 Found 72 hits of Enzyme Inhibition Constant Data
Found 72 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084355
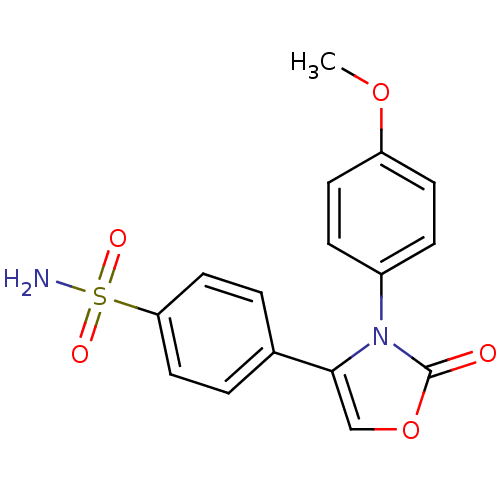
(4-[3-(4-Methoxy-phenyl)-2-oxo-2,3-dihydro-oxazol-4...)Show SMILES COc1ccc(cc1)-n1c(coc1=O)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C16H14N2O5S/c1-22-13-6-4-12(5-7-13)18-15(10-23-16(18)19)11-2-8-14(9-3-11)24(17,20)21/h2-10H,1H3,(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084352

(4-[3-(3,4-Dichloro-phenyl)-5-methyl-2-oxo-2,3-dihy...)Show SMILES Cc1oc(=O)n(c1-c1ccc(cc1)S(N)(=O)=O)-c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C16H12Cl2N2O4S/c1-9-15(10-2-5-12(6-3-10)25(19,22)23)20(16(21)24-9)11-4-7-13(17)14(18)8-11/h2-8H,1H3,(H2,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084366
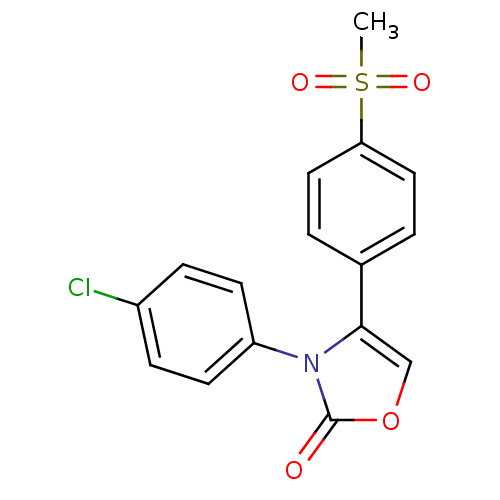
(3-(4-Chloro-phenyl)-4-(4-methanesulfonyl-phenyl)-3...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1coc(=O)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C16H12ClNO4S/c1-23(20,21)14-8-2-11(3-9-14)15-10-22-16(19)18(15)13-6-4-12(17)5-7-13/h2-10H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084359
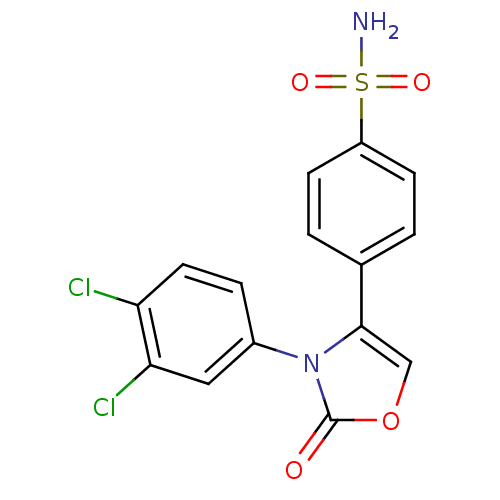
(4-[3-(3,4-Dichloro-phenyl)-2-oxo-2,3-dihydro-oxazo...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1coc(=O)n1-c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C15H10Cl2N2O4S/c16-12-6-3-10(7-13(12)17)19-14(8-23-15(19)20)9-1-4-11(5-2-9)24(18,21)22/h1-8H,(H2,18,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084345
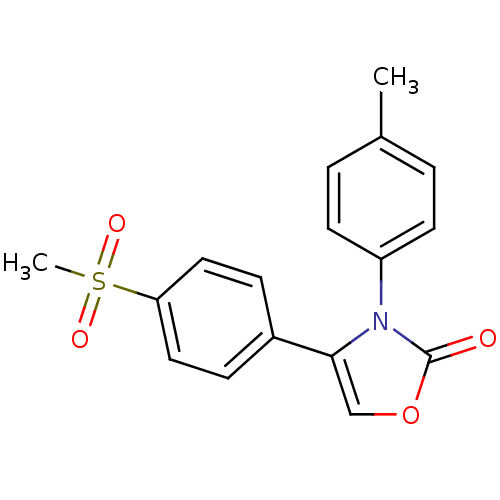
(4-(4-Methanesulfonyl-phenyl)-3-p-tolyl-3H-oxazol-2...)Show SMILES Cc1ccc(cc1)-n1c(coc1=O)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C17H15NO4S/c1-12-3-7-14(8-4-12)18-16(11-22-17(18)19)13-5-9-15(10-6-13)23(2,20)21/h3-11H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084368
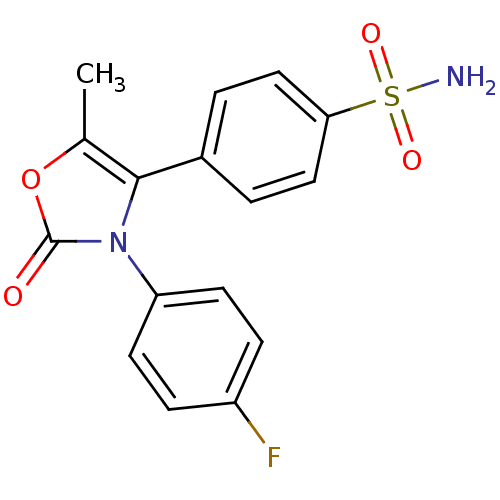
(4-[3-(4-Fluoro-phenyl)-5-methyl-2-oxo-2,3-dihydro-...)Show SMILES Cc1oc(=O)n(c1-c1ccc(cc1)S(N)(=O)=O)-c1ccc(F)cc1 Show InChI InChI=1S/C16H13FN2O4S/c1-10-15(11-2-8-14(9-3-11)24(18,21)22)19(16(20)23-10)13-6-4-12(17)5-7-13/h2-9H,1H3,(H2,18,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084353
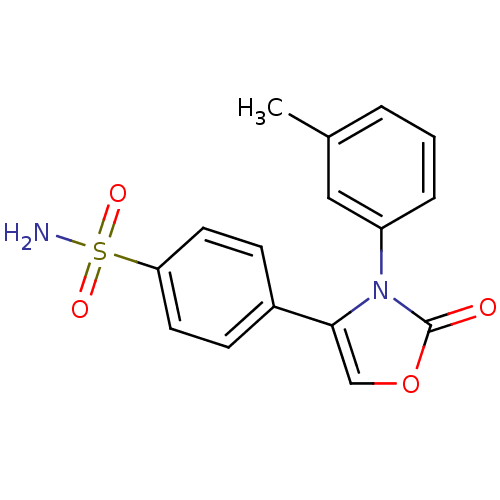
(4-(2-Oxo-3-m-tolyl-2,3-dihydro-oxazol-4-yl)-benzen...)Show SMILES Cc1cccc(c1)-n1c(coc1=O)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C16H14N2O4S/c1-11-3-2-4-13(9-11)18-15(10-22-16(18)19)12-5-7-14(8-6-12)23(17,20)21/h2-10H,1H3,(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084347
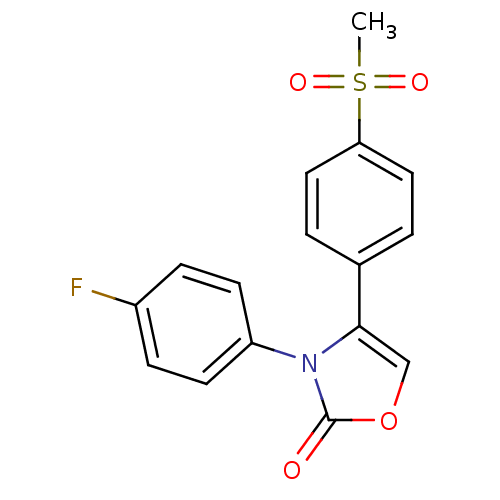
(3-(4-Fluoro-phenyl)-4-(4-methanesulfonyl-phenyl)-3...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1coc(=O)n1-c1ccc(F)cc1 Show InChI InChI=1S/C16H12FNO4S/c1-23(20,21)14-8-2-11(3-9-14)15-10-22-16(19)18(15)13-6-4-12(17)5-7-13/h2-10H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084349
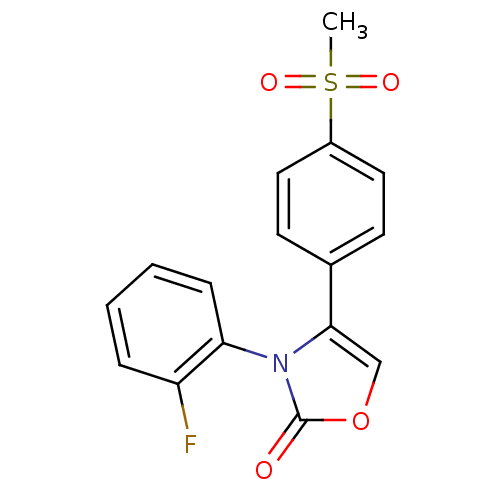
(3-(2-Fluoro-phenyl)-4-(4-methanesulfonyl-phenyl)-3...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1coc(=O)n1-c1ccccc1F |(5.04,-8.06,;6.08,-9.19,;7.18,-10.29,;5.08,-10.45,;7.11,-8.04,;6.63,-6.59,;7.67,-5.45,;9.16,-5.76,;9.64,-7.22,;8.62,-8.36,;10.18,-4.61,;11.71,-4.76,;12.32,-3.35,;11.18,-2.33,;11.34,-.8,;9.85,-3.1,;8.76,-2.01,;9.16,-.51,;8.06,.6,;6.55,.19,;6.15,-1.33,;7.26,-2.42,;6.87,-3.89,)| Show InChI InChI=1S/C16H12FNO4S/c1-23(20,21)12-8-6-11(7-9-12)15-10-22-16(19)18(15)14-5-3-2-4-13(14)17/h2-10H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084346
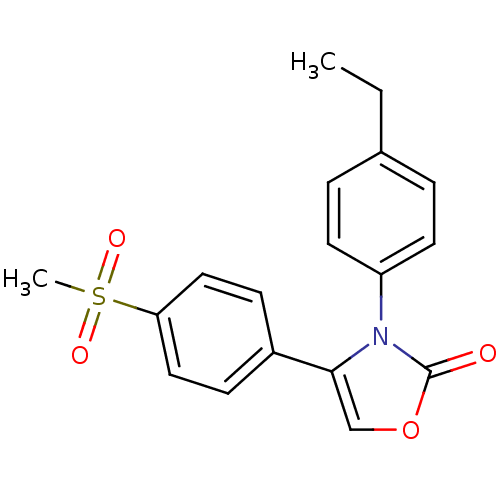
(3-(4-Ethyl-phenyl)-4-(4-methanesulfonyl-phenyl)-3H...)Show SMILES CCc1ccc(cc1)-n1c(coc1=O)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H17NO4S/c1-3-13-4-8-15(9-5-13)19-17(12-23-18(19)20)14-6-10-16(11-7-14)24(2,21)22/h4-12H,3H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084370

(4-[3-(3-Chloro-4-methoxy-phenyl)-2-oxo-2,3-dihydro...)Show SMILES COc1ccc(cc1Cl)-n1c(coc1=O)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C16H13ClN2O5S/c1-23-15-7-4-11(8-13(15)17)19-14(9-24-16(19)20)10-2-5-12(6-3-10)25(18,21)22/h2-9H,1H3,(H2,18,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084364
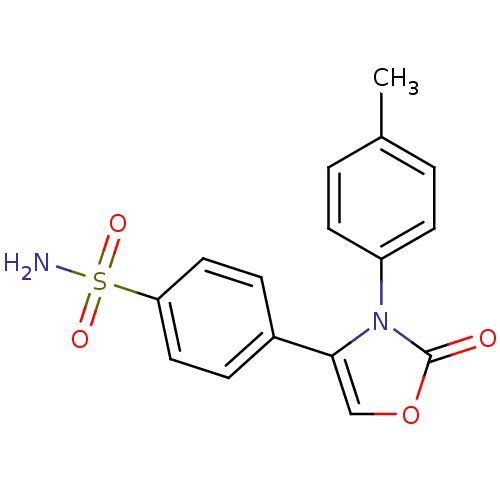
(4-(2-Oxo-3-p-tolyl-2,3-dihydro-oxazol-4-yl)-benzen...)Show SMILES Cc1ccc(cc1)-n1c(coc1=O)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C16H14N2O4S/c1-11-2-6-13(7-3-11)18-15(10-22-16(18)19)12-4-8-14(9-5-12)23(17,20)21/h2-10H,1H3,(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50084355

(4-[3-(4-Methoxy-phenyl)-2-oxo-2,3-dihydro-oxazol-4...)Show SMILES COc1ccc(cc1)-n1c(coc1=O)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C16H14N2O5S/c1-22-13-6-4-12(5-7-13)18-15(10-23-16(18)19)11-2-8-14(9-3-11)24(17,20)21/h2-10H,1H3,(H2,17,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084350

(4-(5-Methyl-2-oxo-3-m-tolyl-2,3-dihydro-oxazol-4-y...)Show SMILES Cc1oc(=O)n(c1-c1ccc(cc1)S(N)(=O)=O)-c1cccc(C)c1 Show InChI InChI=1S/C17H16N2O4S/c1-11-4-3-5-14(10-11)19-16(12(2)23-17(19)20)13-6-8-15(9-7-13)24(18,21)22/h3-10H,1-2H3,(H2,18,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084372
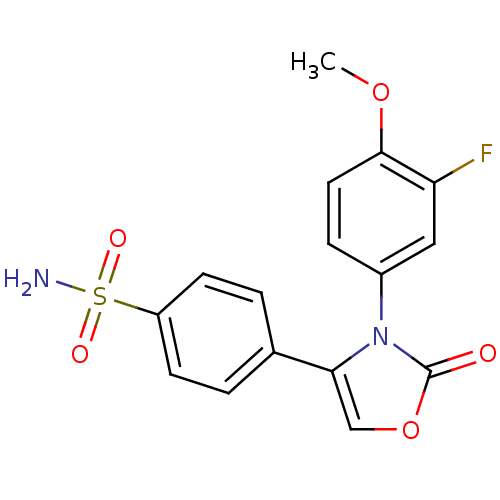
(4-[3-(3-Fluoro-4-methoxy-phenyl)-2-oxo-2,3-dihydro...)Show SMILES COc1ccc(cc1F)-n1c(coc1=O)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C16H13FN2O5S/c1-23-15-7-4-11(8-13(15)17)19-14(9-24-16(19)20)10-2-5-12(6-3-10)25(18,21)22/h2-9H,1H3,(H2,18,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084356

(4-[3-(4-Chloro-phenyl)-2-oxo-2,3-dihydro-oxazol-4-...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1coc(=O)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C15H11ClN2O4S/c16-11-3-5-12(6-4-11)18-14(9-22-15(18)19)10-1-7-13(8-2-10)23(17,20)21/h1-9H,(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084358

(3-(2,4-Difluoro-phenyl)-4-(4-methanesulfonyl-pheny...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1coc(=O)n1-c1ccc(F)cc1F |(3.16,-8.63,;4.23,-9.74,;3.23,-10.97,;5.31,-10.81,;5.24,-8.58,;4.77,-7.14,;5.8,-5.99,;7.28,-6.31,;7.77,-7.75,;6.75,-8.91,;8.3,-5.17,;9.82,-5.31,;10.44,-3.92,;9.3,-2.9,;9.45,-1.37,;7.98,-3.67,;6.89,-2.58,;7.28,-1.07,;6.19,.02,;4.69,-.4,;3.61,.69,;4.29,-1.88,;5.4,-2.97,;5.01,-4.46,)| Show InChI InChI=1S/C16H11F2NO4S/c1-24(21,22)12-5-2-10(3-6-12)15-9-23-16(20)19(15)14-7-4-11(17)8-13(14)18/h2-9H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084361

(4-[3-(4-Fluoro-phenyl)-2-oxo-2,3-dihydro-oxazol-4-...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1coc(=O)n1-c1ccc(F)cc1 Show InChI InChI=1S/C15H11FN2O4S/c16-11-3-5-12(6-4-11)18-14(9-22-15(18)19)10-1-7-13(8-2-10)23(17,20)21/h1-9H,(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50084359

(4-[3-(3,4-Dichloro-phenyl)-2-oxo-2,3-dihydro-oxazo...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1coc(=O)n1-c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C15H10Cl2N2O4S/c16-12-6-3-10(7-13(12)17)19-14(8-23-15(19)20)9-1-4-11(5-2-9)24(18,21)22/h1-8H,(H2,18,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084360
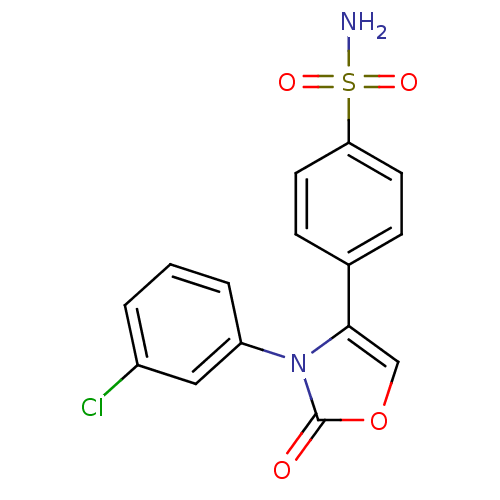
(4-[3-(3-Chloro-phenyl)-2-oxo-2,3-dihydro-oxazol-4-...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1coc(=O)n1-c1cccc(Cl)c1 Show InChI InChI=1S/C15H11ClN2O4S/c16-11-2-1-3-12(8-11)18-14(9-22-15(18)19)10-4-6-13(7-5-10)23(17,20)21/h1-9H,(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084374
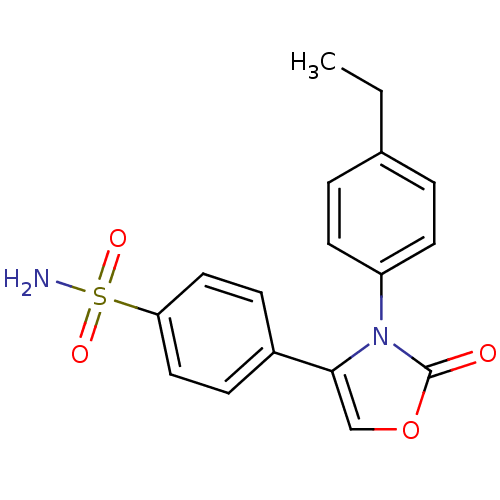
(4-[3-(4-Ethyl-phenyl)-2-oxo-2,3-dihydro-oxazol-4-y...)Show SMILES CCc1ccc(cc1)-n1c(coc1=O)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C17H16N2O4S/c1-2-12-3-7-14(8-4-12)19-16(11-23-17(19)20)13-5-9-15(10-6-13)24(18,21)22/h3-11H,2H2,1H3,(H2,18,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084344

(4-(4-Methanesulfonyl-phenyl)-3-phenyl-3H-oxazol-2-...)Show InChI InChI=1S/C16H13NO4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-16(18)17(15)13-5-3-2-4-6-13/h2-11H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084367

(4-(5-Methyl-2-oxo-3-p-tolyl-2,3-dihydro-oxazol-4-y...)Show SMILES Cc1oc(=O)n(c1-c1ccc(cc1)S(N)(=O)=O)-c1ccc(C)cc1 Show InChI InChI=1S/C17H16N2O4S/c1-11-3-7-14(8-4-11)19-16(12(2)23-17(19)20)13-5-9-15(10-6-13)24(18,21)22/h3-10H,1-2H3,(H2,18,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084362

(4-(5-Methyl-2-oxo-3-phenyl-2,3-dihydro-oxazol-4-yl...)Show SMILES Cc1oc(=O)n(c1-c1ccc(cc1)S(N)(=O)=O)-c1ccccc1 Show InChI InChI=1S/C16H14N2O4S/c1-11-15(12-7-9-14(10-8-12)23(17,20)21)18(16(19)22-11)13-5-3-2-4-6-13/h2-10H,1H3,(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084343

(4-(4-Methanesulfonyl-phenyl)-3-(4-trifluoromethyl-...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1coc(=O)n1-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C17H12F3NO4S/c1-26(23,24)14-8-2-11(3-9-14)15-10-25-16(22)21(15)13-6-4-12(5-7-13)17(18,19)20/h2-10H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084371
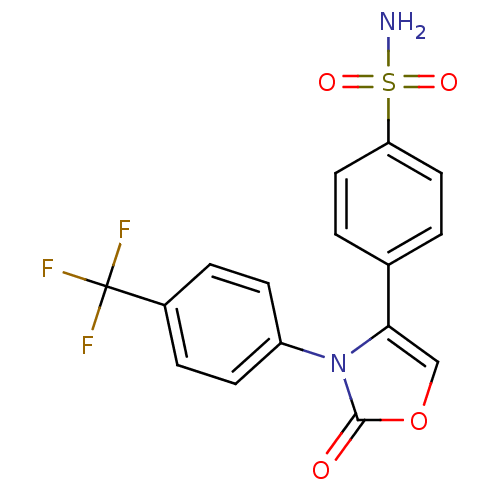
(4-[2-Oxo-3-(4-trifluoromethyl-phenyl)-2,3-dihydro-...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1coc(=O)n1-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C16H11F3N2O4S/c17-16(18,19)11-3-5-12(6-4-11)21-14(9-25-15(21)22)10-1-7-13(8-2-10)26(20,23)24/h1-9H,(H2,20,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084351

(4-[3-(2-Fluoro-phenyl)-2-oxo-2,3-dihydro-oxazol-4-...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1coc(=O)n1-c1ccccc1F |(8.31,-10.38,;9.57,-11.27,;10.84,-12.16,;8.68,-12.53,;10.47,-10.01,;12.02,-10.13,;12.88,-8.85,;12.21,-7.47,;10.67,-7.35,;9.8,-8.61,;13.09,-6.21,;14.62,-6.24,;15.11,-4.81,;13.88,-3.88,;13.91,-2.34,;12.65,-4.74,;11.57,-3.65,;11.97,-2.17,;10.89,-1.08,;9.38,-1.49,;8.99,-2.95,;10.08,-4.04,;9.68,-5.54,)| Show InChI InChI=1S/C15H11FN2O4S/c16-12-3-1-2-4-13(12)18-14(9-22-15(18)19)10-5-7-11(8-6-10)23(17,20)21/h1-9H,(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084363

(4-[3-(2,4-Difluoro-phenyl)-2-oxo-2,3-dihydro-oxazo...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1coc(=O)n1-c1ccc(F)cc1F |(5.11,-6.95,;6.44,-7.71,;7.53,-8.8,;5.44,-8.97,;7.46,-6.58,;8.97,-6.89,;9.99,-5.74,;9.5,-4.29,;8.01,-3.98,;6.99,-5.11,;10.52,-3.14,;12.04,-3.3,;12.66,-1.89,;11.52,-.87,;11.67,.65,;10.2,-1.65,;9.11,-.56,;9.5,.94,;8.41,2.04,;6.9,1.64,;5.83,2.72,;6.51,.13,;7.61,-.96,;7.23,-2.44,)| Show InChI InChI=1S/C15H10F2N2O4S/c16-10-3-6-13(12(17)7-10)19-14(8-23-15(19)20)9-1-4-11(5-2-9)24(18,21)22/h1-8H,(H2,18,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084365
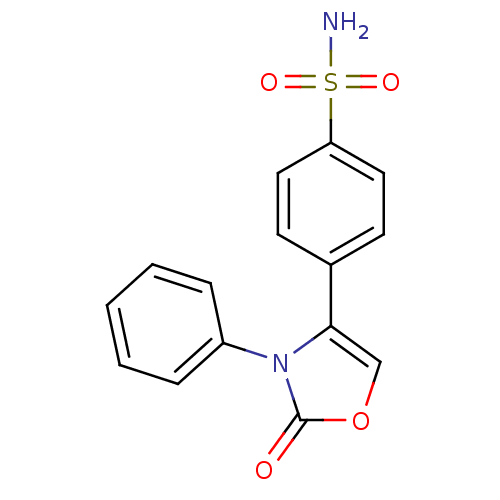
(4-(2-Oxo-3-phenyl-2,3-dihydro-oxazol-4-yl)-benzene...)Show InChI InChI=1S/C15H12N2O4S/c16-22(19,20)13-8-6-11(7-9-13)14-10-21-15(18)17(14)12-4-2-1-3-5-12/h1-10H,(H2,16,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084373

(4-(3-Naphthalen-1-yl-2-oxo-2,3-dihydro-oxazol-4-yl...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1coc(=O)n1-c1cccc2ccccc12 |(3.68,-8.38,;5.02,-9.14,;4.02,-10.39,;6.09,-10.22,;6.03,-8.01,;7.52,-8.32,;8.55,-7.17,;8.07,-5.73,;6.57,-5.42,;5.56,-6.55,;9.08,-4.58,;10.59,-4.74,;11.21,-3.34,;10.06,-2.32,;10.22,-.81,;8.75,-3.1,;7.66,-2.01,;6.17,-2.4,;5.07,-1.32,;5.48,.18,;6.97,.58,;7.36,2.07,;8.85,2.47,;9.95,1.38,;9.55,-.12,;8.05,-.52,)| Show InChI InChI=1S/C19H14N2O4S/c20-26(23,24)15-10-8-14(9-11-15)18-12-25-19(22)21(18)17-7-3-5-13-4-1-2-6-16(13)17/h1-12H,(H2,20,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50084347

(3-(4-Fluoro-phenyl)-4-(4-methanesulfonyl-phenyl)-3...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1coc(=O)n1-c1ccc(F)cc1 Show InChI InChI=1S/C16H12FNO4S/c1-23(20,21)14-8-2-11(3-9-14)15-10-22-16(19)18(15)13-6-4-12(17)5-7-13/h2-10H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50084372

(4-[3-(3-Fluoro-4-methoxy-phenyl)-2-oxo-2,3-dihydro...)Show SMILES COc1ccc(cc1F)-n1c(coc1=O)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C16H13FN2O5S/c1-23-15-7-4-11(8-13(15)17)19-14(9-24-16(19)20)10-2-5-12(6-3-10)25(18,21)22/h2-9H,1H3,(H2,18,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50084370

(4-[3-(3-Chloro-4-methoxy-phenyl)-2-oxo-2,3-dihydro...)Show SMILES COc1ccc(cc1Cl)-n1c(coc1=O)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C16H13ClN2O5S/c1-23-15-7-4-11(8-13(15)17)19-14(9-24-16(19)20)10-2-5-12(6-3-10)25(18,21)22/h2-9H,1H3,(H2,18,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084342
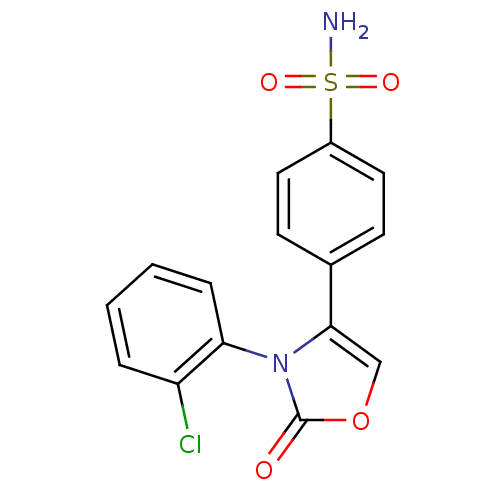
(4-[3-(2-Chloro-phenyl)-2-oxo-2,3-dihydro-oxazol-4-...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1coc(=O)n1-c1ccccc1Cl |(4.41,-9.17,;5.68,-10.06,;6.94,-10.95,;4.78,-11.33,;6.56,-8.8,;8.12,-8.92,;8.99,-7.64,;8.31,-6.26,;6.77,-6.14,;5.9,-7.4,;9.19,-5,;10.72,-5.02,;11.21,-3.6,;9.99,-2.67,;10.02,-1.13,;8.75,-3.53,;7.66,-2.43,;8.07,-.96,;6.98,.13,;5.49,-.28,;5.09,-1.73,;6.18,-2.83,;5.77,-4.32,)| Show InChI InChI=1S/C15H11ClN2O4S/c16-12-3-1-2-4-13(12)18-14(9-22-15(18)19)10-5-7-11(8-6-10)23(17,20)21/h1-9H,(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50084353

(4-(2-Oxo-3-m-tolyl-2,3-dihydro-oxazol-4-yl)-benzen...)Show SMILES Cc1cccc(c1)-n1c(coc1=O)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C16H14N2O4S/c1-11-3-2-4-13(9-11)18-15(10-22-16(18)19)12-5-7-14(8-6-12)23(17,20)21/h2-10H,1H3,(H2,17,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50084358

(3-(2,4-Difluoro-phenyl)-4-(4-methanesulfonyl-pheny...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1coc(=O)n1-c1ccc(F)cc1F |(3.16,-8.63,;4.23,-9.74,;3.23,-10.97,;5.31,-10.81,;5.24,-8.58,;4.77,-7.14,;5.8,-5.99,;7.28,-6.31,;7.77,-7.75,;6.75,-8.91,;8.3,-5.17,;9.82,-5.31,;10.44,-3.92,;9.3,-2.9,;9.45,-1.37,;7.98,-3.67,;6.89,-2.58,;7.28,-1.07,;6.19,.02,;4.69,-.4,;3.61,.69,;4.29,-1.88,;5.4,-2.97,;5.01,-4.46,)| Show InChI InChI=1S/C16H11F2NO4S/c1-24(21,22)12-5-2-10(3-6-12)15-9-23-16(20)19(15)14-7-4-11(17)8-13(14)18/h2-9H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50084356

(4-[3-(4-Chloro-phenyl)-2-oxo-2,3-dihydro-oxazol-4-...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1coc(=O)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C15H11ClN2O4S/c16-11-3-5-12(6-4-11)18-14(9-22-15(18)19)10-1-7-13(8-2-10)23(17,20)21/h1-9H,(H2,17,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50084352

(4-[3-(3,4-Dichloro-phenyl)-5-methyl-2-oxo-2,3-dihy...)Show SMILES Cc1oc(=O)n(c1-c1ccc(cc1)S(N)(=O)=O)-c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C16H12Cl2N2O4S/c1-9-15(10-2-5-12(6-3-10)25(19,22)23)20(16(21)24-9)11-4-7-13(17)14(18)8-11/h2-8H,1H3,(H2,19,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084369
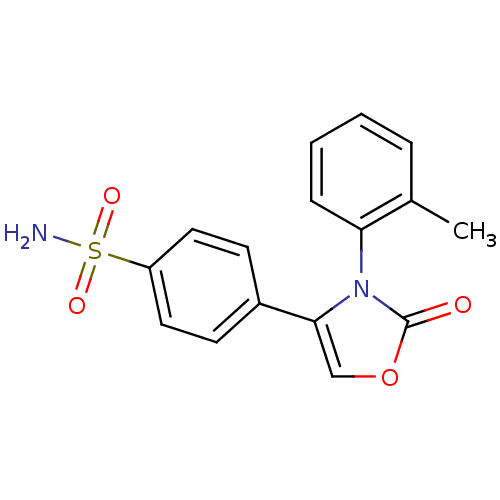
(4-(2-Oxo-3-o-tolyl-2,3-dihydro-oxazol-4-yl)-benzen...)Show SMILES Cc1ccccc1-n1c(coc1=O)-c1ccc(cc1)S(N)(=O)=O |(11.18,-5.11,;11.57,-3.63,;10.47,-2.54,;10.87,-1.03,;12.36,-.63,;13.47,-1.73,;13.06,-3.23,;14.15,-4.32,;14.49,-5.81,;16.01,-5.97,;16.63,-4.56,;15.48,-3.54,;15.64,-2.02,;13.47,-6.97,;13.96,-8.42,;12.92,-9.57,;11.43,-9.25,;10.95,-7.79,;11.97,-6.65,;10.4,-10.39,;9.07,-9.62,;11.48,-11.48,;9.41,-11.64,)| Show InChI InChI=1S/C16H14N2O4S/c1-11-4-2-3-5-14(11)18-15(10-22-16(18)19)12-6-8-13(9-7-12)23(17,20)21/h2-10H,1H3,(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50084374

(4-[3-(4-Ethyl-phenyl)-2-oxo-2,3-dihydro-oxazol-4-y...)Show SMILES CCc1ccc(cc1)-n1c(coc1=O)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C17H16N2O4S/c1-2-12-3-7-14(8-4-12)19-16(11-23-17(19)20)13-5-9-15(10-6-13)24(18,21)22/h3-11H,2H2,1H3,(H2,18,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50084360

(4-[3-(3-Chloro-phenyl)-2-oxo-2,3-dihydro-oxazol-4-...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1coc(=O)n1-c1cccc(Cl)c1 Show InChI InChI=1S/C15H11ClN2O4S/c16-11-2-1-3-12(8-11)18-14(9-22-15(18)19)10-4-6-13(7-5-10)23(17,20)21/h1-9H,(H2,17,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50084364

(4-(2-Oxo-3-p-tolyl-2,3-dihydro-oxazol-4-yl)-benzen...)Show SMILES Cc1ccc(cc1)-n1c(coc1=O)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C16H14N2O4S/c1-11-2-6-13(7-3-11)18-15(10-22-16(18)19)12-4-8-14(9-5-12)23(17,20)21/h2-10H,1H3,(H2,17,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50339185
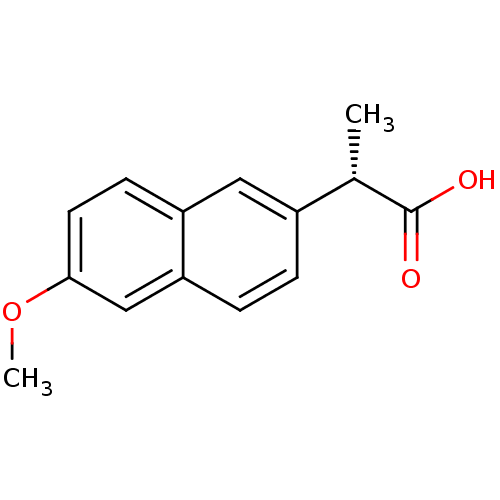
((2S)-2-(6-methoxynaphthalen-2-yl)propanoic acid | ...)Show InChI InChI=1S/C14H14O3/c1-9(14(15)16)10-3-4-12-8-13(17-2)6-5-11(12)7-10/h3-9H,1-2H3,(H,15,16)/t9-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50084345

(4-(4-Methanesulfonyl-phenyl)-3-p-tolyl-3H-oxazol-2...)Show SMILES Cc1ccc(cc1)-n1c(coc1=O)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C17H15NO4S/c1-12-3-7-14(8-4-12)18-16(11-22-17(18)19)13-5-9-15(10-6-13)23(2,20)21/h3-11H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50084361

(4-[3-(4-Fluoro-phenyl)-2-oxo-2,3-dihydro-oxazol-4-...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1coc(=O)n1-c1ccc(F)cc1 Show InChI InChI=1S/C15H11FN2O4S/c16-11-3-5-12(6-4-11)18-14(9-22-15(18)19)10-1-7-13(8-2-10)23(17,20)21/h1-9H,(H2,17,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50084349

(3-(2-Fluoro-phenyl)-4-(4-methanesulfonyl-phenyl)-3...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1coc(=O)n1-c1ccccc1F |(5.04,-8.06,;6.08,-9.19,;7.18,-10.29,;5.08,-10.45,;7.11,-8.04,;6.63,-6.59,;7.67,-5.45,;9.16,-5.76,;9.64,-7.22,;8.62,-8.36,;10.18,-4.61,;11.71,-4.76,;12.32,-3.35,;11.18,-2.33,;11.34,-.8,;9.85,-3.1,;8.76,-2.01,;9.16,-.51,;8.06,.6,;6.55,.19,;6.15,-1.33,;7.26,-2.42,;6.87,-3.89,)| Show InChI InChI=1S/C16H12FNO4S/c1-23(20,21)12-8-6-11(7-9-12)15-10-22-16(19)18(15)14-5-3-2-4-13(14)17/h2-10H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50084366

(3-(4-Chloro-phenyl)-4-(4-methanesulfonyl-phenyl)-3...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1coc(=O)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C16H12ClNO4S/c1-23(20,21)14-8-2-11(3-9-14)15-10-22-16(19)18(15)13-6-4-12(17)5-7-13/h2-10H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50084363

(4-[3-(2,4-Difluoro-phenyl)-2-oxo-2,3-dihydro-oxazo...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1coc(=O)n1-c1ccc(F)cc1F |(5.11,-6.95,;6.44,-7.71,;7.53,-8.8,;5.44,-8.97,;7.46,-6.58,;8.97,-6.89,;9.99,-5.74,;9.5,-4.29,;8.01,-3.98,;6.99,-5.11,;10.52,-3.14,;12.04,-3.3,;12.66,-1.89,;11.52,-.87,;11.67,.65,;10.2,-1.65,;9.11,-.56,;9.5,.94,;8.41,2.04,;6.9,1.64,;5.83,2.72,;6.51,.13,;7.61,-.96,;7.23,-2.44,)| Show InChI InChI=1S/C15H10F2N2O4S/c16-10-3-6-13(12(17)7-10)19-14(8-23-15(19)20)9-1-4-11(5-2-9)24(18,21)22/h1-8H,(H2,18,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50084350

(4-(5-Methyl-2-oxo-3-m-tolyl-2,3-dihydro-oxazol-4-y...)Show SMILES Cc1oc(=O)n(c1-c1ccc(cc1)S(N)(=O)=O)-c1cccc(C)c1 Show InChI InChI=1S/C17H16N2O4S/c1-11-4-3-5-14(10-11)19-16(12(2)23-17(19)20)13-6-8-15(9-7-13)24(18,21)22/h3-10H,1-2H3,(H2,18,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084354
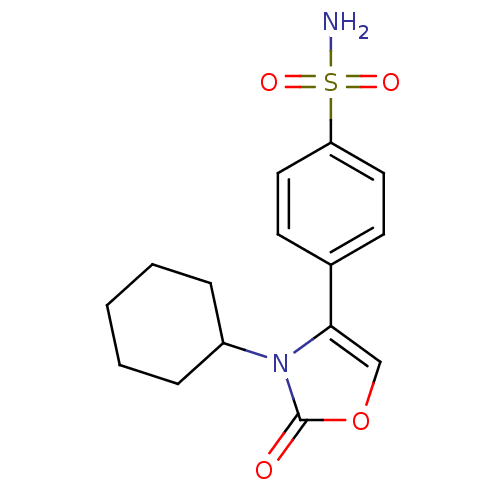
(4-(3-Cyclohexyl-2-oxo-2,3-dihydro-oxazol-4-yl)-ben...)Show InChI InChI=1S/C15H18N2O4S/c16-22(19,20)13-8-6-11(7-9-13)14-10-21-15(18)17(14)12-4-2-1-3-5-12/h6-10,12H,1-5H2,(H2,16,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50339185

((2S)-2-(6-methoxynaphthalen-2-yl)propanoic acid | ...)Show InChI InChI=1S/C14H14O3/c1-9(14(15)16)10-3-4-12-8-13(17-2)6-5-11(12)7-10/h3-9H,1-2H3,(H,15,16)/t9-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 1.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50084365

(4-(2-Oxo-3-phenyl-2,3-dihydro-oxazol-4-yl)-benzene...)Show InChI InChI=1S/C15H12N2O4S/c16-22(19,20)13-8-6-11(7-9-13)14-10-21-15(18)17(14)12-4-2-1-3-5-12/h1-10H,(H2,16,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50084351

(4-[3-(2-Fluoro-phenyl)-2-oxo-2,3-dihydro-oxazol-4-...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1coc(=O)n1-c1ccccc1F |(8.31,-10.38,;9.57,-11.27,;10.84,-12.16,;8.68,-12.53,;10.47,-10.01,;12.02,-10.13,;12.88,-8.85,;12.21,-7.47,;10.67,-7.35,;9.8,-8.61,;13.09,-6.21,;14.62,-6.24,;15.11,-4.81,;13.88,-3.88,;13.91,-2.34,;12.65,-4.74,;11.57,-3.65,;11.97,-2.17,;10.89,-1.08,;9.38,-1.49,;8.99,-2.95,;10.08,-4.04,;9.68,-5.54,)| Show InChI InChI=1S/C15H11FN2O4S/c16-12-3-1-2-4-13(12)18-14(9-22-15(18)19)10-5-7-11(8-6-10)23(17,20)21/h1-9H,(H2,17,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50084369

(4-(2-Oxo-3-o-tolyl-2,3-dihydro-oxazol-4-yl)-benzen...)Show SMILES Cc1ccccc1-n1c(coc1=O)-c1ccc(cc1)S(N)(=O)=O |(11.18,-5.11,;11.57,-3.63,;10.47,-2.54,;10.87,-1.03,;12.36,-.63,;13.47,-1.73,;13.06,-3.23,;14.15,-4.32,;14.49,-5.81,;16.01,-5.97,;16.63,-4.56,;15.48,-3.54,;15.64,-2.02,;13.47,-6.97,;13.96,-8.42,;12.92,-9.57,;11.43,-9.25,;10.95,-7.79,;11.97,-6.65,;10.4,-10.39,;9.07,-9.62,;11.48,-11.48,;9.41,-11.64,)| Show InChI InChI=1S/C16H14N2O4S/c1-11-4-2-3-5-14(11)18-15(10-22-16(18)19)12-6-8-13(9-7-12)23(17,20)21/h2-10H,1H3,(H2,17,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50084344

(4-(4-Methanesulfonyl-phenyl)-3-phenyl-3H-oxazol-2-...)Show InChI InChI=1S/C16H13NO4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-16(18)17(15)13-5-3-2-4-6-13/h2-11H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50084342

(4-[3-(2-Chloro-phenyl)-2-oxo-2,3-dihydro-oxazol-4-...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1coc(=O)n1-c1ccccc1Cl |(4.41,-9.17,;5.68,-10.06,;6.94,-10.95,;4.78,-11.33,;6.56,-8.8,;8.12,-8.92,;8.99,-7.64,;8.31,-6.26,;6.77,-6.14,;5.9,-7.4,;9.19,-5,;10.72,-5.02,;11.21,-3.6,;9.99,-2.67,;10.02,-1.13,;8.75,-3.53,;7.66,-2.43,;8.07,-.96,;6.98,.13,;5.49,-.28,;5.09,-1.73,;6.18,-2.83,;5.77,-4.32,)| Show InChI InChI=1S/C15H11ClN2O4S/c16-12-3-1-2-4-13(12)18-14(9-22-15(18)19)10-5-7-11(8-6-10)23(17,20)21/h1-9H,(H2,17,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50084373

(4-(3-Naphthalen-1-yl-2-oxo-2,3-dihydro-oxazol-4-yl...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1coc(=O)n1-c1cccc2ccccc12 |(3.68,-8.38,;5.02,-9.14,;4.02,-10.39,;6.09,-10.22,;6.03,-8.01,;7.52,-8.32,;8.55,-7.17,;8.07,-5.73,;6.57,-5.42,;5.56,-6.55,;9.08,-4.58,;10.59,-4.74,;11.21,-3.34,;10.06,-2.32,;10.22,-.81,;8.75,-3.1,;7.66,-2.01,;6.17,-2.4,;5.07,-1.32,;5.48,.18,;6.97,.58,;7.36,2.07,;8.85,2.47,;9.95,1.38,;9.55,-.12,;8.05,-.52,)| Show InChI InChI=1S/C19H14N2O4S/c20-26(23,24)15-10-8-14(9-11-15)18-12-25-19(22)21(18)17-7-3-5-13-4-1-2-6-16(13)17/h1-12H,(H2,20,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50084346

(3-(4-Ethyl-phenyl)-4-(4-methanesulfonyl-phenyl)-3H...)Show SMILES CCc1ccc(cc1)-n1c(coc1=O)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H17NO4S/c1-3-13-4-8-15(9-5-13)19-17(12-23-18(19)20)14-6-10-16(11-7-14)24(2,21)22/h4-12H,3H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50084367

(4-(5-Methyl-2-oxo-3-p-tolyl-2,3-dihydro-oxazol-4-y...)Show SMILES Cc1oc(=O)n(c1-c1ccc(cc1)S(N)(=O)=O)-c1ccc(C)cc1 Show InChI InChI=1S/C17H16N2O4S/c1-11-3-7-14(8-4-11)19-16(12(2)23-17(19)20)13-5-9-15(10-6-13)24(18,21)22/h3-10H,1-2H3,(H2,18,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50084368

(4-[3-(4-Fluoro-phenyl)-5-methyl-2-oxo-2,3-dihydro-...)Show SMILES Cc1oc(=O)n(c1-c1ccc(cc1)S(N)(=O)=O)-c1ccc(F)cc1 Show InChI InChI=1S/C16H13FN2O4S/c1-10-15(11-2-8-14(9-3-11)24(18,21)22)19(16(20)23-10)13-6-4-12(17)5-7-13/h2-9H,1H3,(H2,18,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50084362

(4-(5-Methyl-2-oxo-3-phenyl-2,3-dihydro-oxazol-4-yl...)Show SMILES Cc1oc(=O)n(c1-c1ccc(cc1)S(N)(=O)=O)-c1ccccc1 Show InChI InChI=1S/C16H14N2O4S/c1-11-15(12-7-9-14(10-8-12)23(17,20)21)18(16(19)22-11)13-5-3-2-4-6-13/h2-10H,1H3,(H2,17,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084348

(4-[2-Oxo-4-(4-sulfamoyl-phenyl)-oxazol-3-yl]-benzo...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1coc(=O)n1-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C16H12N2O6S/c17-25(22,23)13-7-3-10(4-8-13)14-9-24-16(21)18(14)12-5-1-11(2-6-12)15(19)20/h1-9H,(H,19,20)(H2,17,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50084343

(4-(4-Methanesulfonyl-phenyl)-3-(4-trifluoromethyl-...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1coc(=O)n1-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C17H12F3NO4S/c1-26(23,24)14-8-2-11(3-9-14)15-10-25-16(22)21(15)13-6-4-12(5-7-13)17(18,19)20/h2-10H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084357

(3-[2-Oxo-4-(4-sulfamoyl-phenyl)-oxazol-3-yl]-benzo...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1coc(=O)n1-c1cccc(c1)C(O)=O Show InChI InChI=1S/C16H12N2O6S/c17-25(22,23)13-6-4-10(5-7-13)14-9-24-16(21)18(14)12-3-1-2-11(8-12)15(19)20/h1-9H,(H,19,20)(H2,17,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50084371

(4-[2-Oxo-3-(4-trifluoromethyl-phenyl)-2,3-dihydro-...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1coc(=O)n1-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C16H11F3N2O4S/c17-16(18,19)11-3-5-12(6-4-11)21-14(9-25-15(21)22)10-1-7-13(8-2-10)26(20,23)24/h1-9H,(H2,20,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50084357

(3-[2-Oxo-4-(4-sulfamoyl-phenyl)-oxazol-3-yl]-benzo...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1coc(=O)n1-c1cccc(c1)C(O)=O Show InChI InChI=1S/C16H12N2O6S/c17-25(22,23)13-6-4-10(5-7-13)14-9-24-16(21)18(14)12-3-1-2-11(8-12)15(19)20/h1-9H,(H,19,20)(H2,17,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50084354

(4-(3-Cyclohexyl-2-oxo-2,3-dihydro-oxazol-4-yl)-ben...)Show InChI InChI=1S/C15H18N2O4S/c16-22(19,20)13-8-6-11(7-9-13)14-10-21-15(18)17(14)12-4-2-1-3-5-12/h6-10,12H,1-5H2,(H2,16,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50084348

(4-[2-Oxo-4-(4-sulfamoyl-phenyl)-oxazol-3-yl]-benzo...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1coc(=O)n1-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C16H12N2O6S/c17-25(22,23)13-7-3-10(4-8-13)14-9-24-16(21)18(14)12-5-1-11(2-6-12)15(19)20/h1-9H,(H,19,20)(H2,17,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data