

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50018175 (CHEMBL22389 | N-{1-Benzyl-2-[(pyridine-3-carbonyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
Amherst College Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-catalyzed hydrolysis of BzArg p-nitroanilide by compound at pH 5 | J Med Chem 32: 1253-9 (1989) BindingDB Entry DOI: 10.7270/Q2K64H2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50018174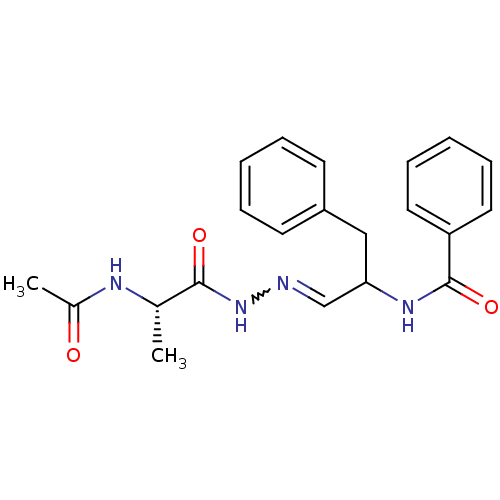 (CHEMBL283109 | N-{2-[(2-Acetylamino-propionyl)-hyd...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
Amherst College Curated by ChEMBL | Assay Description Inhibition of papain-catalyzed hydrolysis of BzArg p-nitroanilide by compound at pH 5.5 | J Med Chem 32: 1253-9 (1989) BindingDB Entry DOI: 10.7270/Q2K64H2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50018175 (CHEMBL22389 | N-{1-Benzyl-2-[(pyridine-3-carbonyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
Amherst College Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-catalyzed hydrolysis of BzArg p-nitroanilide by compound at pH 7.8 | J Med Chem 32: 1253-9 (1989) BindingDB Entry DOI: 10.7270/Q2K64H2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50018173 (CHEMBL22745 | N-{2-[(2-Acetylamino-propionyl)-hydr...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | >8.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
Amherst College Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-catalyzed hydrolysis of BzArg p-nitroanilide by compound at pH 5 | J Med Chem 32: 1253-9 (1989) BindingDB Entry DOI: 10.7270/Q2K64H2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50018174 (CHEMBL283109 | N-{2-[(2-Acetylamino-propionyl)-hyd...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Amherst College Curated by ChEMBL | Assay Description Initial rate of reaction between Chymotrypsinogen and BzArg p-nitro-anilide in the presence of 400 microM of the compound at pH 7 | J Med Chem 32: 1253-9 (1989) BindingDB Entry DOI: 10.7270/Q2K64H2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM113248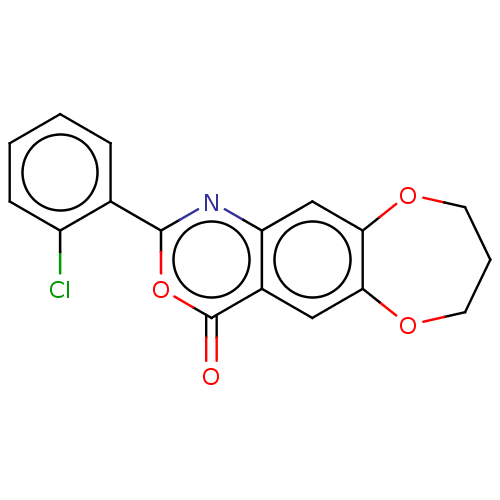 (US9695194, 7) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | <100 | <-41.6 | n/a | n/a | n/a | n/a | n/a | 7.2 | 37 |
SIXERA PHARMA AB US Patent | Assay Description Materials: Chymotrypsin, bovine, 25 ug (Roche, sequence grade), Substrate S-2586 (Chromogenics, cat. no. 82 08 94). Chymotrypsin activity was determi... | US Patent US9695194 (2017) BindingDB Entry DOI: 10.7270/Q2CN723D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM113283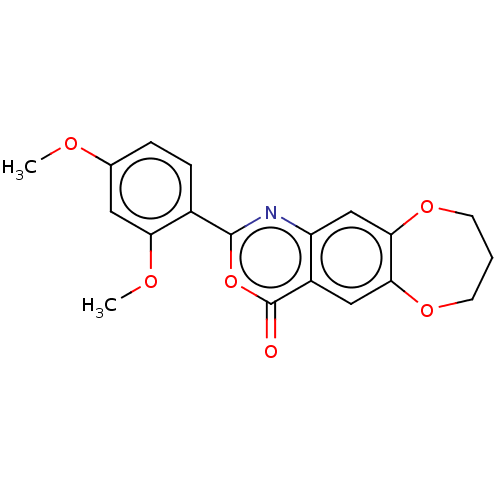 (US9695194, 8) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | <100 | <-41.6 | n/a | n/a | n/a | n/a | n/a | 7.2 | 37 |
SIXERA PHARMA AB US Patent | Assay Description Materials: Chymotrypsin, bovine, 25 ug (Roche, sequence grade), Substrate S-2586 (Chromogenics, cat. no. 82 08 94). Chymotrypsin activity was determi... | US Patent US9695194 (2017) BindingDB Entry DOI: 10.7270/Q2CN723D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM113296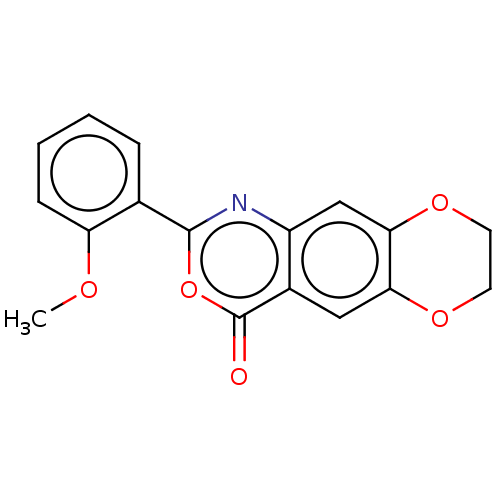 (US9695194, 13) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | <100 | <-41.6 | n/a | n/a | n/a | n/a | n/a | 7.2 | 37 |
SIXERA PHARMA AB US Patent | Assay Description Materials: Chymotrypsin, bovine, 25 ug (Roche, sequence grade), Substrate S-2586 (Chromogenics, cat. no. 82 08 94). Chymotrypsin activity was determi... | US Patent US9695194 (2017) BindingDB Entry DOI: 10.7270/Q2CN723D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM113119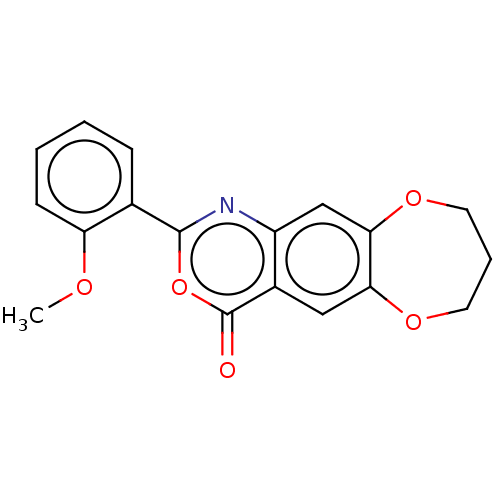 (US9695194, 6) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | <100 | <-41.6 | n/a | n/a | n/a | n/a | n/a | 7.2 | 37 |
SIXERA PHARMA AB US Patent | Assay Description Materials: Chymotrypsin, bovine, 25 ug (Roche, sequence grade), Substrate S-2586 (Chromogenics, cat. no. 82 08 94). Chymotrypsin activity was determi... | US Patent US9695194 (2017) BindingDB Entry DOI: 10.7270/Q2CN723D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM113297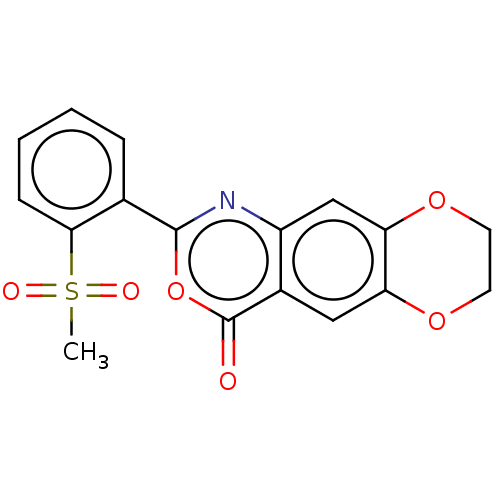 (US9695194, 14) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | <100 | <-41.6 | n/a | n/a | n/a | n/a | n/a | 7.2 | 37 |
SIXERA PHARMA AB US Patent | Assay Description Materials: Chymotrypsin, bovine, 25 ug (Roche, sequence grade), Substrate S-2586 (Chromogenics, cat. no. 82 08 94). Chymotrypsin activity was determi... | US Patent US9695194 (2017) BindingDB Entry DOI: 10.7270/Q2CN723D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM113300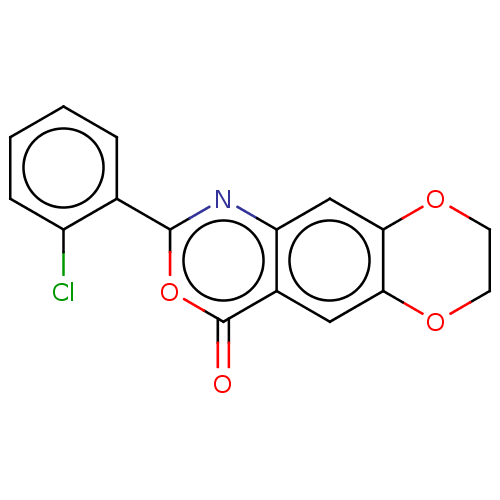 (US9695194, 16) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | <100 | <-41.6 | n/a | n/a | n/a | n/a | n/a | 7.2 | 37 |
SIXERA PHARMA AB US Patent | Assay Description Materials: Chymotrypsin, bovine, 25 ug (Roche, sequence grade), Substrate S-2586 (Chromogenics, cat. no. 82 08 94). Chymotrypsin activity was determi... | US Patent US9695194 (2017) BindingDB Entry DOI: 10.7270/Q2CN723D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM113299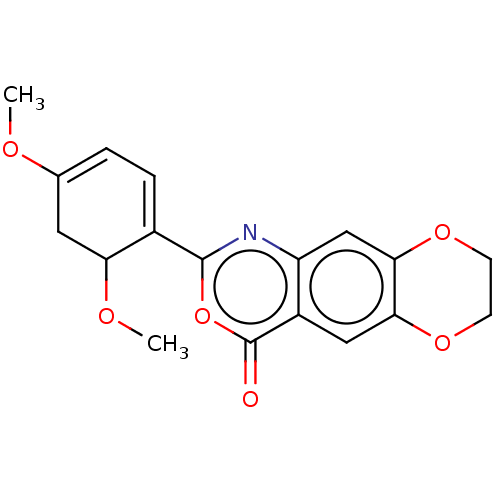 (US9695194, 15) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <100 | <-41.6 | n/a | n/a | n/a | n/a | n/a | 7.2 | 37 |
SIXERA PHARMA AB US Patent | Assay Description Materials: Chymotrypsin, bovine, 25 ug (Roche, sequence grade), Substrate S-2586 (Chromogenics, cat. no. 82 08 94). Chymotrypsin activity was determi... | US Patent US9695194 (2017) BindingDB Entry DOI: 10.7270/Q2CN723D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM113293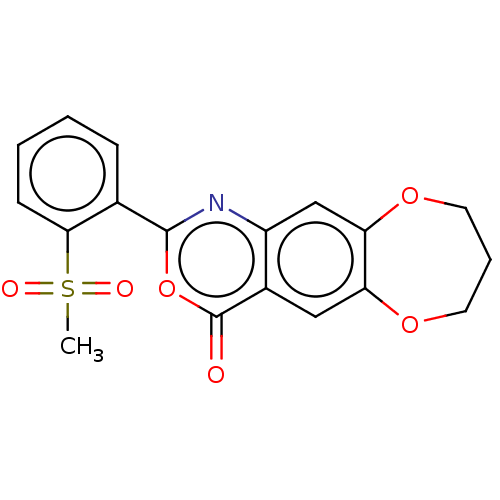 (US9695194, 10) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 200 | -39.8 | n/a | n/a | n/a | n/a | n/a | 7.2 | 37 |
SIXERA PHARMA AB US Patent | Assay Description Materials: Chymotrypsin, bovine, 25 ug (Roche, sequence grade), Substrate S-2586 (Chromogenics, cat. no. 82 08 94). Chymotrypsin activity was determi... | US Patent US9695194 (2017) BindingDB Entry DOI: 10.7270/Q2CN723D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM113292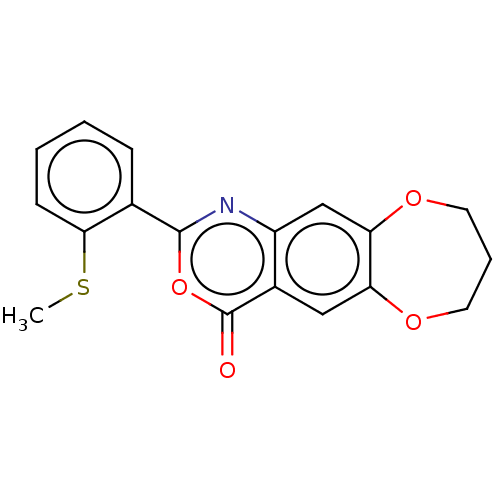 (US9695194, 9) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 400 | -38.0 | n/a | n/a | n/a | n/a | n/a | 7.2 | 37 |
SIXERA PHARMA AB US Patent | Assay Description Materials: Chymotrypsin, bovine, 25 ug (Roche, sequence grade), Substrate S-2586 (Chromogenics, cat. no. 82 08 94). Chymotrypsin activity was determi... | US Patent US9695194 (2017) BindingDB Entry DOI: 10.7270/Q2CN723D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM113295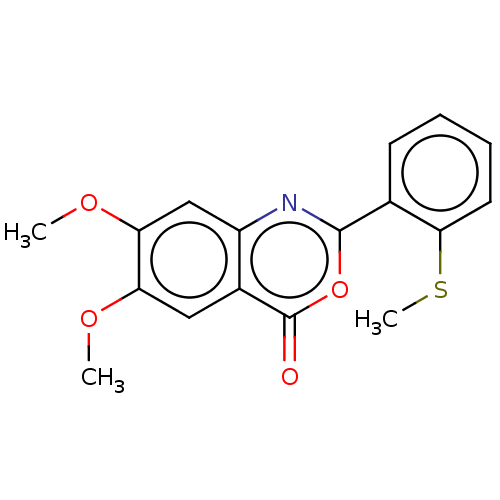 (US9695194, 12) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 600 | -36.9 | n/a | n/a | n/a | n/a | n/a | 7.2 | 37 |
SIXERA PHARMA AB US Patent | Assay Description Materials: Chymotrypsin, bovine, 25 ug (Roche, sequence grade), Substrate S-2586 (Chromogenics, cat. no. 82 08 94). Chymotrypsin activity was determi... | US Patent US9695194 (2017) BindingDB Entry DOI: 10.7270/Q2CN723D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM113294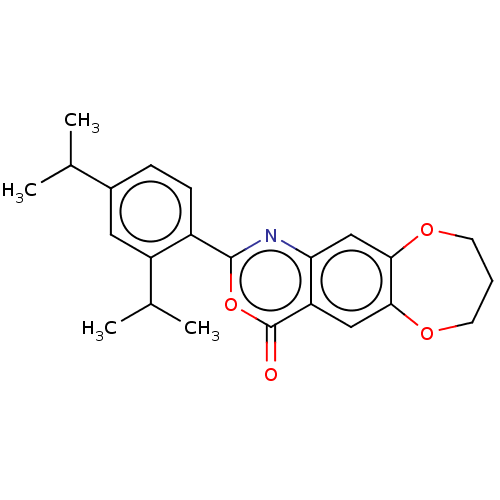 (US9695194, 11) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 1.00E+3 | -35.6 | n/a | n/a | n/a | n/a | n/a | 7.2 | 37 |
SIXERA PHARMA AB US Patent | Assay Description Materials: Chymotrypsin, bovine, 25 ug (Roche, sequence grade), Substrate S-2586 (Chromogenics, cat. no. 82 08 94). Chymotrypsin activity was determi... | US Patent US9695194 (2017) BindingDB Entry DOI: 10.7270/Q2CN723D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM113301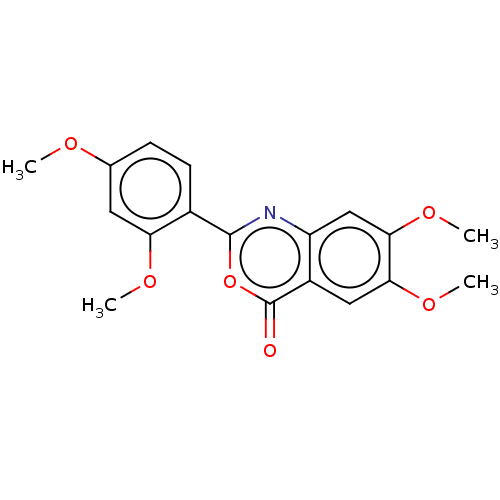 (US9695194, 17) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 1.20E+3 | -35.2 | n/a | n/a | n/a | n/a | n/a | 7.2 | 37 |
SIXERA PHARMA AB US Patent | Assay Description Materials: Chymotrypsin, bovine, 25 ug (Roche, sequence grade), Substrate S-2586 (Chromogenics, cat. no. 82 08 94). Chymotrypsin activity was determi... | US Patent US9695194 (2017) BindingDB Entry DOI: 10.7270/Q2CN723D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50289001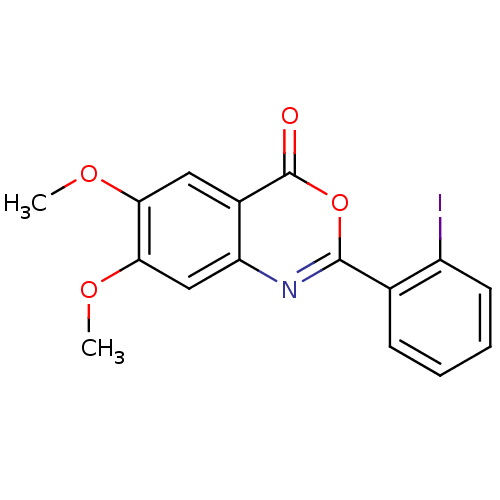 (2-(2-Iodo-phenyl)-6,7-dimethoxy-benzo[d][1,3]oxazi...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | >1.00E+4 | >-29.7 | n/a | n/a | n/a | n/a | n/a | 7.2 | 37 |
SIXERA PHARMA AB US Patent | Assay Description Materials: Chymotrypsin, bovine, 25 ug (Roche, sequence grade), Substrate S-2586 (Chromogenics, cat. no. 82 08 94). Chymotrypsin activity was determi... | US Patent US9695194 (2017) BindingDB Entry DOI: 10.7270/Q2CN723D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM113069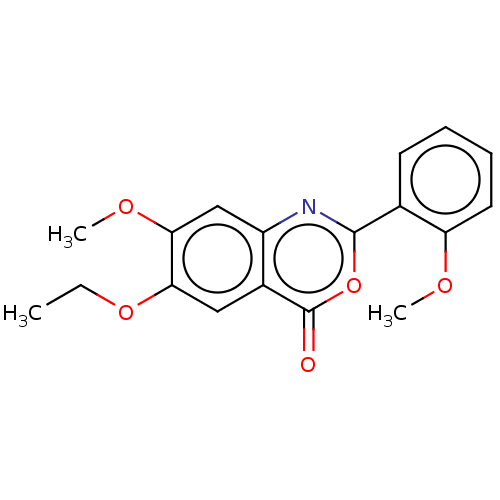 (US9695194, 5) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | >1.00E+4 | >-29.7 | n/a | n/a | n/a | n/a | n/a | 7.2 | 37 |
SIXERA PHARMA AB US Patent | Assay Description Materials: Chymotrypsin, bovine, 25 ug (Roche, sequence grade), Substrate S-2586 (Chromogenics, cat. no. 82 08 94). Chymotrypsin activity was determi... | US Patent US9695194 (2017) BindingDB Entry DOI: 10.7270/Q2CN723D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM113068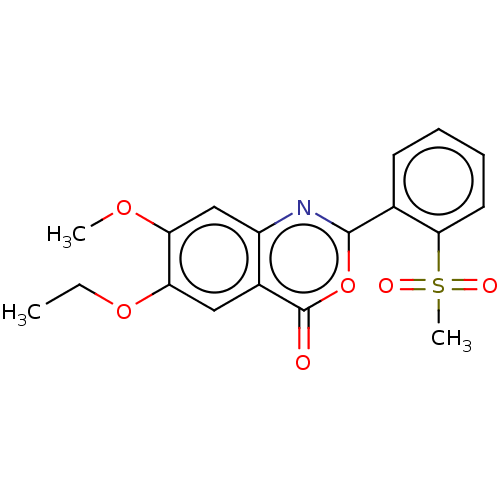 (US9695194, 4) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | >1.00E+4 | >-29.7 | n/a | n/a | n/a | n/a | n/a | 7.2 | 37 |
SIXERA PHARMA AB US Patent | Assay Description Materials: Chymotrypsin, bovine, 25 ug (Roche, sequence grade), Substrate S-2586 (Chromogenics, cat. no. 82 08 94). Chymotrypsin activity was determi... | US Patent US9695194 (2017) BindingDB Entry DOI: 10.7270/Q2CN723D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM113067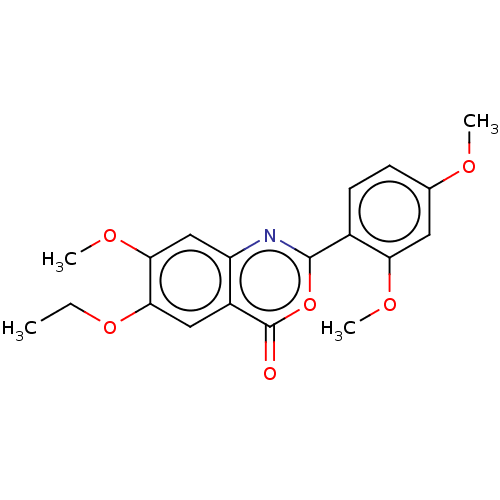 (US9695194, 3) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | >1.00E+4 | >-29.7 | n/a | n/a | n/a | n/a | n/a | 7.2 | 37 |
SIXERA PHARMA AB US Patent | Assay Description Materials: Chymotrypsin, bovine, 25 ug (Roche, sequence grade), Substrate S-2586 (Chromogenics, cat. no. 82 08 94). Chymotrypsin activity was determi... | US Patent US9695194 (2017) BindingDB Entry DOI: 10.7270/Q2CN723D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM113060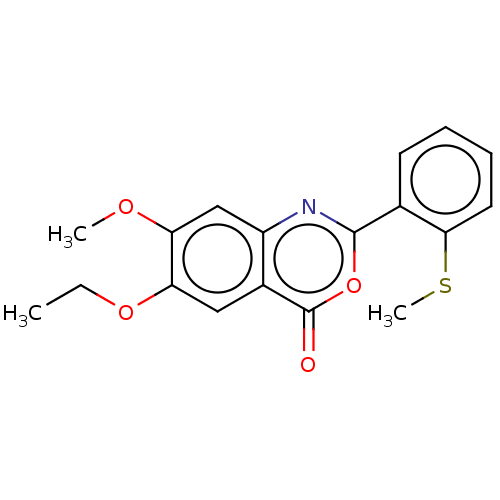 (US9695194, 1) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | >1.00E+4 | >-29.7 | n/a | n/a | n/a | n/a | n/a | 7.2 | 37 |
SIXERA PHARMA AB US Patent | Assay Description Materials: Chymotrypsin, bovine, 25 ug (Roche, sequence grade), Substrate S-2586 (Chromogenics, cat. no. 82 08 94). Chymotrypsin activity was determi... | US Patent US9695194 (2017) BindingDB Entry DOI: 10.7270/Q2CN723D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM113065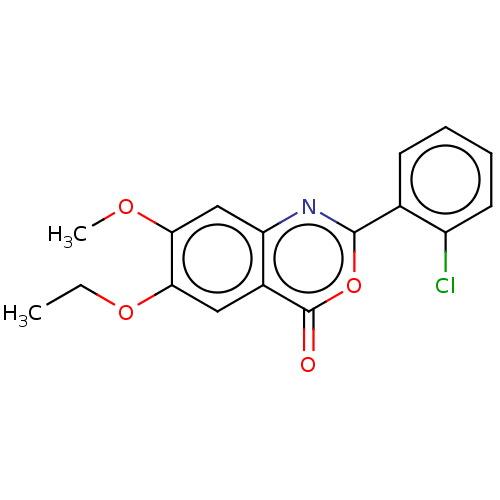 (US9695194, 2) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | >1.00E+4 | >-29.7 | n/a | n/a | n/a | n/a | n/a | 7.2 | 37 |
SIXERA PHARMA AB US Patent | Assay Description Materials: Chymotrypsin, bovine, 25 ug (Roche, sequence grade), Substrate S-2586 (Chromogenics, cat. no. 82 08 94). Chymotrypsin activity was determi... | US Patent US9695194 (2017) BindingDB Entry DOI: 10.7270/Q2CN723D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM105233 (US8580800, 31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 28 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US8580800 (2013) BindingDB Entry DOI: 10.7270/Q2WM1C18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM166433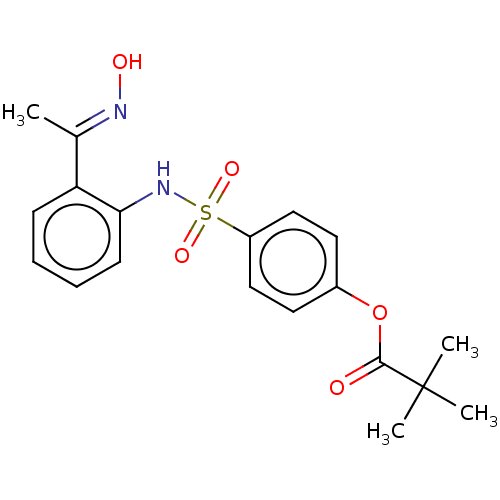 (US9073833, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.13E+4 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Chang Gung University US Patent | Assay Description Each of Compounds (1) to (7) was added into 50 μL buffer A (containing 20 mM Tris-HCl (pH 7.4), 0.1% NaN3 and 5 mM CaCl2) to obtain compound sol... | US Patent US9073833 (2015) BindingDB Entry DOI: 10.7270/Q2TT4PQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM166434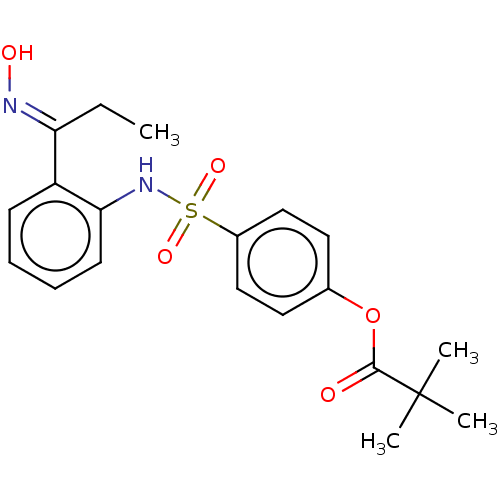 (US9073833, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.79E+5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Chang Gung University US Patent | Assay Description Each of Compounds (1) to (7) was added into 50 μL buffer A (containing 20 mM Tris-HCl (pH 7.4), 0.1% NaN3 and 5 mM CaCl2) to obtain compound sol... | US Patent US9073833 (2015) BindingDB Entry DOI: 10.7270/Q2TT4PQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM166435 (US9073833, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.68E+5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Chang Gung University US Patent | Assay Description Each of Compounds (1) to (7) was added into 50 μL buffer A (containing 20 mM Tris-HCl (pH 7.4), 0.1% NaN3 and 5 mM CaCl2) to obtain compound sol... | US Patent US9073833 (2015) BindingDB Entry DOI: 10.7270/Q2TT4PQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM166436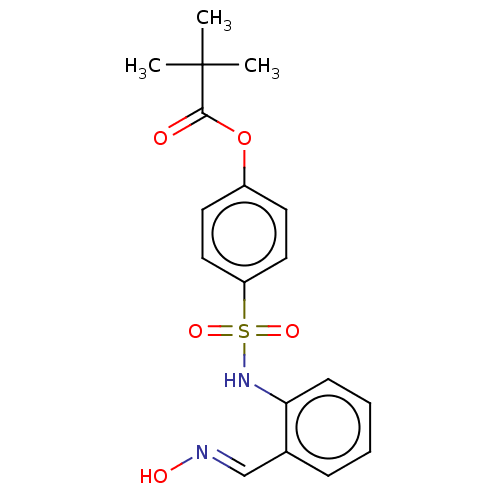 (US9073833, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.94E+5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Chang Gung University US Patent | Assay Description Each of Compounds (1) to (7) was added into 50 μL buffer A (containing 20 mM Tris-HCl (pH 7.4), 0.1% NaN3 and 5 mM CaCl2) to obtain compound sol... | US Patent US9073833 (2015) BindingDB Entry DOI: 10.7270/Q2TT4PQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM166437 (US9073833, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.24E+5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Chang Gung University US Patent | Assay Description Each of Compounds (1) to (7) was added into 50 μL buffer A (containing 20 mM Tris-HCl (pH 7.4), 0.1% NaN3 and 5 mM CaCl2) to obtain compound sol... | US Patent US9073833 (2015) BindingDB Entry DOI: 10.7270/Q2TT4PQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM166438 (US9073833, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.67E+5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Chang Gung University US Patent | Assay Description Each of Compounds (1) to (7) was added into 50 μL buffer A (containing 20 mM Tris-HCl (pH 7.4), 0.1% NaN3 and 5 mM CaCl2) to obtain compound sol... | US Patent US9073833 (2015) BindingDB Entry DOI: 10.7270/Q2TT4PQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM166440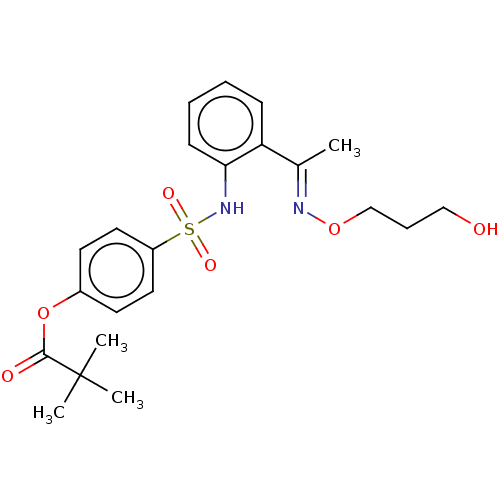 (US9073833, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.59E+5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Chang Gung University US Patent | Assay Description Each of Compounds (1) to (7) was added into 50 μL buffer A (containing 20 mM Tris-HCl (pH 7.4), 0.1% NaN3 and 5 mM CaCl2) to obtain compound sol... | US Patent US9073833 (2015) BindingDB Entry DOI: 10.7270/Q2TT4PQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM166439 (US9073833, Sivelestat) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Chang Gung University US Patent | Assay Description Each of Compounds (1) to (7) was added into 50 μL buffer A (containing 20 mM Tris-HCl (pH 7.4), 0.1% NaN3 and 5 mM CaCl2) to obtain compound sol... | US Patent US9073833 (2015) BindingDB Entry DOI: 10.7270/Q2TT4PQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM93332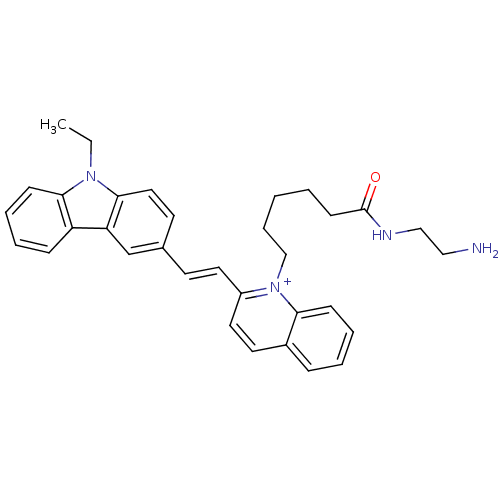 (QN-33) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 2.53E+4 | n/a | n/a | n/a | 7.4 | n/a |
New York University | Assay Description Fluorescence titration using alpha-chymotrypsin. | J Comb Chem 10: 460-5 (2008) Article DOI: 10.1021/cc700189b BindingDB Entry DOI: 10.7270/Q2V123CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM93333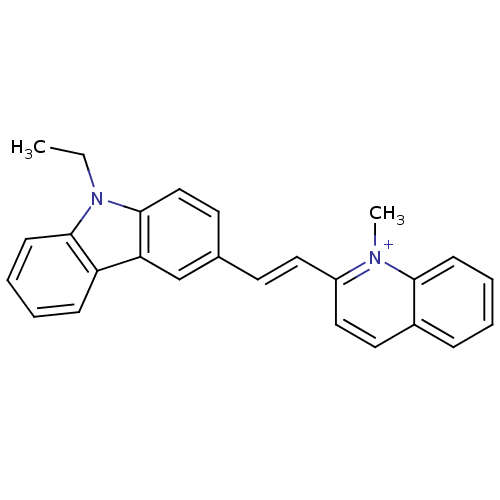 (Met-33) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 1.10E+5 | n/a | n/a | n/a | 7.4 | n/a |
New York University | Assay Description Fluorescence titration using alpha-chymotrypsin. | J Comb Chem 10: 460-5 (2008) Article DOI: 10.1021/cc700189b BindingDB Entry DOI: 10.7270/Q2V123CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM93334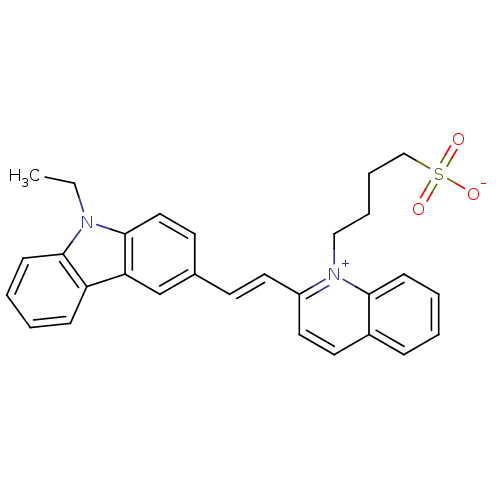 (Sul-33) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 3.51E+5 | n/a | n/a | n/a | 7.4 | n/a |
New York University | Assay Description Fluorescence titration using alpha-chymotrypsin. | J Comb Chem 10: 460-5 (2008) Article DOI: 10.1021/cc700189b BindingDB Entry DOI: 10.7270/Q2V123CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM93335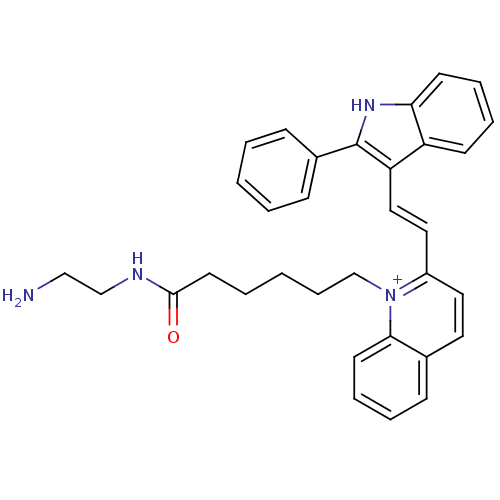 (QN-49) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 2.24E+4 | n/a | n/a | n/a | 7.4 | n/a |
New York University | Assay Description Fluorescence titration using alpha-chymotrypsin. | J Comb Chem 10: 460-5 (2008) Article DOI: 10.1021/cc700189b BindingDB Entry DOI: 10.7270/Q2V123CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM93336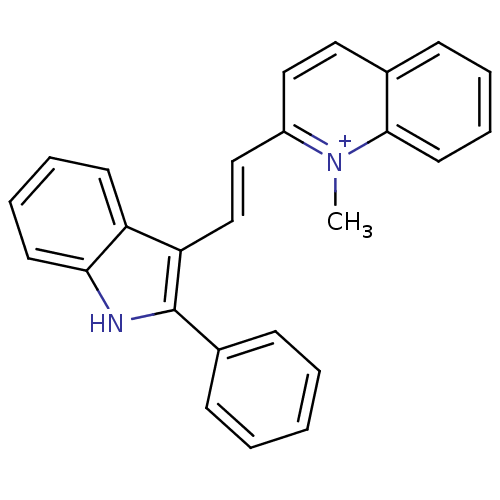 (Met-49) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 4.10E+4 | n/a | n/a | n/a | 7.4 | n/a |
New York University | Assay Description Fluorescence titration using alpha-chymotrypsin. | J Comb Chem 10: 460-5 (2008) Article DOI: 10.1021/cc700189b BindingDB Entry DOI: 10.7270/Q2V123CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118029 (3-Isopropyl-1-methanesulfonyl-4-(4-piperidin-1-yl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | n/a | 2.06E+6 | n/a | 7.4 | n/a |
GSK Curated by ChEMBL | Assay Description Association rate constant at pH 7.4.against Elastase | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118029 (3-Isopropyl-1-methanesulfonyl-4-(4-piperidin-1-yl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 6.63E+3 | 7.4 | n/a |
GSK Curated by ChEMBL | Assay Description Dissociation rate constant at pH 7.4 against Elastase | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118028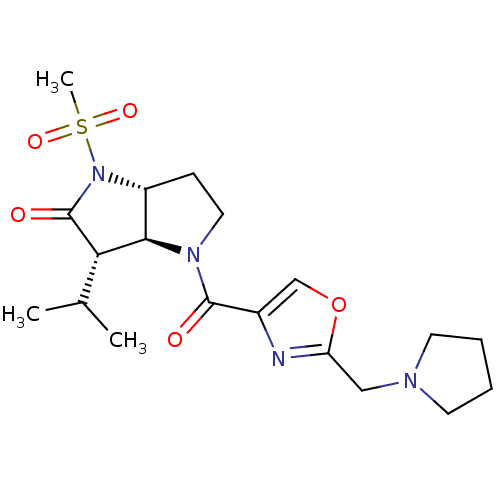 (3-Isopropyl-1-methanesulfonyl-4-(2-pyrrolidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 3.05E+4 | 7.4 | n/a |
GSK Curated by ChEMBL | Assay Description Dissociation rate constant at pH 7.4 against Elastase | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118027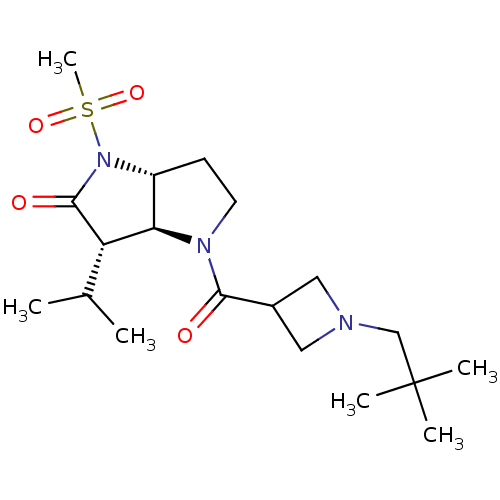 (4-[1-(2,2-Dimethyl-propyl)-azetidine-3-carbonyl]-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | n/a | 1.09E+7 | n/a | 7.4 | n/a |
GSK Curated by ChEMBL | Assay Description Association rate constant at pH 7.4 against Elastase | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118030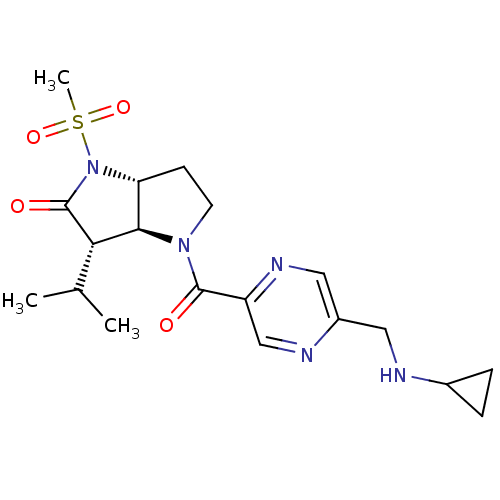 (4-(5-Cyclopropylaminomethyl-pyrazine-2-carbonyl)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | n/a | 3.30E+6 | n/a | 7.4 | n/a |
GSK Curated by ChEMBL | Assay Description Association rate constant at pH 7.4 against Elastase | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118030 (4-(5-Cyclopropylaminomethyl-pyrazine-2-carbonyl)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 2.52E+5 | 7.4 | n/a |
GSK Curated by ChEMBL | Assay Description Dissociation rate constant at pH 7.4 against Elastase | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189810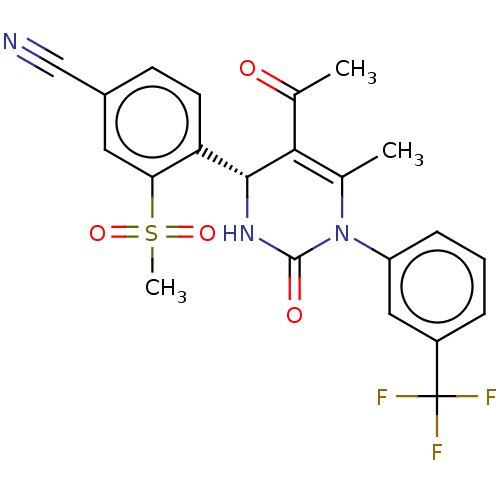 (US9174997, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189811 (US9174997, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189812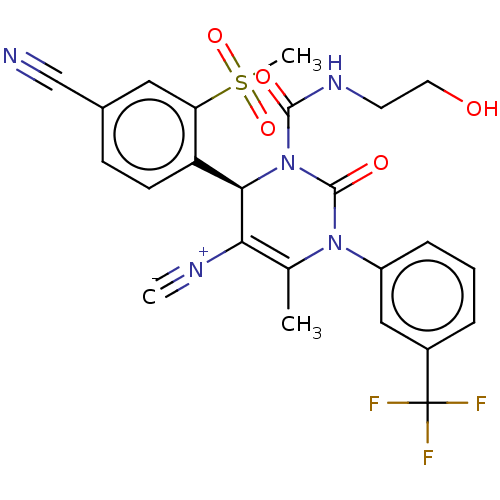 (US9174997, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189813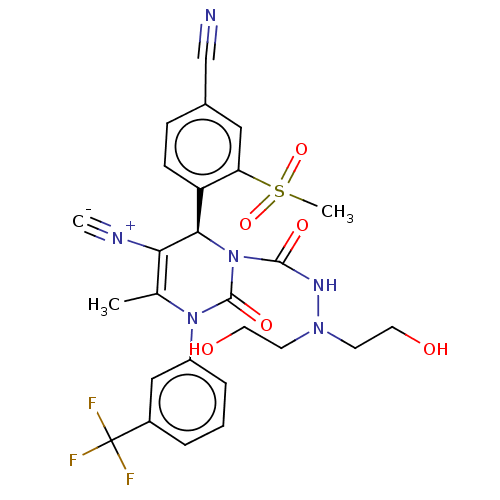 (US9174997, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189814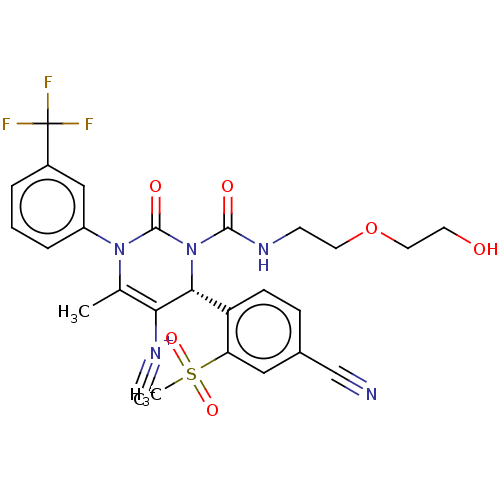 (US9174997, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189815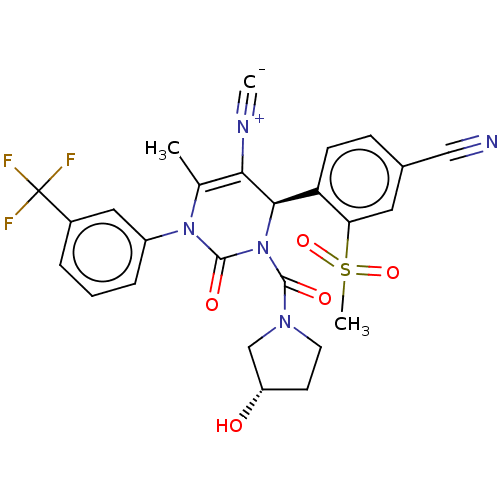 (US9174997, 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189816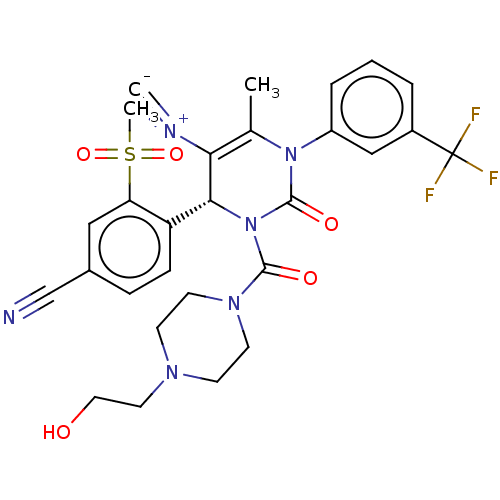 (US9174997, 20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 5513 total ) | Next | Last >> |