

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290224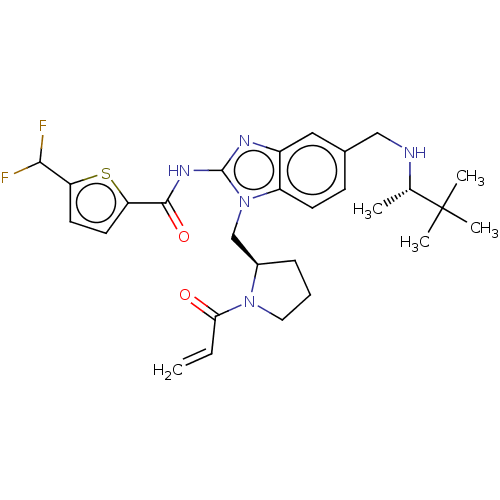 (US9573958, Compound 59) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290222 (US9573958, Compound 20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290254 (US9573958, Compound 63) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290231 (US9573958, Compound 22) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290167 (US9573958, Compound 113) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290232 (US9573958, Compound 61) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290242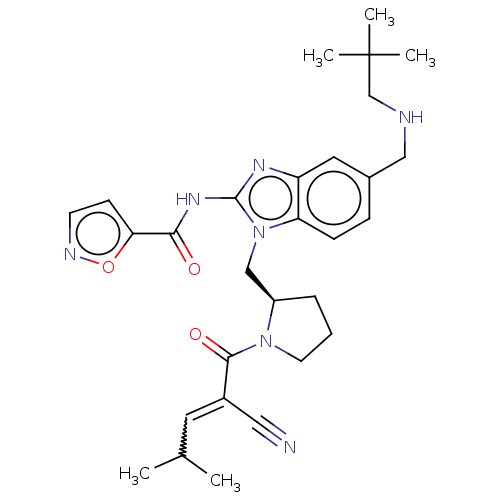 (US9573958, Compound 134) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290226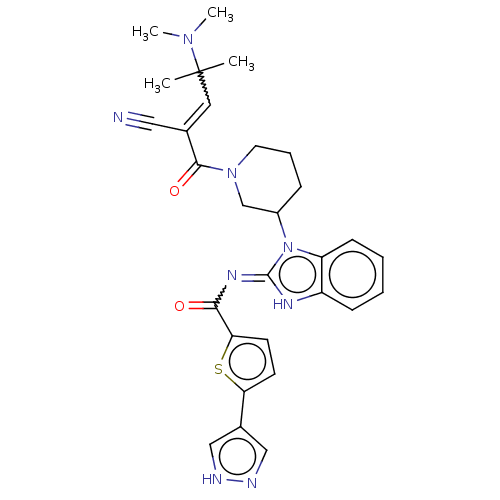 (US9573958, Compound 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290235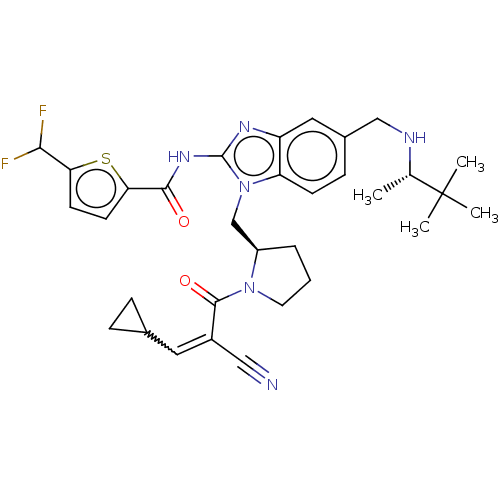 (US9573958, Compound 62) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290229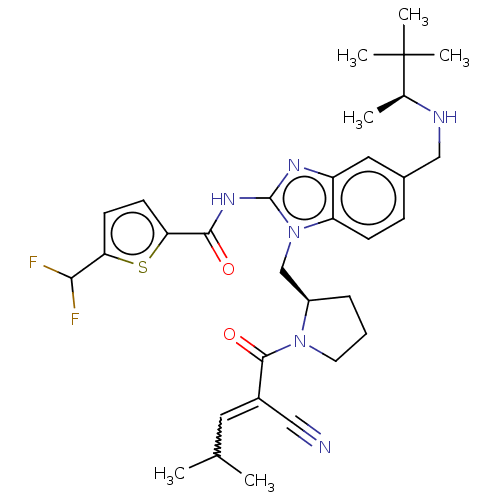 (US9573958, Compound 60) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290185 (US9573958, Compound 49) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290257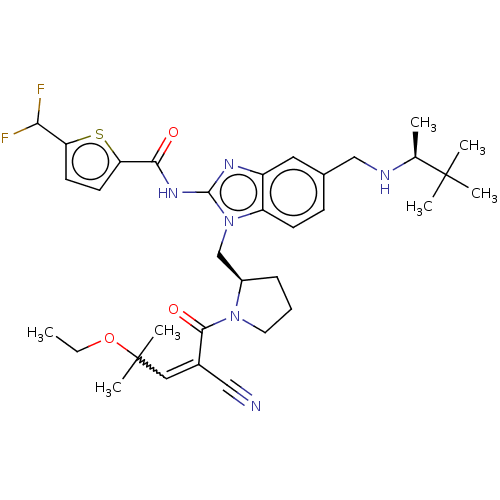 (US9573958, Compound 64) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290164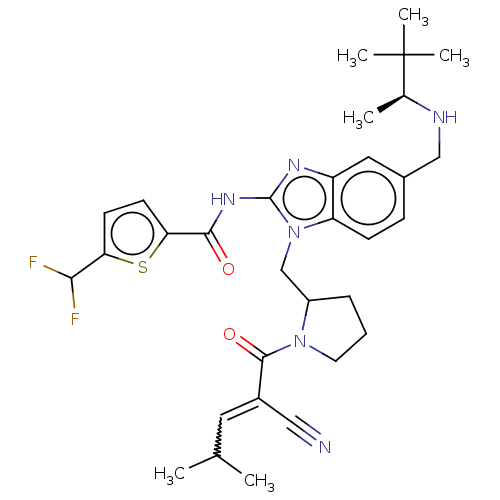 (US9573958, Compound 40) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290248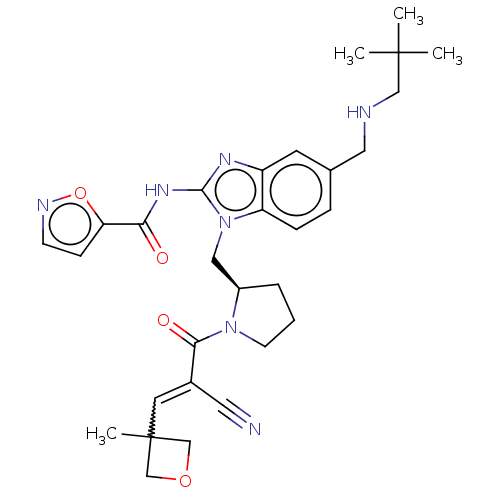 (US9573958, Compound 136) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290217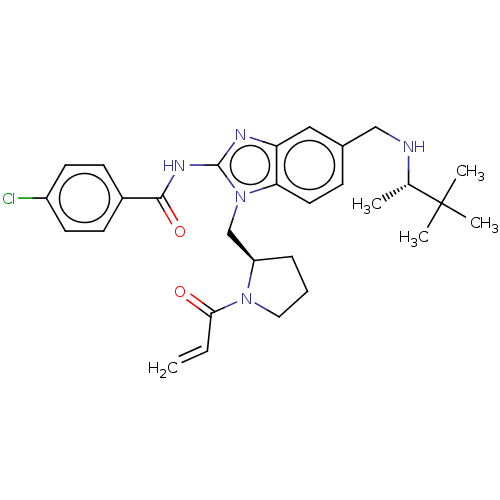 (US9573958, Compound 58) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290149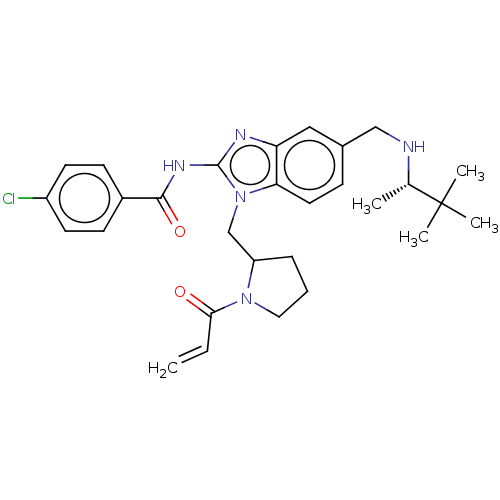 (US9573958, Compound 33) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290258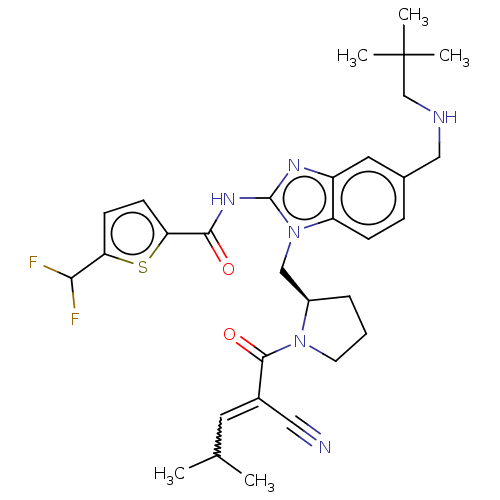 (US9573958, Compound 85) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290142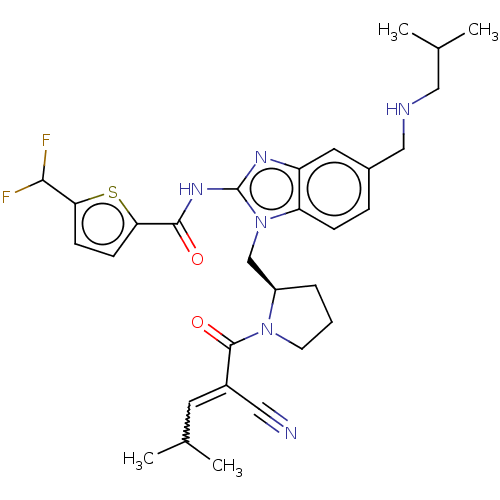 (US9573958, Compound 102) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290181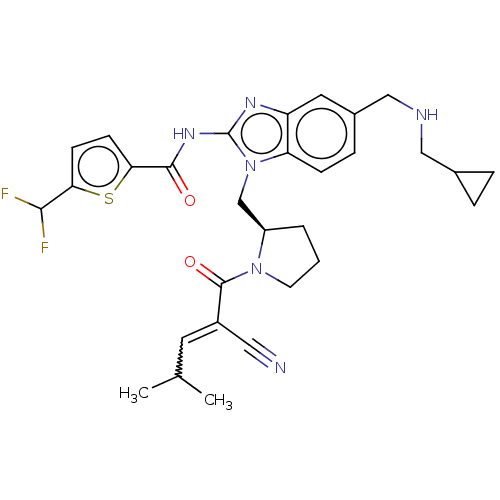 (US9573958, Compound 117) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290216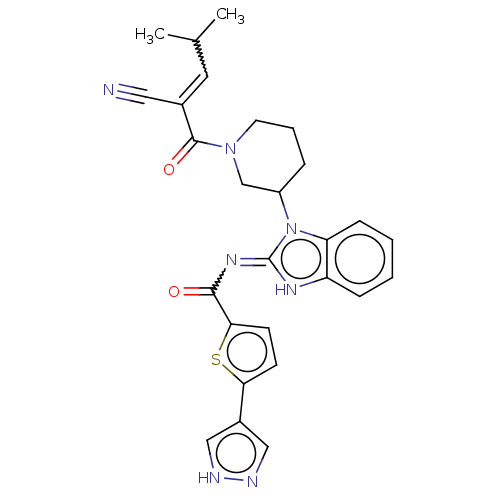 (US9573958, Compound 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290171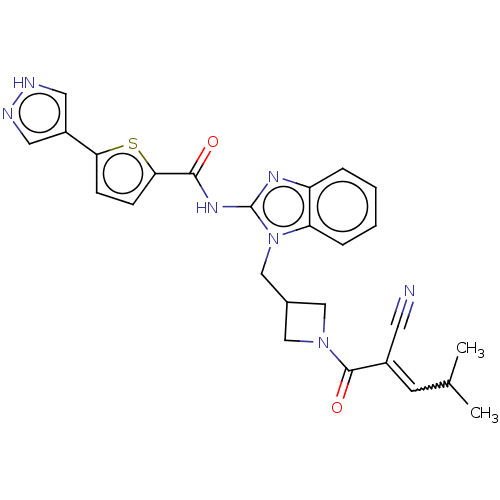 (US9573958, Compound 42) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290173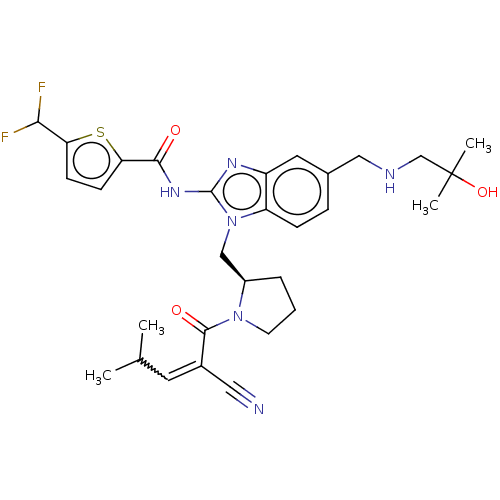 (US9573958, Compound 116) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10.3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290186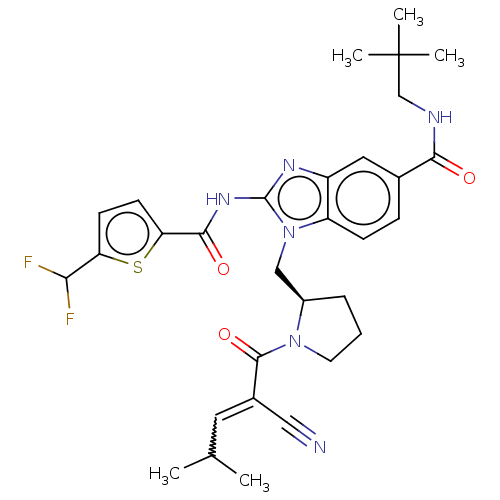 (US9573958, Compound 118) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11.8 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290243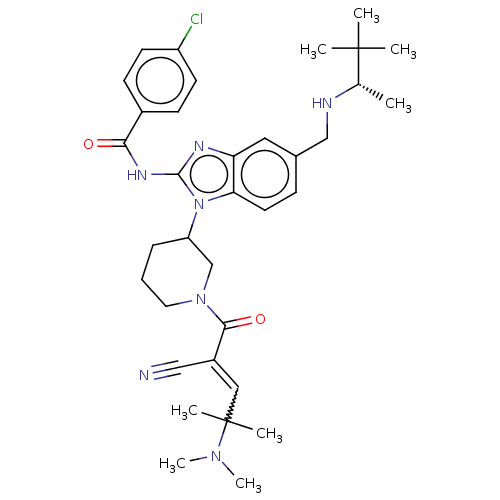 (US9573958, Compound 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290193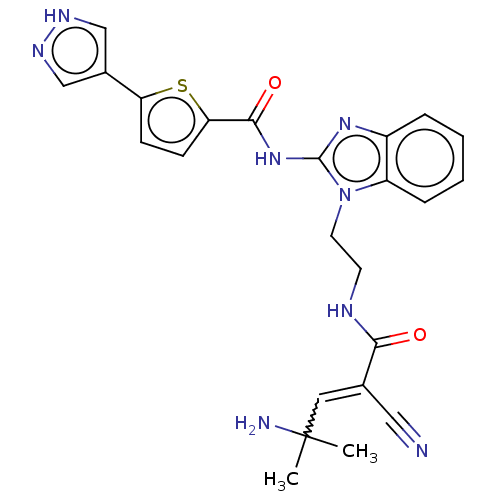 (US9573958, Compound 51) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290233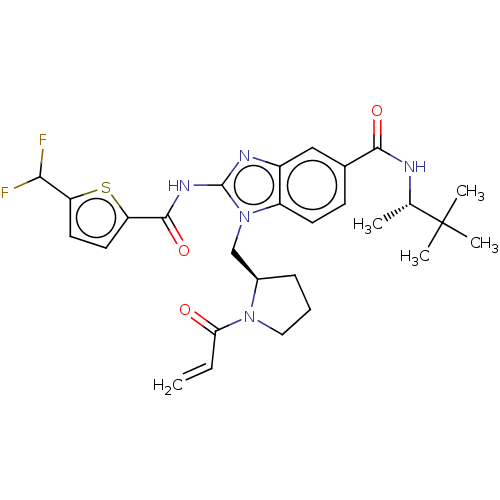 (US9573958, Compound 128) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13.7 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290161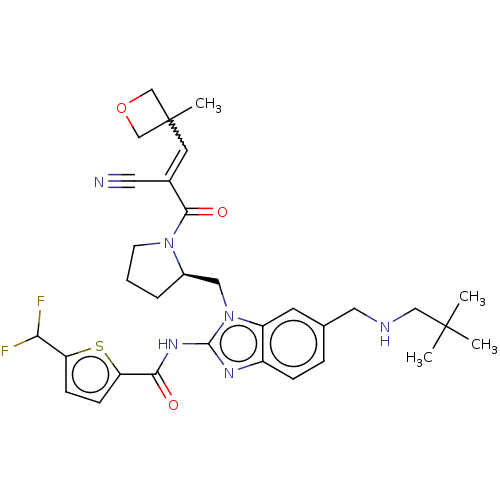 (US9573958, Compound 115) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14.5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290230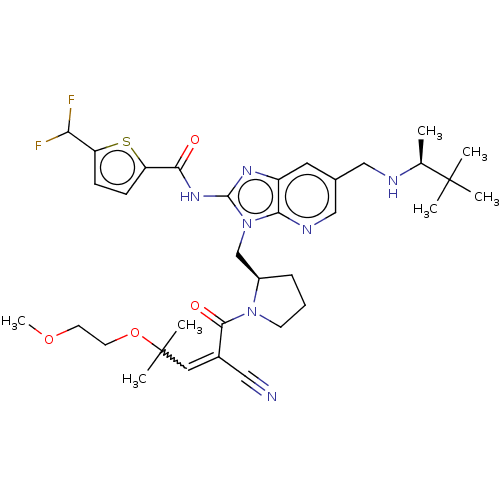 (US9573958, Compound 127) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 18 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290237 (US9573958, Compound 24) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290245 (US9573958, Compound 135) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290179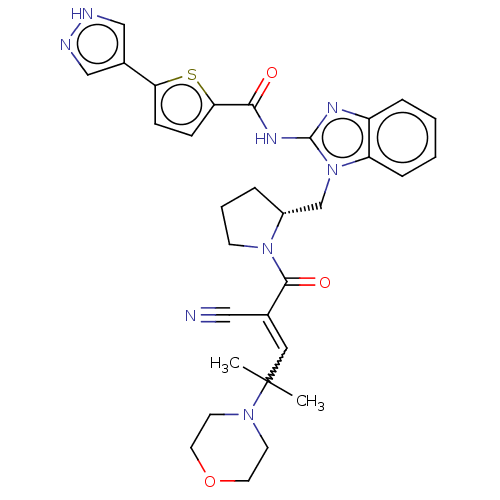 (US9573958, Compound 44) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19.9 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290158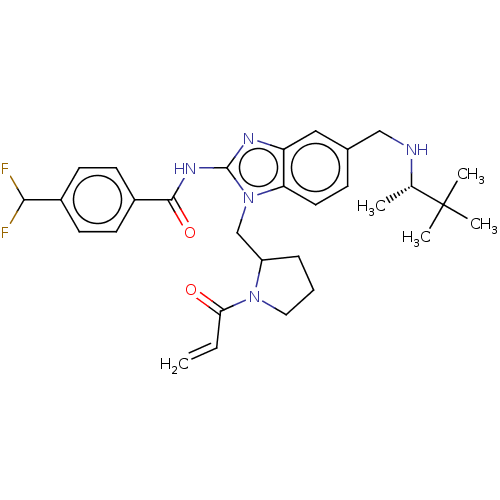 (US9573958, Compound 37) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290234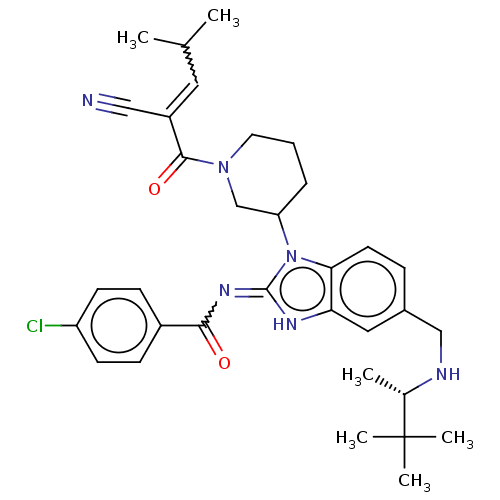 (US9573958, Compound 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290256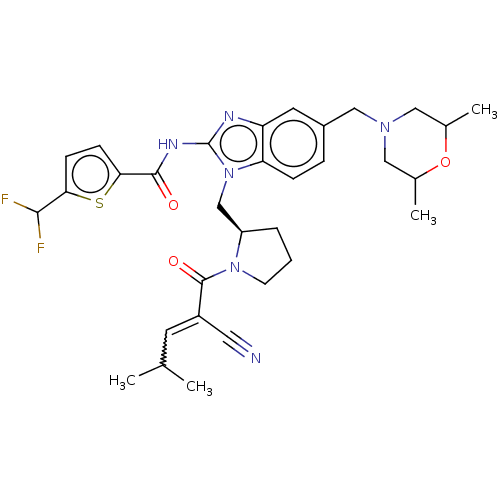 (US9573958, Compound 87) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24.8 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290150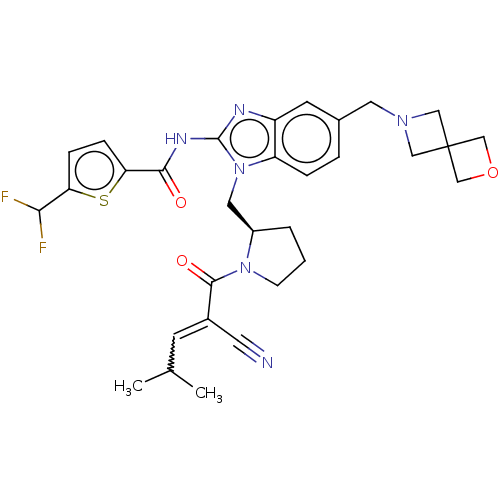 (US9573958, Compound 108) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 26 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290239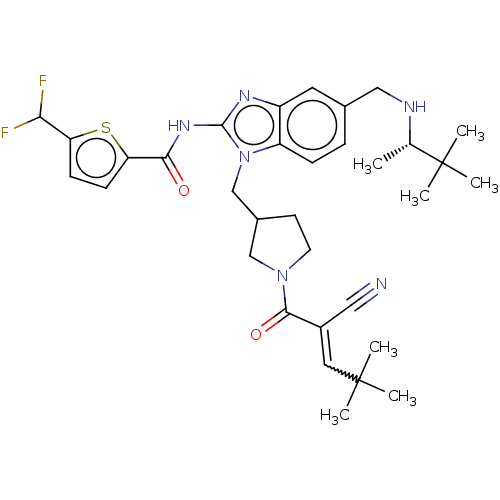 (US9573958, Compound 132) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 28 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290203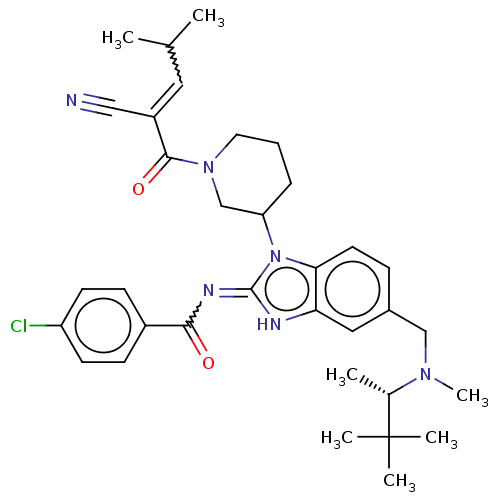 (US9573958, Compound 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 28 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290194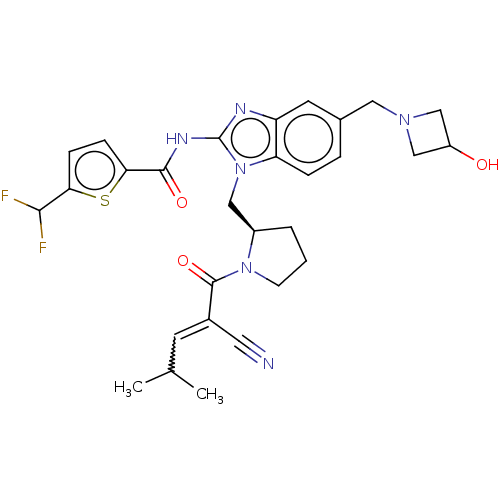 (US9573958, Compound 121) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290189 (US9573958, Compound 50) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 37 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290155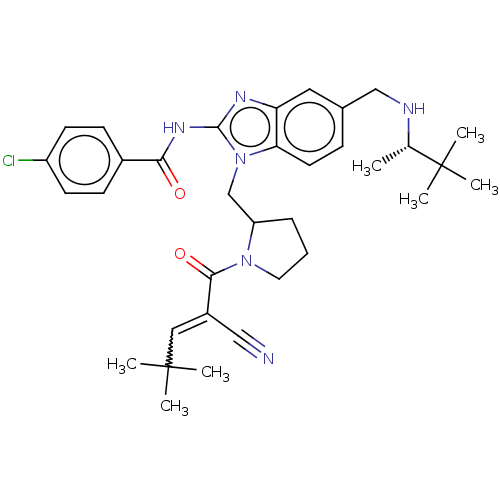 (US9573958, Compound 36) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290255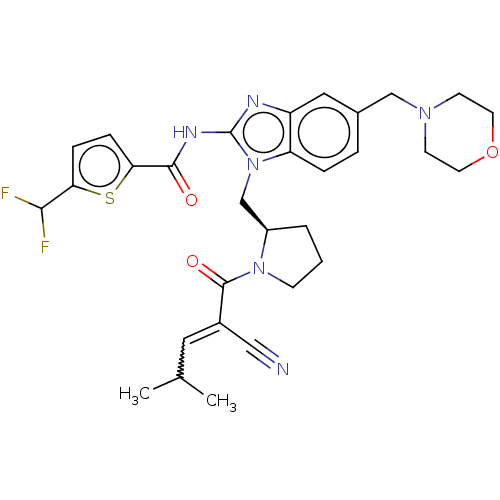 (US9573958, Compound 86) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 44 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290253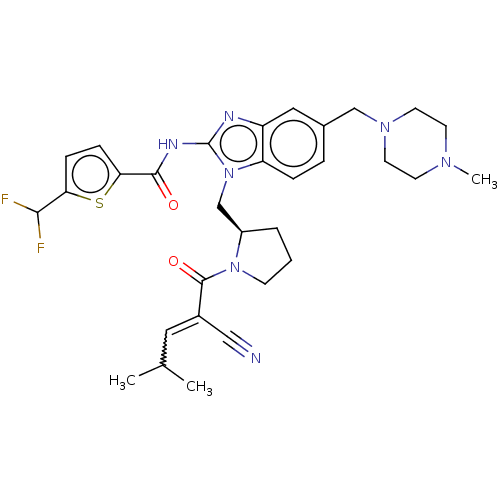 (US9573958, Compound 89) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 49 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290198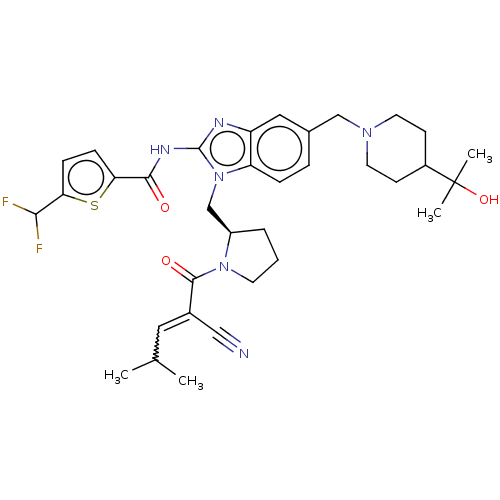 (US9573958, Compound 122) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 52 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290251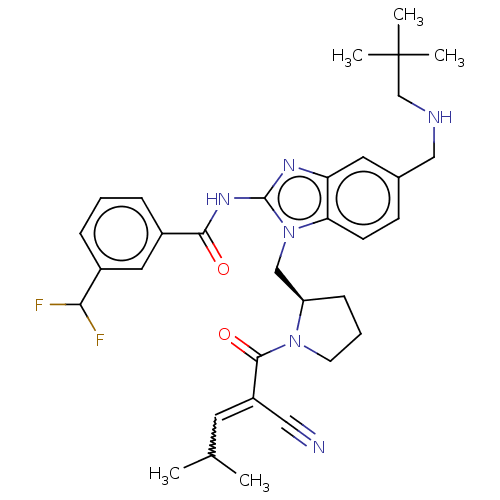 (US9573958, Compound 138) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 68 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290183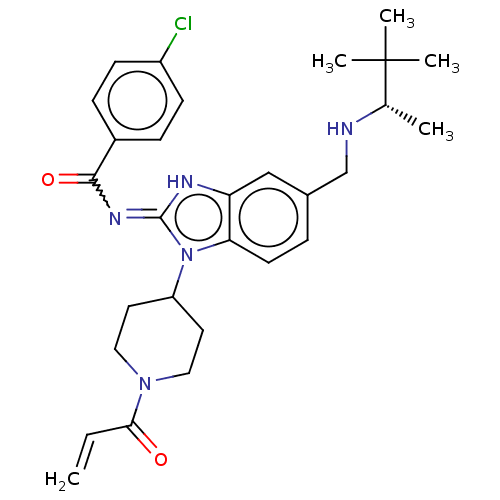 (US9573958, Compound 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290197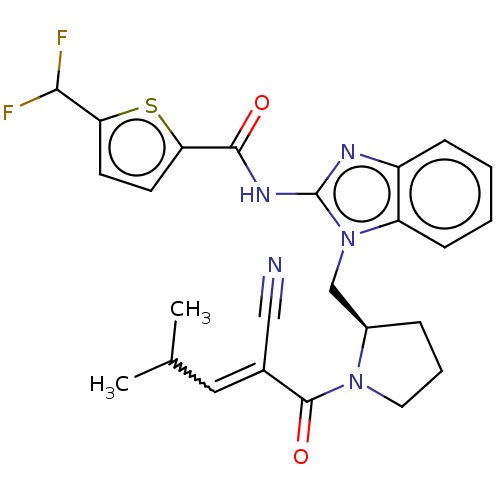 (US9573958, Compound 53) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290238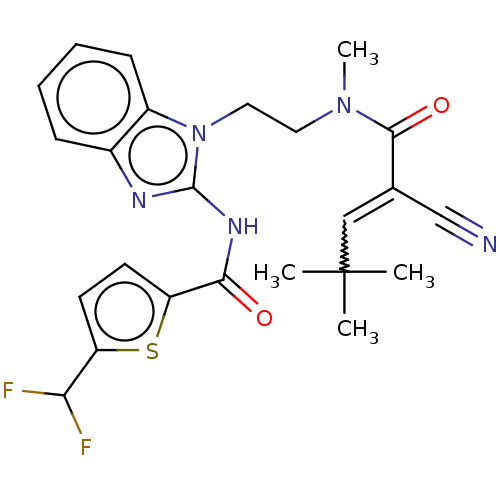 (US9573958, Compound 68) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 127 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290195 (US9573958, Compound 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290240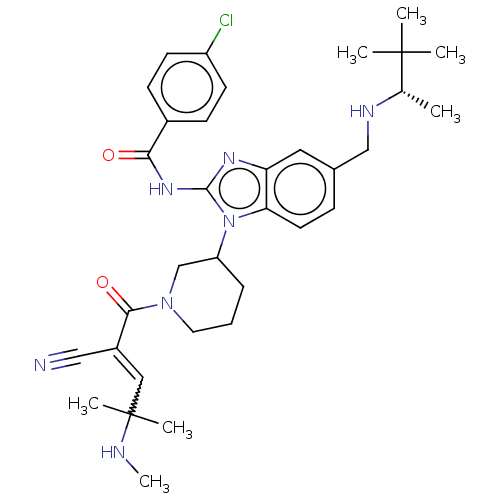 (US9573958, Compound 25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 137 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290134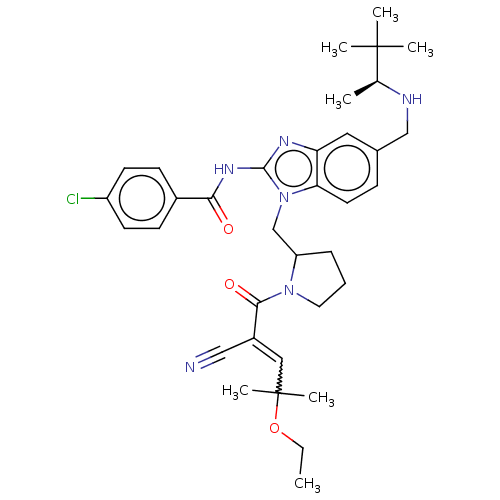 (US9573958, Compound 32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 142 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290207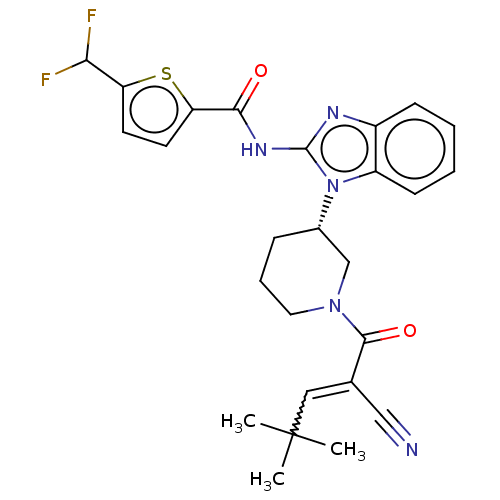 (US9573958, Compound 124) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 170 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290156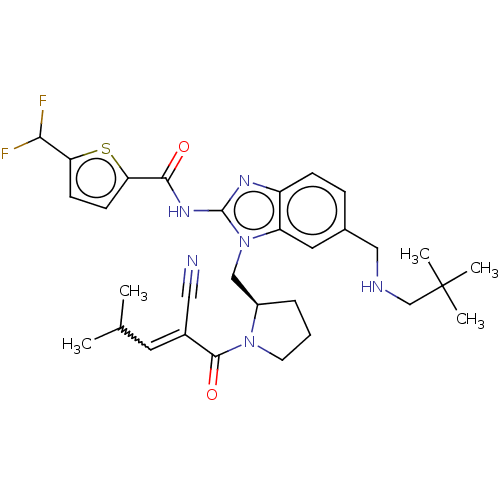 (US9573958, Compound 114) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 179 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290225 (US9573958, Compound 126) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 215 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290246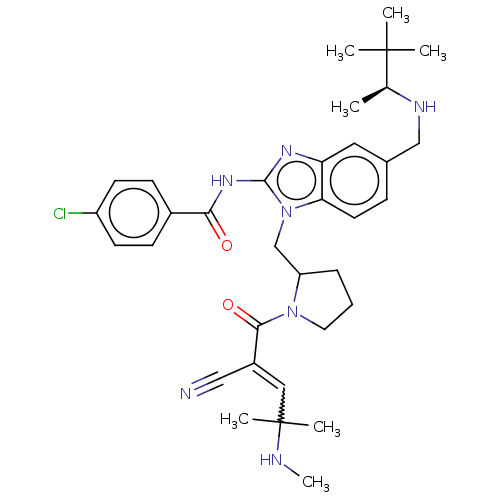 (US9573958, Compound 30) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 312 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290192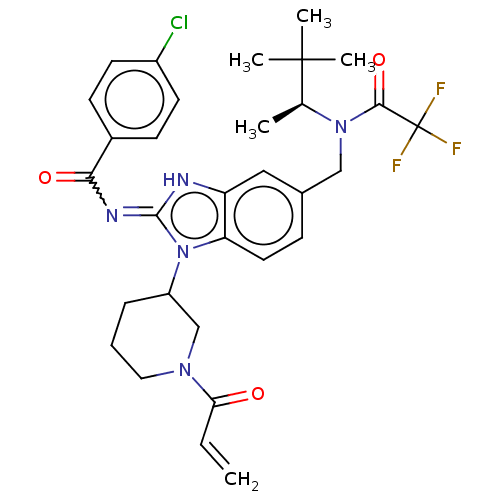 (US9573958, Compound 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 320 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290153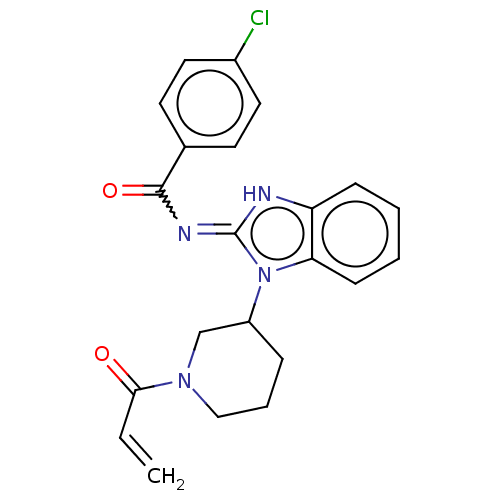 (US9573958, Compound 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 530 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290241 (US9573958, Compound 69) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 571 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290162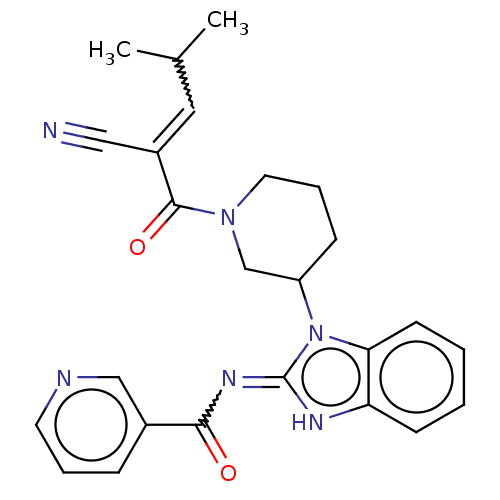 (US9573958, Compound 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 670 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290236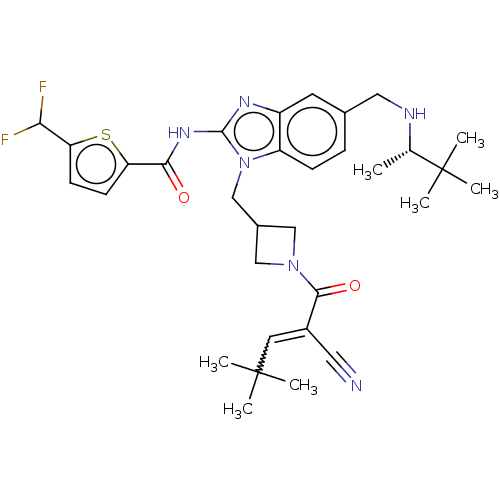 (US9573958, Compound 129) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 680 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290252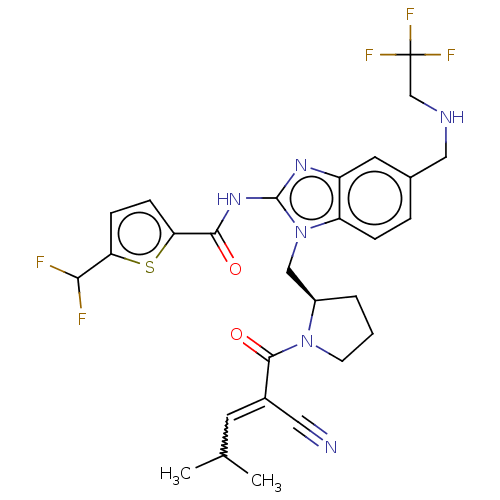 (US9573958, Compound 88) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 743 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290218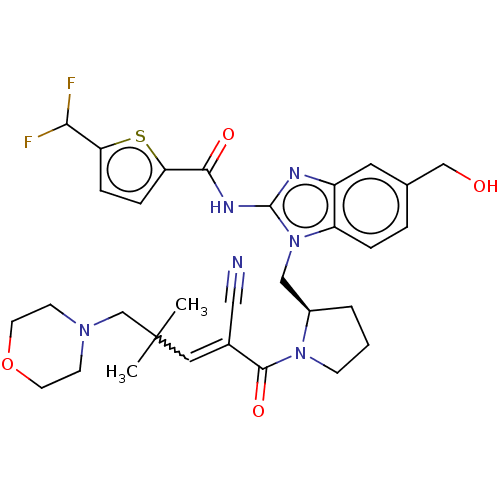 (US9573958, Compound 125) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 770 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290191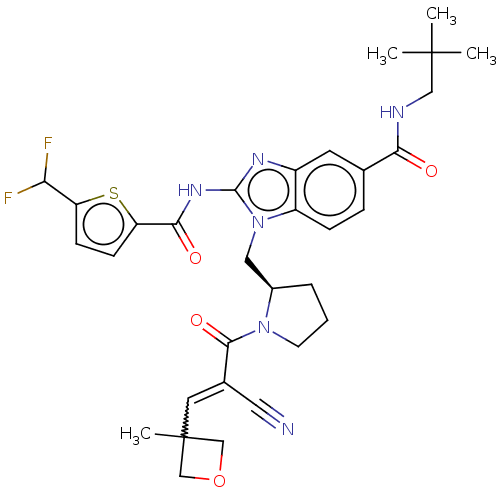 (US9573958, Compound 119) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 920 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290125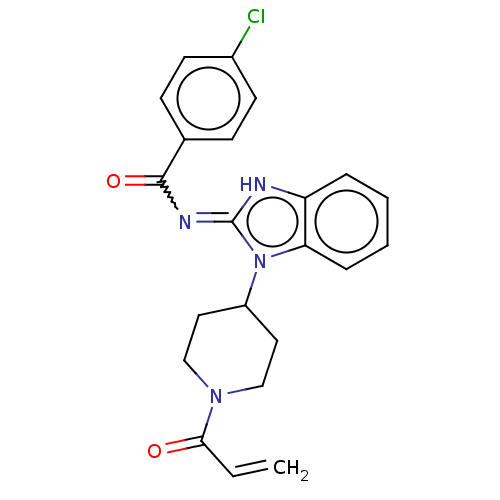 (US9573958, Compound 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 929 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290249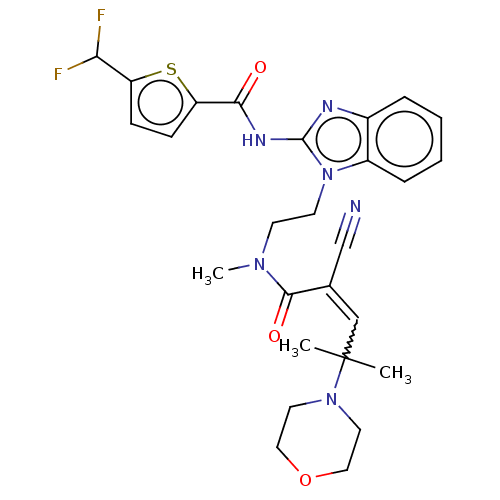 (US9573958, Compound 80) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 959 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290259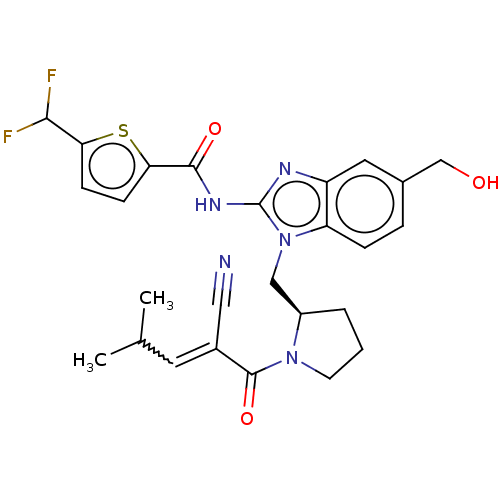 (US9573958, Compound 84) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 973 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290204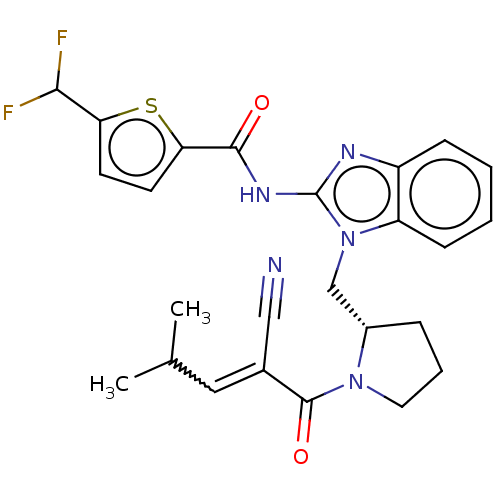 (US9573958, Compound 54 | US9573958, Compound 79) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290175 (US9573958, Compound 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.72E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290148 (US9573958, Compound 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.78E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290204 (US9573958, Compound 54 | US9573958, Compound 79) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.89E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290187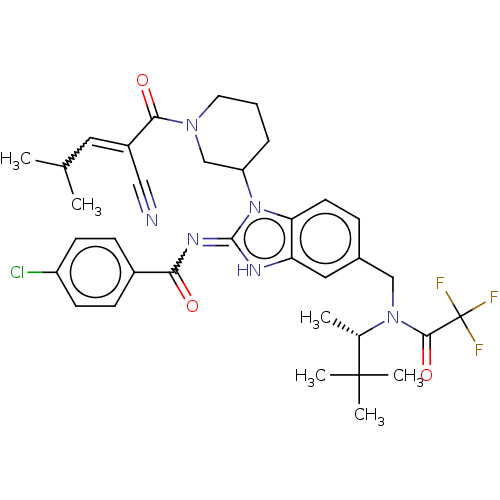 (US9573958, Compound 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >4.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290170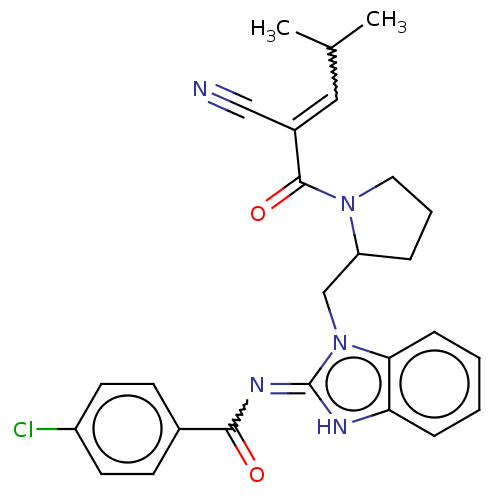 (US9573958, Compound 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290244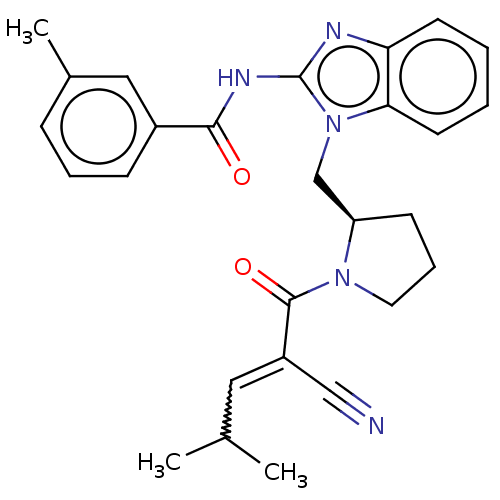 (US9573958, Compound 72) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290247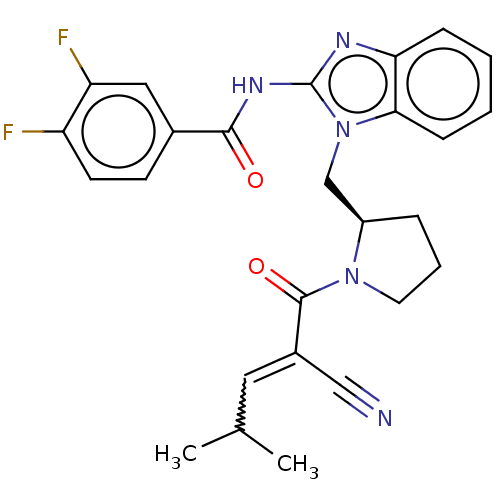 (US9573958, Compound 73) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM290157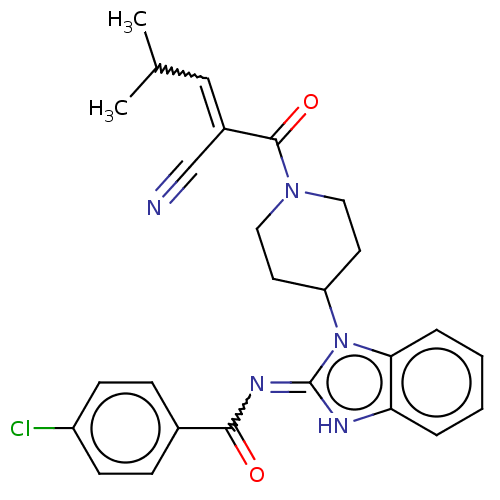 (US9573958, Compound 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC. US Patent | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | US Patent US9573958 (2017) BindingDB Entry DOI: 10.7270/Q2M047GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||