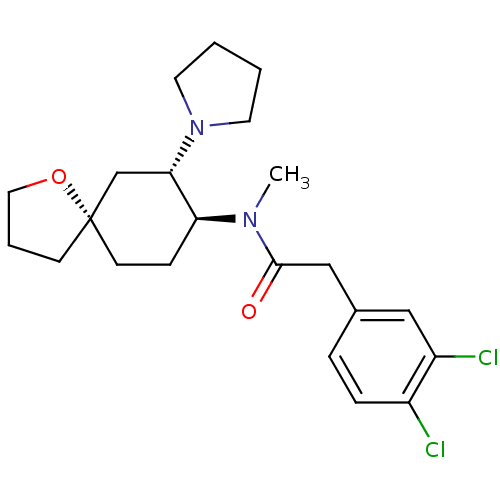
(2-(3,4-dichlorophenyl)-N-methyl-N-[(5R,7S,8S)-7-(1...)Show SMILES CN([C@H]1CC[C@@]2(CCCO2)C[C@@H]1N1CCCC1)C(=O)Cc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C22H30Cl2N2O2/c1-25(21(27)14-16-5-6-17(23)18(24)13-16)19-7-9-22(8-4-12-28-22)15-20(19)26-10-2-3-11-26/h5-6,13,19-20H,2-4,7-12,14-15H2,1H3/t19-,20-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 45: 330-4 (1994)
Article DOI: 10.1016/j.bioorg.2015.02.008
BindingDB Entry DOI: 10.7270/Q21Z42X1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50013388
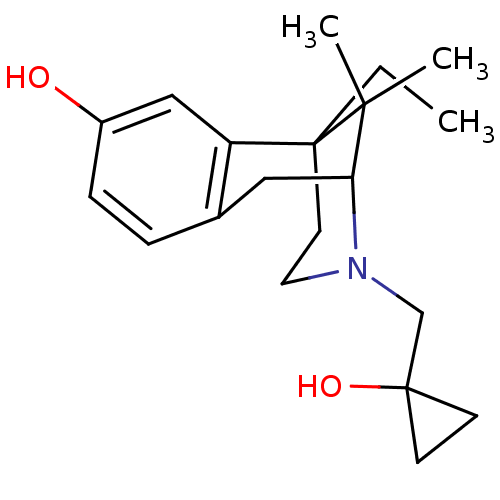
(6-Ethyl-3-(1-hydroxy-cyclopropylmethyl)-11,11-dime...)Show SMILES CCC12CCN(CC3(O)CC3)C(Cc3ccc(O)cc13)C2(C)C |TLB:6:5:20:19.13.12| Show InChI InChI=1S/C20H29NO2/c1-4-20-9-10-21(13-19(23)7-8-19)17(18(20,2)3)11-14-5-6-15(22)12-16(14)20/h5-6,12,17,22-23H,4,7-11,13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International
Curated by PDSP Ki Database
| |
NIDA Res Monogr 178: 440-66 (1998)
BindingDB Entry DOI: 10.7270/Q23J3BH2 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50130563

((3R)-7-Hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphe...)Show SMILES CC(C)[C@@H](CN1CC[C@](C)([C@@H](C)C1)c1cccc(O)c1)NC(=O)[C@H]1Cc2ccc(O)cc2CN1 |r| Show InChI InChI=1S/C28H39N3O3/c1-18(2)26(30-27(34)25-13-20-8-9-24(33)12-21(20)15-29-25)17-31-11-10-28(4,19(3)16-31)22-6-5-7-23(32)14-22/h5-9,12,14,18-19,25-26,29,32-33H,10-11,13,15-17H2,1-4H3,(H,30,34)/t19-,25+,26+,28+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Caen Basse-Normandie
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human recombinant mu opioid receptor expressed in HEK cells after 60 mins |
Eur J Med Chem 90: 742-50 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.016
BindingDB Entry DOI: 10.7270/Q2TQ636H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50346951
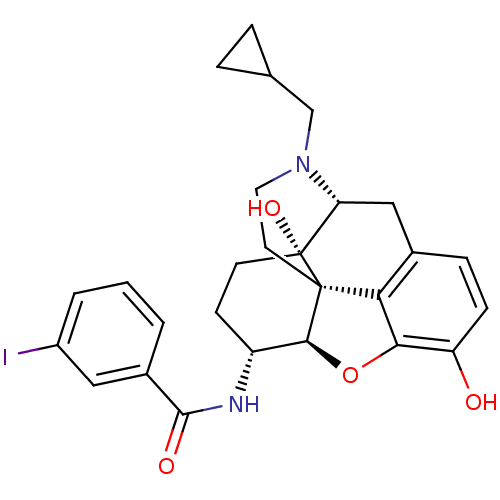
(CHEMBL1795711 | CHEMBL1795714)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)[C@@H](CC[C@@]35O)NC(=O)c1cccc(I)c1 |r| Show InChI InChI=1S/C27H29IN2O4/c28-18-3-1-2-17(12-18)25(32)29-19-8-9-27(33)21-13-16-6-7-20(31)23-22(16)26(27,24(19)34-23)10-11-30(21)14-15-4-5-15/h1-3,6-7,12,15,19,21,24,31,33H,4-5,8-11,13-14H2,(H,29,32)/t19-,21-,24+,26+,27-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-BNtxA from mouse cloned KOR-1 expressed in CHO cell membrane after 90 mins |
J Med Chem 55: 6352-62 (2012)
Article DOI: 10.1021/jm300305c
BindingDB Entry DOI: 10.7270/Q2930V8C |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50303629
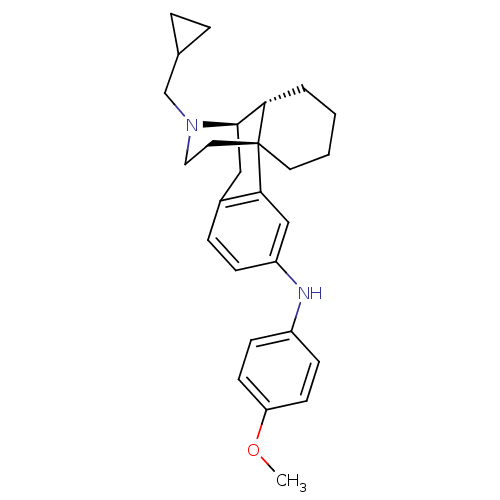
(17-(Cyclopropylmethyl)-N-(4-methoxyphenyl)morphina...)Show SMILES COc1ccc(Nc2ccc3C[C@@H]4[C@@H]5CCCC[C@]5(CCN4CC4CC4)c3c2)cc1 |r| Show InChI InChI=1S/C27H34N2O/c1-30-23-11-9-21(10-12-23)28-22-8-7-20-16-26-24-4-2-3-13-27(24,25(20)17-22)14-15-29(26)18-19-5-6-19/h7-12,17,19,24,26,28H,2-6,13-16,18H2,1H3/t24-,26+,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cells |
J Med Chem 53: 402-18 (2010)
Article DOI: 10.1021/jm9013482
BindingDB Entry DOI: 10.7270/Q2668D84 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM364552

(N-((4bR,6S,8aS,9R)-11- (cyclopropylmethyl)- 3-meth...)Show SMILES COc1ccc2C[C@H]3N(CC4CC4)CC[C@@]4(CC5(CC[C@@]34OC5)NC(=O)c3ccccc3)c2c1 |r,TLB:9:8:20:32.5.6| Show InChI InChI=1S/C29H34N2O3/c1-33-23-10-9-22-15-25-29-12-11-27(19-34-29,30-26(32)21-5-3-2-4-6-21)18-28(29,24(22)16-23)13-14-31(25)17-20-7-8-20/h2-6,9-10,16,20,25H,7-8,11-15,17-19H2,1H3,(H,30,32)/t25-,27?,28-,29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF
| Assay Description
Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb... |
J Med Chem 50: 4255-9 (2007)
BindingDB Entry DOI: 10.7270/Q2222X2R |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50174573

(CHEMBL3808477)Show SMILES Clc1ccc2C(=O)CC(C(=O)N3CCc4ccccc4C3CN3CCCC3)c2c1 Show InChI InChI=1S/C24H25ClN2O2/c25-17-7-8-19-20(13-17)21(14-23(19)28)24(29)27-12-9-16-5-1-2-6-18(16)22(27)15-26-10-3-4-11-26/h1-2,5-8,13,21-22H,3-4,9-12,14-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes after 30 mins by liquid scintillation counting ana... |
Bioorg Med Chem 24: 2964-2970 (2016)
Article DOI: 10.1016/j.bmc.2016.05.002
BindingDB Entry DOI: 10.7270/Q2P84DTT |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50430593
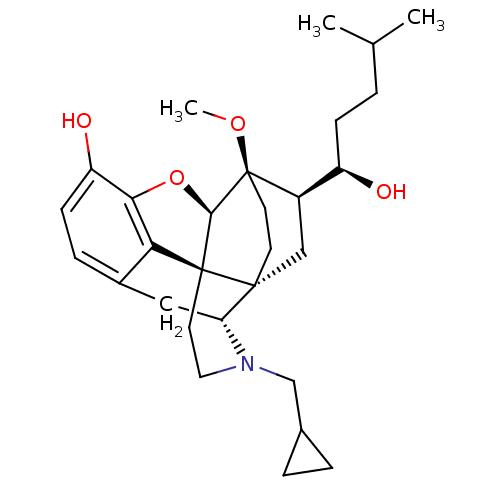
(CHEMBL2338739)Show SMILES CO[C@]12CC[C@@]3(C[C@@H]1[C@H](O)CCC(C)C)[C@H]1Cc4ccc(O)c5O[C@@H]2[C@]3(CCN1CC1CC1)c45 |r,THB:8:7:25.24:4.3| Show InChI InChI=1S/C29H41NO4/c1-17(2)4-8-21(31)20-15-27-10-11-29(20,33-3)26-28(27)12-13-30(16-18-5-6-18)23(27)14-19-7-9-22(32)25(34-26)24(19)28/h7,9,17-18,20-21,23,26,31-32H,4-6,8,10-16H2,1-3H3/t20-,21-,23-,26-,27-,28+,29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human KOR expressed in CHO cells |
J Med Chem 56: 3207-16 (2013)
Article DOI: 10.1021/jm301543e
BindingDB Entry DOI: 10.7270/Q28G8N2Q |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50135800

((-)-3-Hydroxy-N-cycloproypylmethylmorphinan Mandel...)Show SMILES Oc1ccc2C[C@@H]3[C@@H]4CCCC[C@]4(CCN3CC3CC3)c2c1 Show InChI InChI=1S/C20H27NO/c22-16-7-6-15-11-19-17-3-1-2-8-20(17,18(15)12-16)9-10-21(19)13-14-4-5-14/h6-7,12,14,17,19,22H,1-5,8-11,13H2/t17-,19+,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69593 from human kappa opioid receptors expressed in CHO cell membrane |
Bioorg Med Chem Lett 17: 1508-11 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.013
BindingDB Entry DOI: 10.7270/Q2WS8V2W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50135800

((-)-3-Hydroxy-N-cycloproypylmethylmorphinan Mandel...)Show SMILES Oc1ccc2C[C@@H]3[C@@H]4CCCC[C@]4(CCN3CC3CC3)c2c1 Show InChI InChI=1S/C20H27NO/c22-16-7-6-15-11-19-17-3-1-2-8-20(17,18(15)12-16)9-10-21(19)13-14-4-5-14/h6-7,12,14,17,19,22H,1-5,8-11,13H2/t17-,19+,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Displacement of [3H]-U69,593 from human kappa opioid receptor expressed in CHO cells after 60 mins by scintillation counting |
J Med Chem 55: 3878-90 (2012)
Article DOI: 10.1021/jm3001086
BindingDB Entry DOI: 10.7270/Q28053P6 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105483
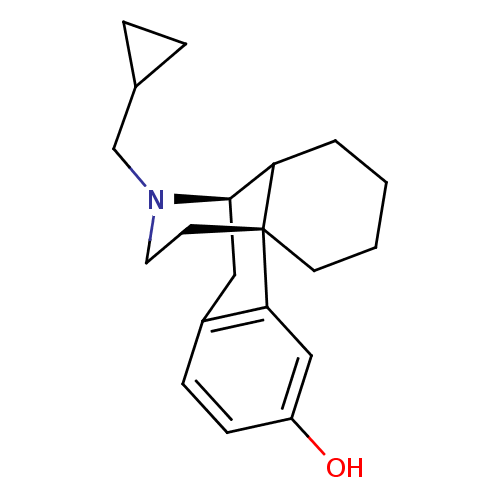
((-)-cyclorphan | 17-cyclopropylmethyl-(1R,9R)-17-a...)Show SMILES Oc1ccc2C[C@@H]3C4CCCC[C@]4(CCN3CC3CC3)c2c1 |TLB:8:7:20.4.5:15.13.14| Show InChI InChI=1S/C20H27NO/c22-16-7-6-15-11-19-17-3-1-2-8-20(17,18(15)12-16)9-10-21(19)13-14-4-5-14/h6-7,12,14,17,19,22H,1-5,8-11,13H2/t17?,19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Agonistic activity against kappa opioid receptor in Chinese hamster ovary membranes |
J Med Chem 47: 1886-8 (2004)
Article DOI: 10.1021/jm049978n
BindingDB Entry DOI: 10.7270/Q2N58N4X |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50135800

((-)-3-Hydroxy-N-cycloproypylmethylmorphinan Mandel...)Show SMILES Oc1ccc2C[C@@H]3[C@@H]4CCCC[C@]4(CCN3CC3CC3)c2c1 Show InChI InChI=1S/C20H27NO/c22-16-7-6-15-11-19-17-3-1-2-8-20(17,18(15)12-16)9-10-21(19)13-14-4-5-14/h6-7,12,14,17,19,22H,1-5,8-11,13H2/t17-,19+,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cells after 60 mins by scintillation counting |
J Med Chem 54: 1903-13 (2011)
Article DOI: 10.1021/jm101542c
BindingDB Entry DOI: 10.7270/Q2028SJ1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105483

((-)-cyclorphan | 17-cyclopropylmethyl-(1R,9R)-17-a...)Show SMILES Oc1ccc2C[C@@H]3C4CCCC[C@]4(CCN3CC3CC3)c2c1 |TLB:8:7:20.4.5:15.13.14| Show InChI InChI=1S/C20H27NO/c22-16-7-6-15-11-19-17-3-1-2-8-20(17,18(15)12-16)9-10-21(19)13-14-4-5-14/h6-7,12,14,17,19,22H,1-5,8-11,13H2/t17?,19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cells |
J Med Chem 49: 256-62 (2006)
Article DOI: 10.1021/jm050577x
BindingDB Entry DOI: 10.7270/Q2HQ40QF |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50135800

((-)-3-Hydroxy-N-cycloproypylmethylmorphinan Mandel...)Show SMILES Oc1ccc2C[C@@H]3[C@@H]4CCCC[C@]4(CCN3CC3CC3)c2c1 Show InChI InChI=1S/C20H27NO/c22-16-7-6-15-11-19-17-3-1-2-8-20(17,18(15)12-16)9-10-21(19)13-14-4-5-14/h6-7,12,14,17,19,22H,1-5,8-11,13H2/t17-,19+,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cells |
J Med Chem 53: 402-18 (2010)
Article DOI: 10.1021/jm9013482
BindingDB Entry DOI: 10.7270/Q2668D84 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50135800

((-)-3-Hydroxy-N-cycloproypylmethylmorphinan Mandel...)Show SMILES Oc1ccc2C[C@@H]3[C@@H]4CCCC[C@]4(CCN3CC3CC3)c2c1 Show InChI InChI=1S/C20H27NO/c22-16-7-6-15-11-19-17-3-1-2-8-20(17,18(15)12-16)9-10-21(19)13-14-4-5-14/h6-7,12,14,17,19,22H,1-5,8-11,13H2/t17-,19+,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Opioid receptor kappa 1 in chinese Hamster Ovary (CHO) cell membranes was determined using [3H]U-69593 radioligand |
J Med Chem 46: 5162-70 (2003)
Article DOI: 10.1021/jm030139v
BindingDB Entry DOI: 10.7270/Q2GX4C9C |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50135800

((-)-3-Hydroxy-N-cycloproypylmethylmorphinan Mandel...)Show SMILES Oc1ccc2C[C@@H]3[C@@H]4CCCC[C@]4(CCN3CC3CC3)c2c1 Show InChI InChI=1S/C20H27NO/c22-16-7-6-15-11-19-17-3-1-2-8-20(17,18(15)12-16)9-10-21(19)13-14-4-5-14/h6-7,12,14,17,19,22H,1-5,8-11,13H2/t17-,19+,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO membrane |
Bioorg Med Chem 15: 4106-12 (2007)
Article DOI: 10.1016/j.bmc.2007.03.076
BindingDB Entry DOI: 10.7270/Q2D50MN1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50596293
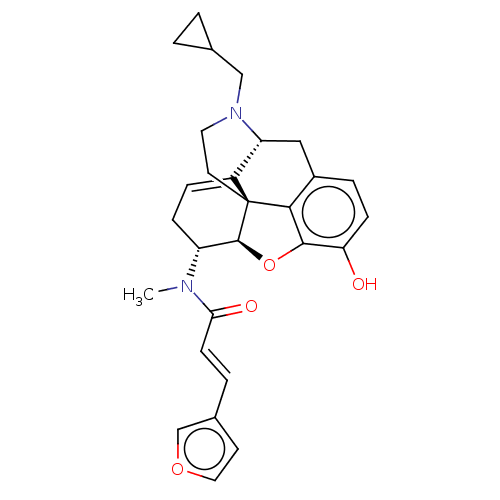
(CHEMBL5188658)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14C5=CC[C@H]2N(C)C(=O)\C=C\c1ccoc1)ccc3O |r,t:21,THB:10:9:17:4.5.6| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0356 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128527
BindingDB Entry DOI: 10.7270/Q2HT2TDM |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM82551

(C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)c1[nH]c2[C@@H]4Oc6c7c(C[C@H]8N(CC9CC9)CC[C@@]47[C@@]8(O)Cc2c1C[C@@]35O)ccc6O |r| Show InChI InChI=1S/C40H43N3O6/c44-25-7-5-21-13-27-39(46)15-23-24-16-40(47)28-14-22-6-8-26(45)34-30(22)38(40,10-12-43(28)18-20-3-4-20)36(49-34)32(24)41-31(23)35-37(39,29(21)33(25)48-35)9-11-42(27)17-19-1-2-19/h5-8,19-20,27-28,35-36,41,44-47H,1-4,9-18H2/t27-,28-,35+,36+,37+,38+,39-,40-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity by displacing [3H]U-69593 to human cloned Kappa opioid receptor transfected into CHO cells using [35S... |
Bioorg Med Chem Lett 10: 2259-61 (2001)
BindingDB Entry DOI: 10.7270/Q2WS8TRF |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50240954
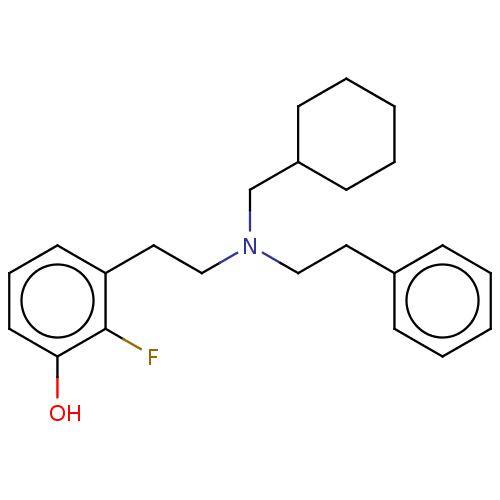
(CHEMBL4101576)Show InChI InChI=1S/C23H30FNO/c24-23-21(12-7-13-22(23)26)15-17-25(18-20-10-5-2-6-11-20)16-14-19-8-3-1-4-9-19/h1,3-4,7-9,12-13,20,26H,2,5-6,10-11,14-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membranes after 60 mins by liquid scintillation counting method |
J Med Chem 60: 7579-7590 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00981
BindingDB Entry DOI: 10.7270/Q21C202C |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM3916
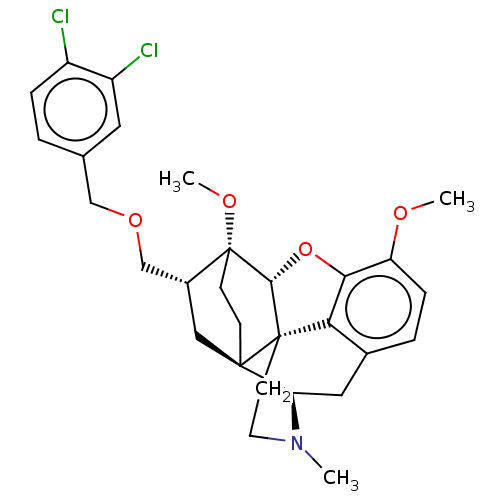
(US8530494, 206)Show SMILES COc1ccc2C[C@H]3N(C)CC[C@@]45[C@@H](Oc1c24)[C@]1(CC[C@@]35C[C@@H]1COCc1ccc(Cl)c(Cl)c1)OC |r,TLB:23:22:12.13:19.18| Show InChI InChI=1S/C29H33Cl2NO4/c1-32-11-10-28-24-18-5-7-22(33-2)25(24)36-26(28)29(34-3)9-8-27(28,23(32)13-18)14-19(29)16-35-15-17-4-6-20(30)21(31)12-17/h4-7,12,19,23,26H,8-11,13-16H2,1-3H3/t19-,23-,26-,27-,28+,29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
US Patent
| Assay Description
Binding assay of certain compounds of this invention to the opioid receptor was determine using radioligand binding assay (screening and dose-displac... |
US Patent US8530494 (2013)
BindingDB Entry DOI: 10.7270/Q2JS9P3J |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50026603

(Buprenorphine | CHEBI:3216)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@]([H])(C1)[C@](C)(O)C(C)(C)C)ccc3O |r,TLB:25:17:4.5.6:9.15.14,18:17:4.5.6:9.15.14,THB:10:9:17:4.5.6,3:4:17:9.15.14,26:23:16.1:18.19| Show InChI InChI=1S/C29H41NO4/c1-25(2,3)26(4,32)20-15-27-10-11-29(20,33-5)24-28(27)12-13-30(16-17-6-7-17)21(27)14-18-8-9-19(31)23(34-24)22(18)28/h8-9,17,20-21,24,31-32H,6-7,10-16H2,1-5H3/t20-,21-,24-,26+,27-,28+,29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology
Curated by ChEMBL
| Assay Description
Antagonist activity at kappa opioid receptor (unknown origin) assessed as stimulation of [35S]GTPgammaS |
Eur J Med Chem 141: 632-647 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.012
BindingDB Entry DOI: 10.7270/Q2V98BQ2 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50090760
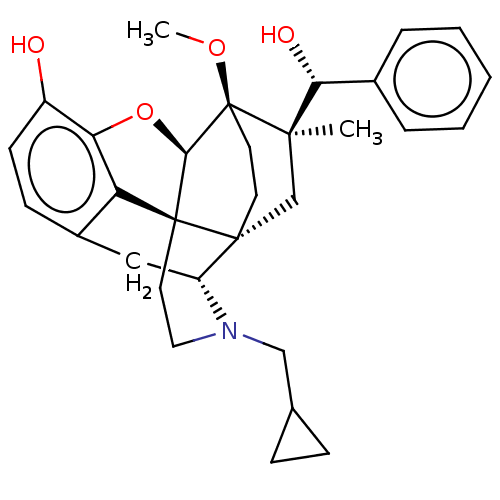
(CHEMBL3581750 | US9259422, 30, R = Ph-BU10119 | US...)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(C1)[C@H](O)c1ccccc1)ccc3O |r,TLB:26:23:16.1:18.19,THB:10:9:17:4.5.6,24:23:16.1:18.19| Show InChI InChI=1S/C31H37NO4/c1-28(26(34)20-6-4-3-5-7-20)18-29-12-13-31(28,35-2)27-30(29)14-15-32(17-19-8-9-19)23(29)16-21-10-11-22(33)25(36-27)24(21)30/h3-7,10-11,19,23,26-27,33-34H,8-9,12-18H2,1-2H3/t23-,26-,27-,28-,29-,30+,31+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of Bath
US Patent
| Assay Description
As Alt et al., 2002. Membranes (20 μg) are incubated in 50 mM Tris-HCl, pH 7.4 with [3H]diprenorphine or [3H]nociceptin in the absence or presen... |
US Patent US9480684 (2016)
BindingDB Entry DOI: 10.7270/Q29885ZT |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50090731
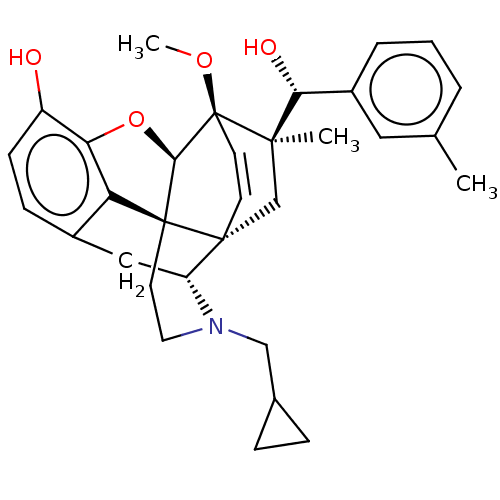
(CHEMBL3581741 | US9259422, 22, R = 3-MePh- BU10112...)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@]51C[C@](C)([C@H](O)c4cccc(C)c4)[C@]2(OC)C=C1)ccc3O |r,wU:17.21,16.16,19.23,1.0,wD:30.36,21.25,7.7,c:39,TLB:21:19:16.1:34.33,THB:10:9:17:4.5.6,20:19:16.1:34.33,(-3.17,.26,;-2.2,.57,;-3.38,2.21,;-2.2,3.8,;-.81,2.99,;.55,3.8,;1.94,2.99,;1.94,1.4,;2.83,1.9,;3.33,.57,;4.33,-.58,;5.84,-.3,;7.2,-.82,;6.92,.69,;2.73,2.15,;.51,2.18,;-.81,1.4,;.55,.57,;.55,-.99,;-.81,-1.79,;-1.89,-2.38,;.53,-2.51,;1.56,-1.84,;.61,-4.05,;1.98,-4.74,;2.06,-6.28,;.77,-7.12,;-.6,-6.42,;-1.63,-7.1,;-.68,-4.89,;-2.2,-.99,;-3.6,-1.56,;-4.57,-.8,;-.88,-.72,;-.88,.28,;.55,5.4,;-.81,6.2,;-2.2,5.4,;-3.26,6.01,)| Show InChI InChI=1S/C55H75N17O13/c1-29(2)19-38(49(80)67-37(9-5-17-60-55(57)58)54(85)72-18-6-10-43(72)53(84)62-25-44(56)75)66-46(77)26-63-47(78)39(20-30-11-13-33(74)14-12-30)68-52(83)42(27-73)71-50(81)40(21-31-23-61-35-8-4-3-7-34(31)35)69-51(82)41(22-32-24-59-28-64-32)70-48(79)36-15-16-45(76)65-36/h3-4,7-8,11-14,23-24,28-29,36-43,61,73-74H,5-6,9-10,15-22,25-27H2,1-2H3,(H2,56,75)(H,59,64)(H,62,84)(H,63,78)(H,65,76)(H,66,77)(H,67,80)(H,68,83)(H,69,82)(H,70,79)(H,71,81)(H4,57,58,60)/t36?,37-,38-,39-,40-,41-,42-,43+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of Bath
US Patent
| Assay Description
As Alt et al., 2002. Membranes (20 μg) are incubated in 50 mM Tris-HCl, pH 7.4 with [3H]diprenorphine or [3H]nociceptin in the absence or presen... |
US Patent US9480684 (2016)
BindingDB Entry DOI: 10.7270/Q29885ZT |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50354578

(BUPRENORPHINE | US10752592, Compound buprenorphine...)Show SMILES CO[C@@]12CC[C@@]3(C[C@@H]1[C@](C)(O)C(C)(C)C)[C@H]1Cc4ccc(O)c5O[C@@H]2[C@]3(CCN1CC1CC1)c45 |r| Show InChI InChI=1S/C29H41NO4/c1-25(2,3)26(4,32)20-15-27-10-11-29(20,33-5)24-28(27)12-13-30(16-17-6-7-17)21(27)14-18-8-9-19(31)23(34-24)22(18)28/h8-9,17,20-21,24,31-32H,6-7,10-16H2,1-5H3/t20-,21-,24-,26+,27-,28+,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| 0.0400 | -59.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Rhodes Technologies
US Patent
| Assay Description
Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recombin... |
US Patent US9315514 (2016)
BindingDB Entry DOI: 10.7270/Q2KW5DWC |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50090760

(CHEMBL3581750 | US9259422, 30, R = Ph-BU10119 | US...)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(C1)[C@H](O)c1ccccc1)ccc3O |r,TLB:26:23:16.1:18.19,THB:10:9:17:4.5.6,24:23:16.1:18.19| Show InChI InChI=1S/C31H37NO4/c1-28(26(34)20-6-4-3-5-7-20)18-29-12-13-31(28,35-2)27-30(29)14-15-32(17-19-8-9-19)23(29)16-21-10-11-22(33)25(36-27)24(21)30/h3-7,10-11,19,23,26-27,33-34H,8-9,12-18H2,1-2H3/t23-,26-,27-,28-,29-,30+,31+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0400 | -59.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
The University of Bath
US Patent
| Assay Description
As Alt et al., 2002. Membranes (20 ug) are incubated in 50 mM Tris-HCl,
pH 7.4 with [3H]diprenorphine or [3H]nociceptin in the absence or
presence ... |
US Patent US9259422 (2016)
BindingDB Entry DOI: 10.7270/Q2DJ5DG0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50090731

(CHEMBL3581741 | US9259422, 22, R = 3-MePh- BU10112...)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@]51C[C@](C)([C@H](O)c4cccc(C)c4)[C@]2(OC)C=C1)ccc3O |r,wU:17.21,16.16,19.23,1.0,wD:30.36,21.25,7.7,c:39,TLB:21:19:16.1:34.33,THB:10:9:17:4.5.6,20:19:16.1:34.33,(-3.17,.26,;-2.2,.57,;-3.38,2.21,;-2.2,3.8,;-.81,2.99,;.55,3.8,;1.94,2.99,;1.94,1.4,;2.83,1.9,;3.33,.57,;4.33,-.58,;5.84,-.3,;7.2,-.82,;6.92,.69,;2.73,2.15,;.51,2.18,;-.81,1.4,;.55,.57,;.55,-.99,;-.81,-1.79,;-1.89,-2.38,;.53,-2.51,;1.56,-1.84,;.61,-4.05,;1.98,-4.74,;2.06,-6.28,;.77,-7.12,;-.6,-6.42,;-1.63,-7.1,;-.68,-4.89,;-2.2,-.99,;-3.6,-1.56,;-4.57,-.8,;-.88,-.72,;-.88,.28,;.55,5.4,;-.81,6.2,;-2.2,5.4,;-3.26,6.01,)| Show InChI InChI=1S/C55H75N17O13/c1-29(2)19-38(49(80)67-37(9-5-17-60-55(57)58)54(85)72-18-6-10-43(72)53(84)62-25-44(56)75)66-46(77)26-63-47(78)39(20-30-11-13-33(74)14-12-30)68-52(83)42(27-73)71-50(81)40(21-31-23-61-35-8-4-3-7-34(31)35)69-51(82)41(22-32-24-59-28-64-32)70-48(79)36-15-16-45(76)65-36/h3-4,7-8,11-14,23-24,28-29,36-43,61,73-74H,5-6,9-10,15-22,25-27H2,1-2H3,(H2,56,75)(H,59,64)(H,62,84)(H,63,78)(H,65,76)(H,66,77)(H,67,80)(H,68,83)(H,69,82)(H,70,79)(H,71,81)(H4,57,58,60)/t36?,37-,38-,39-,40-,41-,42-,43+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0400 | -59.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
The University of Bath
US Patent
| Assay Description
As Alt et al., 2002. Membranes (20 ug) are incubated in 50 mM Tris-HCl,
pH 7.4 with [3H]diprenorphine or [3H]nociceptin in the absence or
presence ... |
US Patent US9259422 (2016)
BindingDB Entry DOI: 10.7270/Q2DJ5DG0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM199363
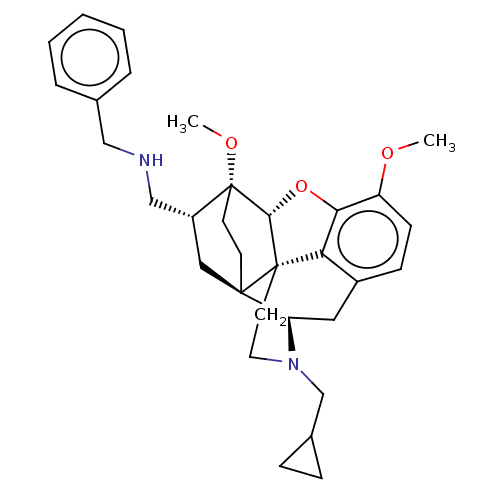
(US9221831, 28)Show SMILES COc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)[C@]1(CC[C@@]35C[C@@H]1CNCc1ccccc1)OC |r,THB:26:25:15.16:22.21| Show InChI InChI=1S/C32H40N2O3/c1-35-25-11-10-23-16-26-30-12-13-32(36-2,24(17-30)19-33-18-21-6-4-3-5-7-21)29-31(30,27(23)28(25)37-29)14-15-34(26)20-22-8-9-22/h3-7,10-11,22,24,26,29,33H,8-9,12-20H2,1-2H3/t24-,26-,29-,30-,31+,32-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Purdue Pharma, L.P.
US Patent
| Assay Description
Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb... |
US Patent US9221831 (2015)
BindingDB Entry DOI: 10.7270/Q2T72G8M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data