

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50122764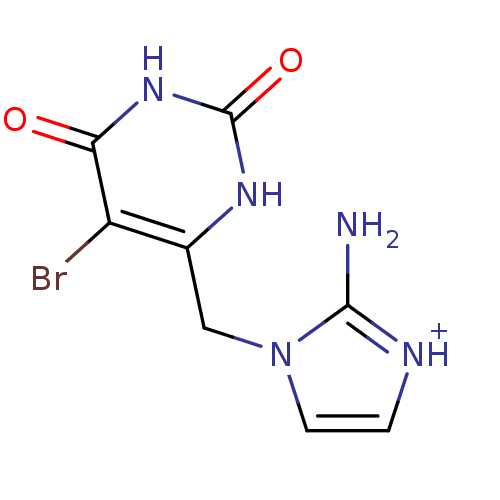 (2-Amino-1-(5-bromo-2,6-dioxo-1,2,3,6-tetrahydro-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against recombinant purified E. coli Thymidine Phosphorylase | J Med Chem 46: 207-9 (2003) Article DOI: 10.1021/jm020964w BindingDB Entry DOI: 10.7270/Q24X574R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20079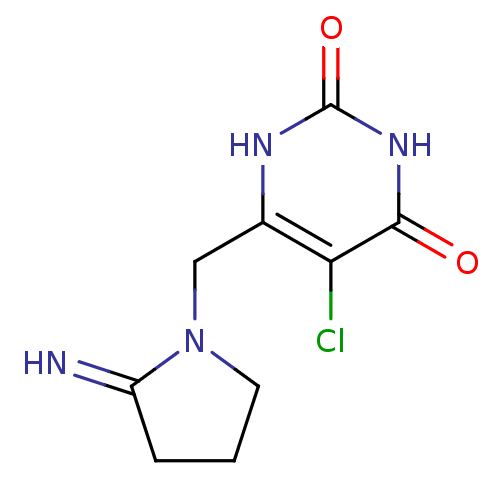 (5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]-1,2,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against recombinant purified E. coli Thymidine Phosphorylase | J Med Chem 46: 207-9 (2003) Article DOI: 10.1021/jm020964w BindingDB Entry DOI: 10.7270/Q24X574R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50122771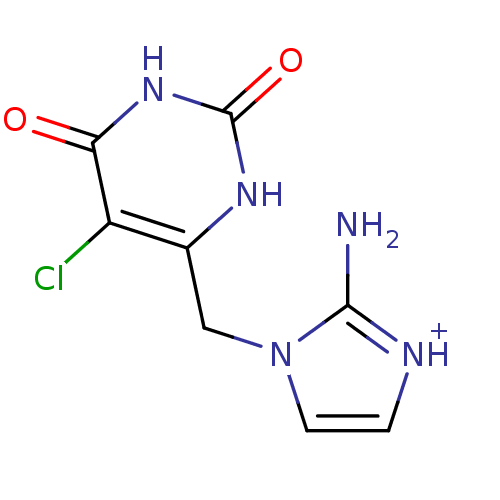 (2-Amino-1-(5-chloro-2,6-dioxo-1,2,3,6-tetrahydro-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against recombinant purified E. coli Thymidine Phosphorylase | J Med Chem 46: 207-9 (2003) Article DOI: 10.1021/jm020964w BindingDB Entry DOI: 10.7270/Q24X574R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50122768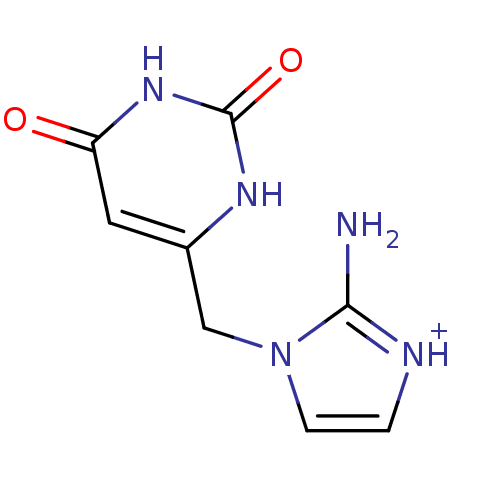 (2-Amino-1-(2,6-dioxo-1,2,3,6-tetrahydro-pyrimidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against recombinant purified E. coli Thymidine Phosphorylase | J Med Chem 46: 207-9 (2003) Article DOI: 10.1021/jm020964w BindingDB Entry DOI: 10.7270/Q24X574R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50122770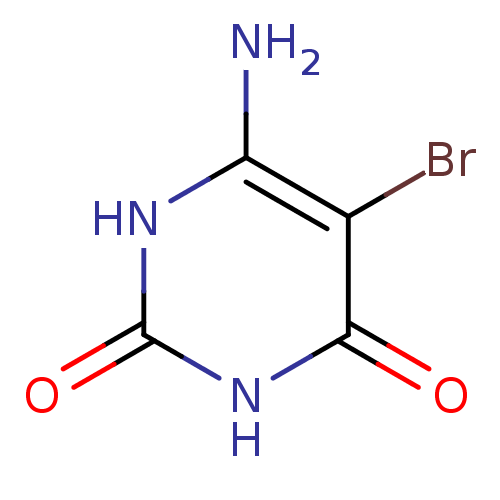 (6-Amino-5-bromo-1H-pyrimidine-2,4-dione | 6-Amino-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against recombinant purified E. coli Thymidine Phosphorylase | J Med Chem 46: 207-9 (2003) Article DOI: 10.1021/jm020964w BindingDB Entry DOI: 10.7270/Q24X574R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50122765 (6-(2-Nitro-imidazol-1-ylmethyl)-1H-pyrimidine-2,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against recombinant purified E. coli Thymidine Phosphorylase | J Med Chem 46: 207-9 (2003) Article DOI: 10.1021/jm020964w BindingDB Entry DOI: 10.7270/Q24X574R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50122767 (5-Chloro-6-(2-nitro-imidazol-1-ylmethyl)-1H-pyrimi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against recombinant purified E. coli Thymidine Phosphorylase | J Med Chem 46: 207-9 (2003) Article DOI: 10.1021/jm020964w BindingDB Entry DOI: 10.7270/Q24X574R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50122766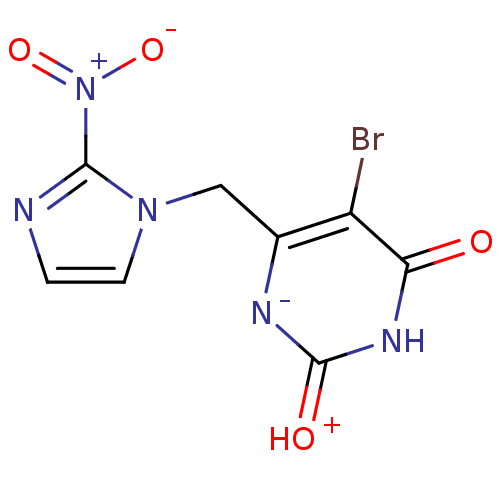 (5-Bromo-6-(2-nitro-imidazol-1-ylmethyl)-1H-pyrimid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against recombinant purified E. coli Thymidine Phosphorylase | J Med Chem 46: 207-9 (2003) Article DOI: 10.1021/jm020964w BindingDB Entry DOI: 10.7270/Q24X574R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||