

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hormone-sensitive lipase (Rattus norvegicus (Rat)) | BDBM141543 (US8921404, 10) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 670 | n/a | n/a | n/a | n/a | 7.0 | 37 |
Sanofi US Patent | Assay Description To prepare the substrate, 25-50 uCi of [3H]trioleoylglycerol (in toluene), 6.8 umol of unlabeled trioleoylglycerol and 0.6 mg of phospholipids (phosp... | US Patent US8921404 (2014) BindingDB Entry DOI: 10.7270/Q24748KQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Rattus norvegicus (Rat)) | BDBM141540 (US8921404, 5) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.0 | 37 |
Sanofi US Patent | Assay Description To prepare the substrate, 25-50 uCi of [3H]trioleoylglycerol (in toluene), 6.8 umol of unlabeled trioleoylglycerol and 0.6 mg of phospholipids (phosp... | US Patent US8921404 (2014) BindingDB Entry DOI: 10.7270/Q24748KQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Rattus norvegicus (Rat)) | BDBM141539 (US8921404, 4) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.0 | 37 |
Sanofi US Patent | Assay Description To prepare the substrate, 25-50 uCi of [3H]trioleoylglycerol (in toluene), 6.8 umol of unlabeled trioleoylglycerol and 0.6 mg of phospholipids (phosp... | US Patent US8921404 (2014) BindingDB Entry DOI: 10.7270/Q24748KQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Rattus norvegicus (Rat)) | BDBM141538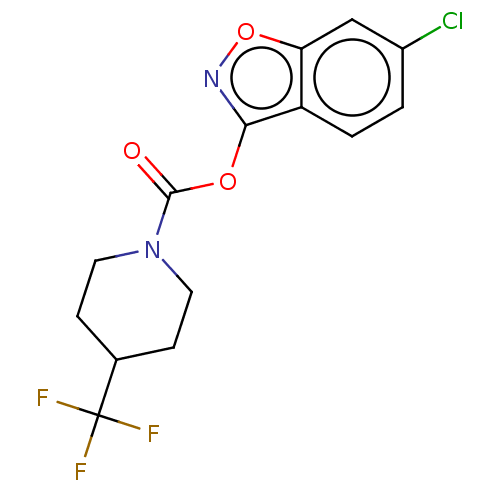 (US8921404, 3) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.0 | 37 |
Sanofi US Patent | Assay Description To prepare the substrate, 25-50 uCi of [3H]trioleoylglycerol (in toluene), 6.8 umol of unlabeled trioleoylglycerol and 0.6 mg of phospholipids (phosp... | US Patent US8921404 (2014) BindingDB Entry DOI: 10.7270/Q24748KQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Rattus norvegicus (Rat)) | BDBM141537 (US8921404, 2) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 7.0 | 37 |
Sanofi US Patent | Assay Description To prepare the substrate, 25-50 uCi of [3H]trioleoylglycerol (in toluene), 6.8 umol of unlabeled trioleoylglycerol and 0.6 mg of phospholipids (phosp... | US Patent US8921404 (2014) BindingDB Entry DOI: 10.7270/Q24748KQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Rattus norvegicus (Rat)) | BDBM141536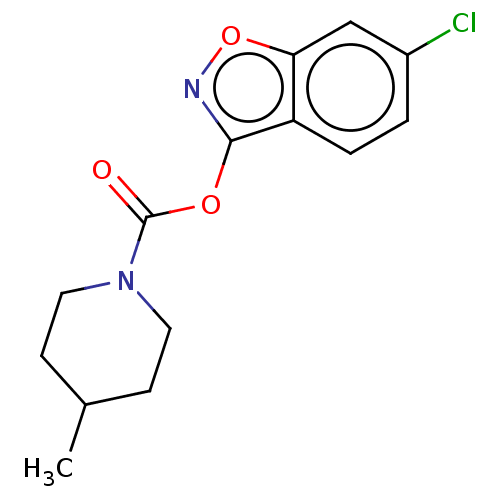 (US8921404, 1) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 7.0 | 37 |
Sanofi US Patent | Assay Description To prepare the substrate, 25-50 uCi of [3H]trioleoylglycerol (in toluene), 6.8 umol of unlabeled trioleoylglycerol and 0.6 mg of phospholipids (phosp... | US Patent US8921404 (2014) BindingDB Entry DOI: 10.7270/Q24748KQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Rattus norvegicus (Rat)) | BDBM141544 (US8921404, 11) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 260 | n/a | n/a | n/a | n/a | 7.0 | 37 |
Sanofi US Patent | Assay Description To prepare the substrate, 25-50 uCi of [3H]trioleoylglycerol (in toluene), 6.8 umol of unlabeled trioleoylglycerol and 0.6 mg of phospholipids (phosp... | US Patent US8921404 (2014) BindingDB Entry DOI: 10.7270/Q24748KQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM97632 (US8470843, 8) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, MO) as a substrate. | US Patent US8470843 (2013) BindingDB Entry DOI: 10.7270/Q2WH2NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM97633 (US8470843, 9) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, MO) as a substrate. | US Patent US8470843 (2013) BindingDB Entry DOI: 10.7270/Q2WH2NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM97634 (US8470843, 10) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, MO) as a substrate. | US Patent US8470843 (2013) BindingDB Entry DOI: 10.7270/Q2WH2NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM97635 (US8470843, 11) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, MO) as a substrate. | US Patent US8470843 (2013) BindingDB Entry DOI: 10.7270/Q2WH2NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM97636 (US8470843, 12) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, MO) as a substrate. | US Patent US8470843 (2013) BindingDB Entry DOI: 10.7270/Q2WH2NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM1664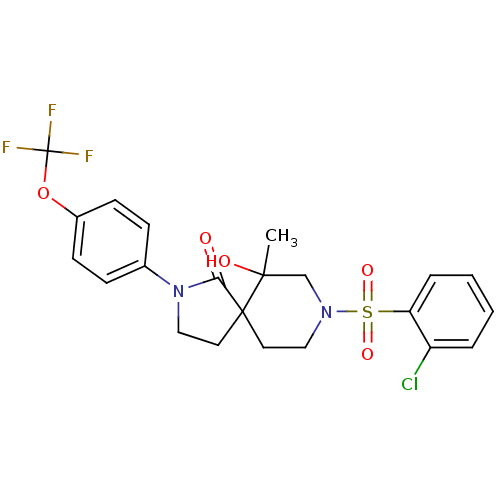 (US8470843, 13 | US8470843, 14) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, MO) as a substrate. | US Patent US8470843 (2013) BindingDB Entry DOI: 10.7270/Q2WH2NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM97638 (US8470843, 15) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 430 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, MO) as a substrate. | US Patent US8470843 (2013) BindingDB Entry DOI: 10.7270/Q2WH2NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM120365 (US8703807, 1) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8703807 (2014) BindingDB Entry DOI: 10.7270/Q2RN36HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM120366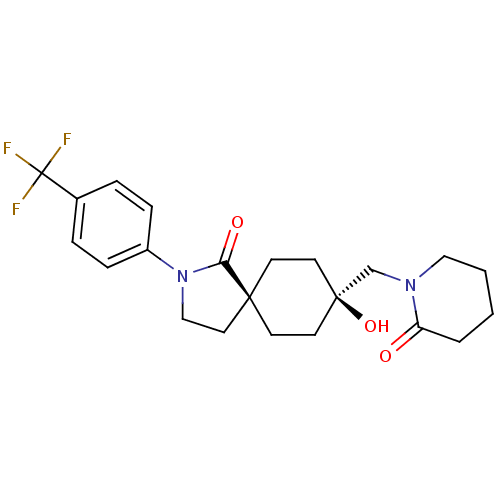 (US8703807, 2) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 68.1 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8703807 (2014) BindingDB Entry DOI: 10.7270/Q2RN36HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM120367 (US8703807, 3) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50.9 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8703807 (2014) BindingDB Entry DOI: 10.7270/Q2RN36HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM120368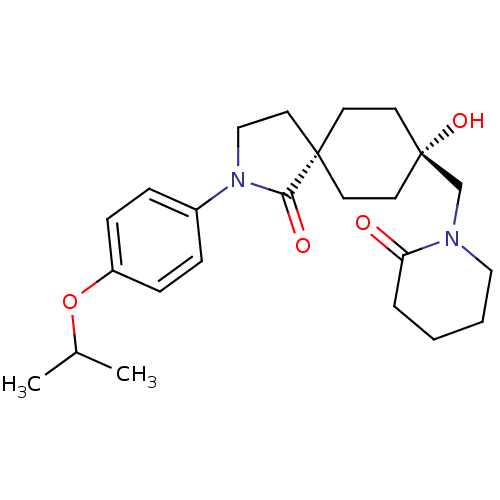 (US8703807, 4) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 27.4 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8703807 (2014) BindingDB Entry DOI: 10.7270/Q2RN36HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM120369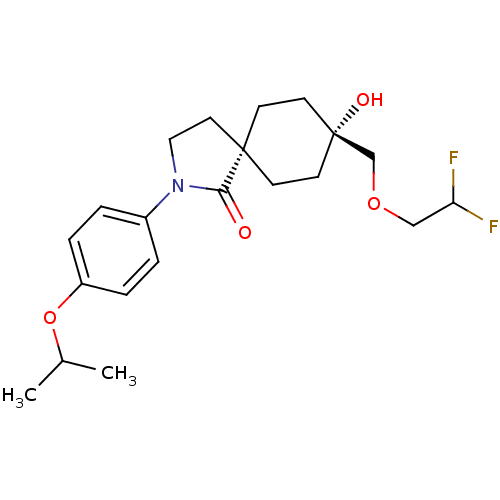 (US8703807, 5) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 16.3 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8703807 (2014) BindingDB Entry DOI: 10.7270/Q2RN36HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM120370 (US8703807, 6) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 264 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8703807 (2014) BindingDB Entry DOI: 10.7270/Q2RN36HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM120371 (US8703807, 7) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 13.7 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8703807 (2014) BindingDB Entry DOI: 10.7270/Q2RN36HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM120372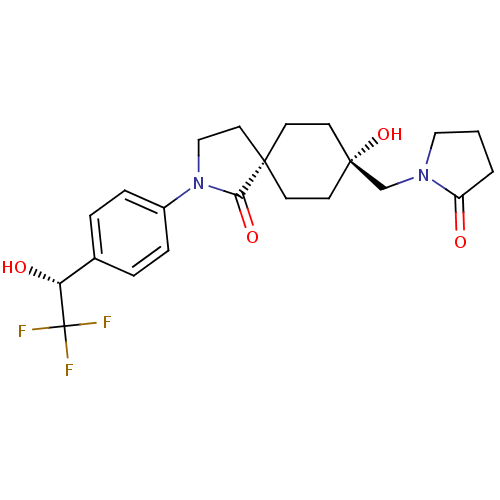 (US8703807, 8) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 213 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8703807 (2014) BindingDB Entry DOI: 10.7270/Q2RN36HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM120373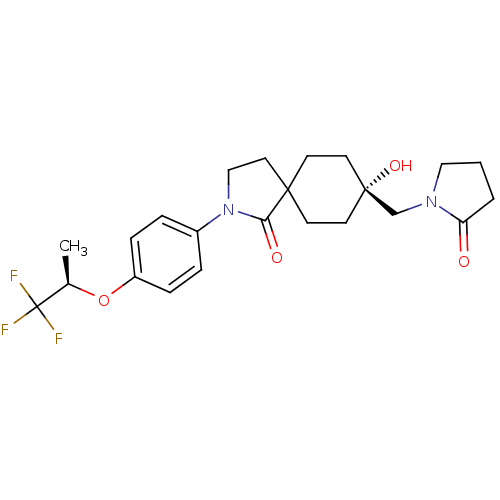 (US8703807, 9) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30.6 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8703807 (2014) BindingDB Entry DOI: 10.7270/Q2RN36HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM120374 (US8703807, 10) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 41.8 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8703807 (2014) BindingDB Entry DOI: 10.7270/Q2RN36HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM120375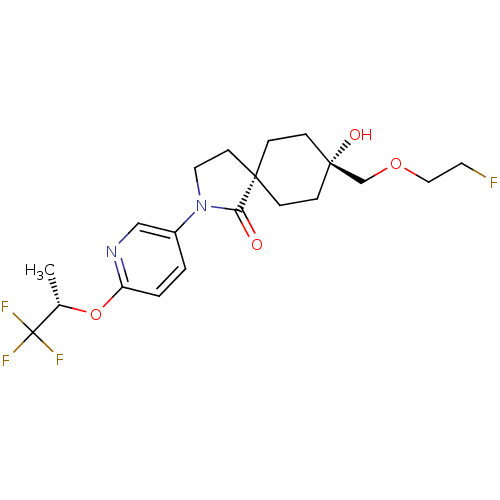 (US8703807, 11) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8703807 (2014) BindingDB Entry DOI: 10.7270/Q2RN36HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM120376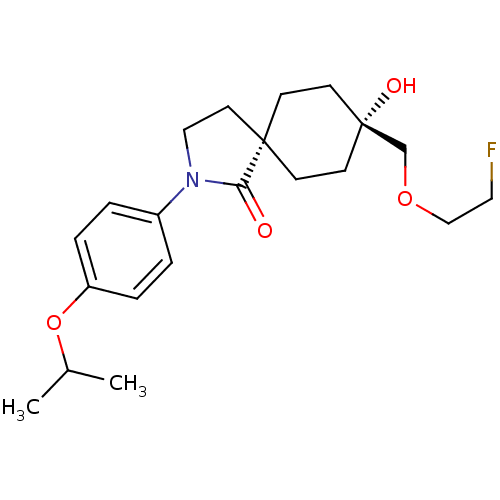 (US8703807, 12) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 37 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8703807 (2014) BindingDB Entry DOI: 10.7270/Q2RN36HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM121010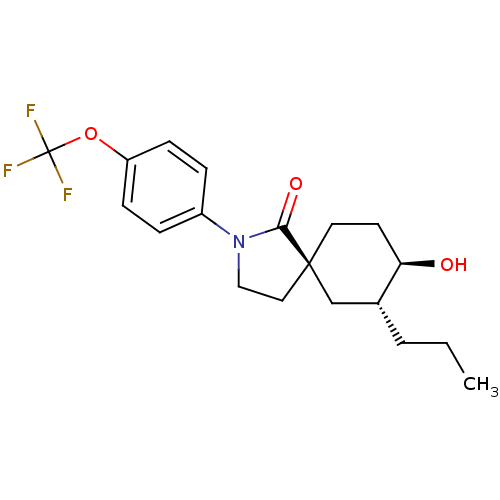 (US8722721, 1) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 67 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8722721 (2014) BindingDB Entry DOI: 10.7270/Q2F76B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM121011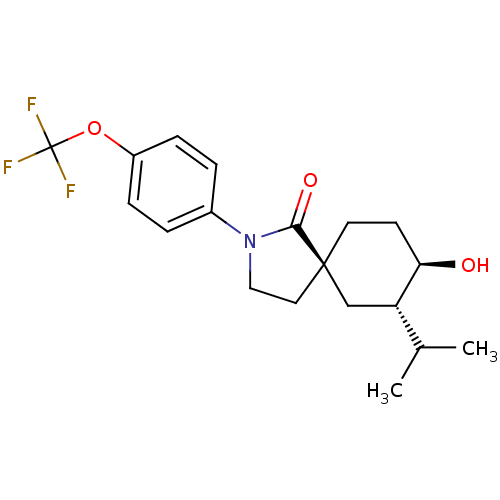 (US8722721, 2) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 16.2 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8722721 (2014) BindingDB Entry DOI: 10.7270/Q2F76B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM121012 (US8722721, 3) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 189 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8722721 (2014) BindingDB Entry DOI: 10.7270/Q2F76B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM121013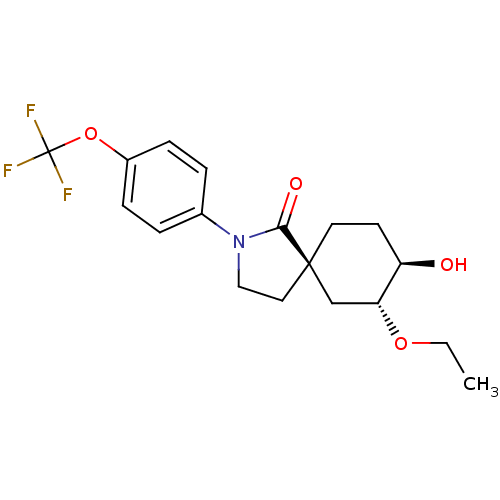 (US8722721, 4) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 198 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8722721 (2014) BindingDB Entry DOI: 10.7270/Q2F76B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM121014 (US8722721, 5 | US8722721, 6) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 66.4 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8722721 (2014) BindingDB Entry DOI: 10.7270/Q2F76B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM121014 (US8722721, 5 | US8722721, 6) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 158 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8722721 (2014) BindingDB Entry DOI: 10.7270/Q2F76B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM121027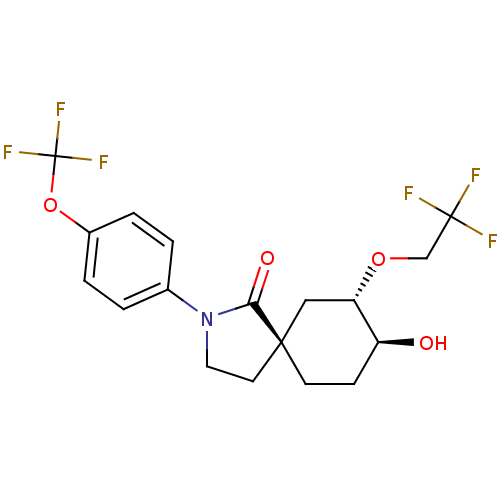 (US8722721, 7) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 42.1 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8722721 (2014) BindingDB Entry DOI: 10.7270/Q2F76B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM121016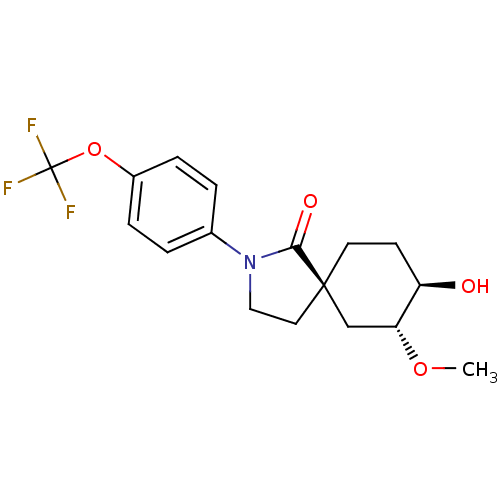 (US8722721, 8) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 343 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8722721 (2014) BindingDB Entry DOI: 10.7270/Q2F76B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM121017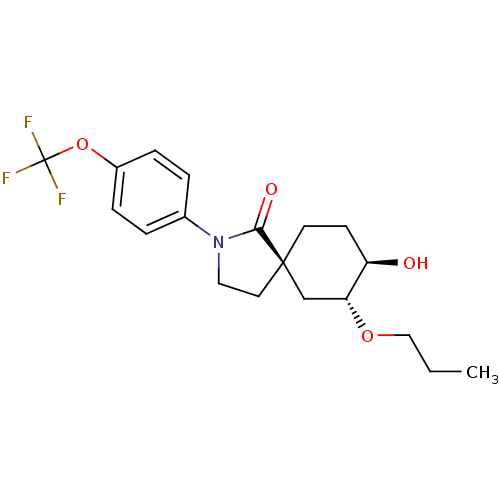 (US8722721, 9) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 73.3 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8722721 (2014) BindingDB Entry DOI: 10.7270/Q2F76B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM121018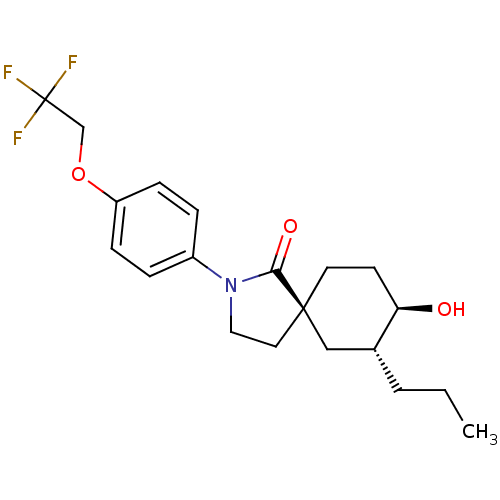 (US8722721, 10) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8722721 (2014) BindingDB Entry DOI: 10.7270/Q2F76B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM121019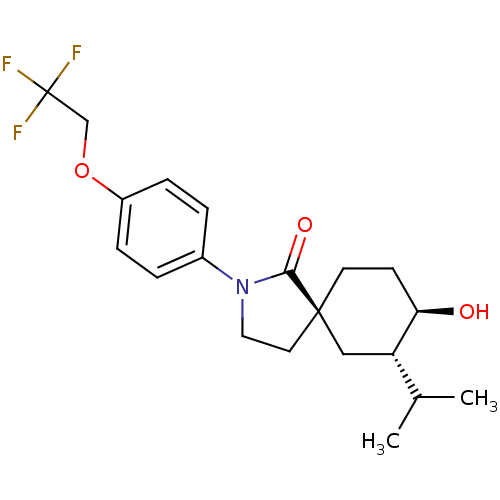 (US8722721, 11) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8722721 (2014) BindingDB Entry DOI: 10.7270/Q2F76B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM121020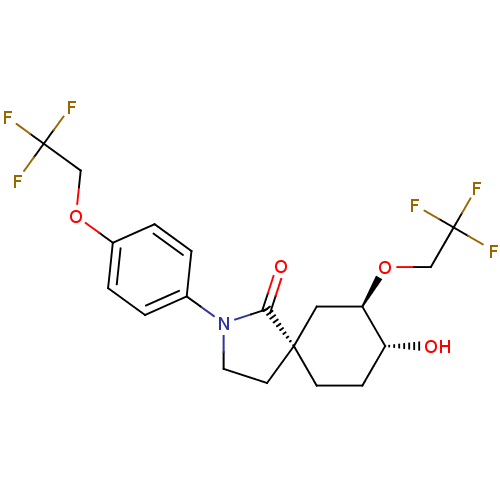 (US8722721, 12) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 350 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8722721 (2014) BindingDB Entry DOI: 10.7270/Q2F76B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM121021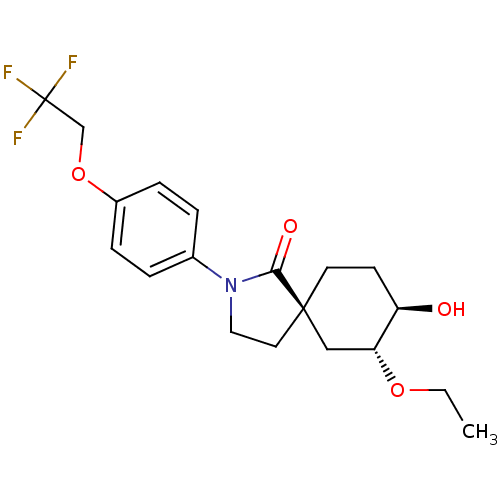 (US8722721, 13) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 994 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8722721 (2014) BindingDB Entry DOI: 10.7270/Q2F76B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM121022 (US8722721, 14) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 422 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8722721 (2014) BindingDB Entry DOI: 10.7270/Q2F76B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM121023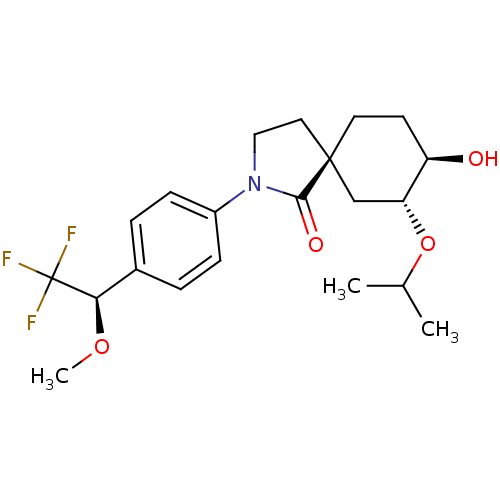 (US8722721, 15) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 136 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8722721 (2014) BindingDB Entry DOI: 10.7270/Q2F76B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM121024 (US8722721, 16) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 175 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8722721 (2014) BindingDB Entry DOI: 10.7270/Q2F76B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM121025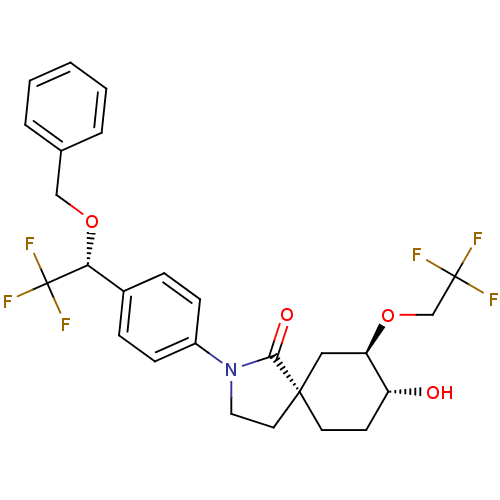 (US8722721, 17) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8722721 (2014) BindingDB Entry DOI: 10.7270/Q2F76B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM121026 (US8722721, 18) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 185 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8722721 (2014) BindingDB Entry DOI: 10.7270/Q2F76B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM97631 (US8470843, 7) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, MO) as a substrate. | US Patent US8470843 (2013) BindingDB Entry DOI: 10.7270/Q2WH2NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM97630 (US8470843, 6) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 90 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, MO) as a substrate. | US Patent US8470843 (2013) BindingDB Entry DOI: 10.7270/Q2WH2NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM97629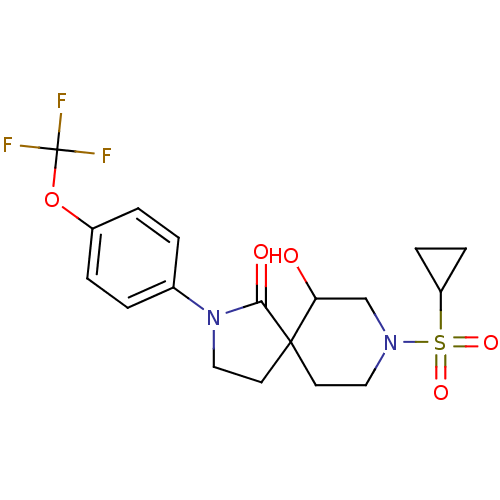 (US8470843, 5) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 160 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, MO) as a substrate. | US Patent US8470843 (2013) BindingDB Entry DOI: 10.7270/Q2WH2NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM97628 (US8470843, 4) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, MO) as a substrate. | US Patent US8470843 (2013) BindingDB Entry DOI: 10.7270/Q2WH2NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM97627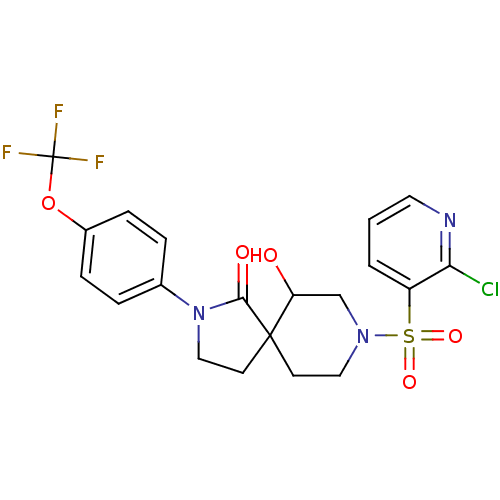 (US8470843, 3) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, MO) as a substrate. | US Patent US8470843 (2013) BindingDB Entry DOI: 10.7270/Q2WH2NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM97626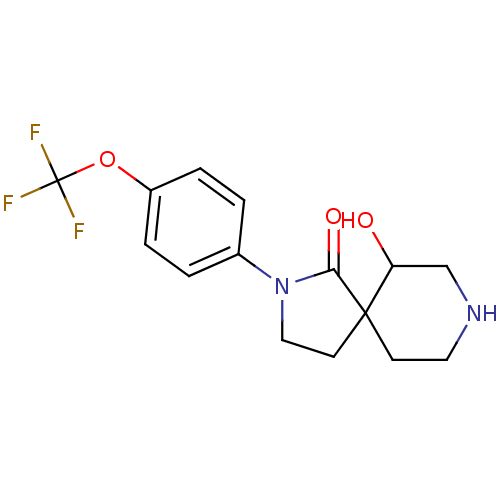 (US8470841, 2) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, MO) as a substrate. | US Patent US8470843 (2013) BindingDB Entry DOI: 10.7270/Q2WH2NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 593 total ) | Next | Last >> |