 Found 49 hits of Enzyme Inhibition Constant Data
Found 49 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50123324

(7-Methoxy-6-oxazol-5-yl-2-phenyl-1H-quinolin-4-one...)Show InChI InChI=1S/C19H14N2O3/c1-23-18-9-16-13(7-14(18)19-10-20-11-24-19)17(22)8-15(21-16)12-5-3-2-4-6-12/h2-11H,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50112565
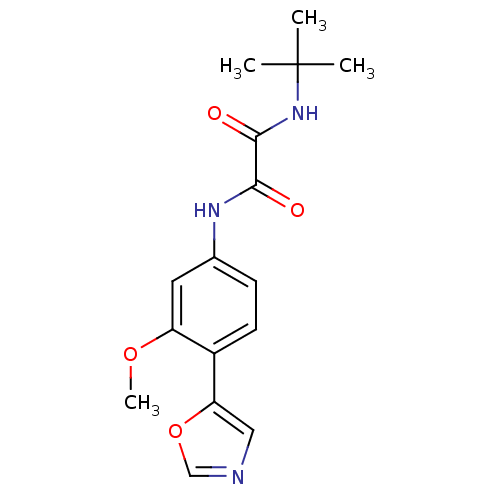
(CHEMBL25138 | N-tert-Butyl-N'-(3-methoxy-4-oxazol-...)Show InChI InChI=1S/C16H19N3O4/c1-16(2,3)19-15(21)14(20)18-10-5-6-11(12(7-10)22-4)13-8-17-9-23-13/h5-9H,1-4H3,(H,18,20)(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50113230
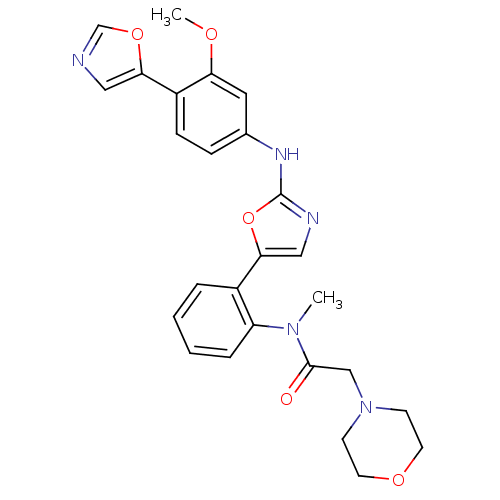
(BMS-337197 | CHEMBL64830 | N-{2-[2-(3-Methoxy-4-ox...)Show SMILES COc1cc(Nc2ncc(o2)-c2ccccc2N(C)C(=O)CN2CCOCC2)ccc1-c1cnco1 Show InChI InChI=1S/C26H27N5O5/c1-30(25(32)16-31-9-11-34-12-10-31)21-6-4-3-5-19(21)24-15-28-26(36-24)29-18-7-8-20(22(13-18)33-2)23-14-27-17-35-23/h3-8,13-15,17H,9-12,16H2,1-2H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50119045

(1-(3-Methoxy-4-oxazol-5-yl-phenyl)-3-m-tolyl-urea ...)Show InChI InChI=1S/C18H17N3O3/c1-12-4-3-5-13(8-12)20-18(22)21-14-6-7-15(16(9-14)23-2)17-10-19-11-24-17/h3-11H,1-2H3,(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50113224
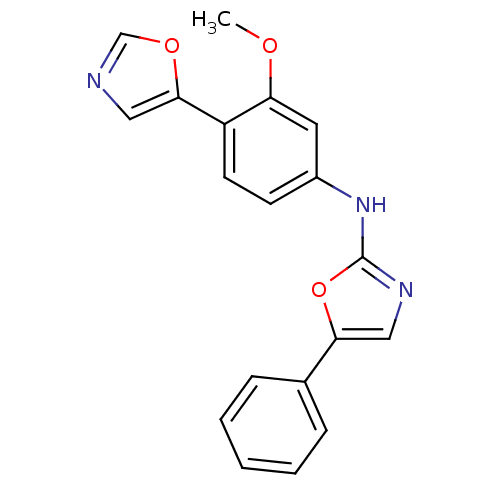
((3-Methoxy-4-oxazol-5-yl-phenyl)-(5-phenyl-oxazol-...)Show InChI InChI=1S/C19H15N3O3/c1-23-16-9-14(7-8-15(16)18-10-20-12-24-18)22-19-21-11-17(25-19)13-5-3-2-4-6-13/h2-12H,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50129116

(2-Hydroxy-N-{2-[2-(3-methoxy-4-oxazol-5-yl-phenyla...)Show SMILES COc1cc(Nc2ncc(o2)-c2ccccc2N(C)C(=O)CO)ccc1-c1cnco1 Show InChI InChI=1S/C22H20N4O5/c1-26(21(28)12-27)17-6-4-3-5-15(17)20-11-24-22(31-20)25-14-7-8-16(18(9-14)29-2)19-10-23-13-30-19/h3-11,13,27H,12H2,1-2H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50129109
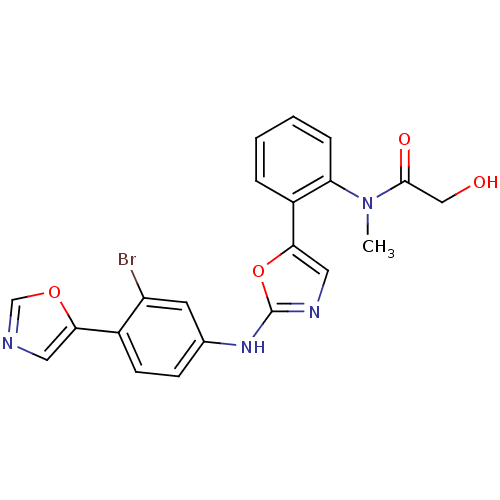
(CHEMBL62748 | N-{2-[2-(3-Bromo-4-oxazol-5-yl-pheny...)Show SMILES CN(C(=O)CO)c1ccccc1-c1cnc(Nc2ccc(-c3cnco3)c(Br)c2)o1 Show InChI InChI=1S/C21H17BrN4O4/c1-26(20(28)11-27)17-5-3-2-4-15(17)19-10-24-21(30-19)25-13-6-7-14(16(22)8-13)18-9-23-12-29-18/h2-10,12,27H,11H2,1H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50119033

((E)-3-Furan-2-yl-N-(3-methoxy-4-oxazol-5-yl-phenyl...)Show InChI InChI=1S/C17H14N2O4/c1-21-15-9-12(4-6-14(15)16-10-18-11-23-16)19-17(20)7-5-13-3-2-8-22-13/h2-11H,1H3,(H,19,20)/b7-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50129090

(CHEMBL305374 | N-{2-[2-(3-Bromo-4-oxazol-5-yl-phen...)Show SMILES CN(C(=O)CN1CCOCC1)c1ccccc1-c1cnc(Nc2ccc(-c3cnco3)c(Br)c2)o1 Show InChI InChI=1S/C25H24BrN5O4/c1-30(24(32)15-31-8-10-33-11-9-31)21-5-3-2-4-19(21)23-14-28-25(35-23)29-17-6-7-18(20(26)12-17)22-13-27-16-34-22/h2-7,12-14,16H,8-11,15H2,1H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50129106

(CHEMBL63122 | N-{2-[2-(3-Chloro-4-oxazol-5-yl-phen...)Show SMILES CN(C(=O)CO)c1ccccc1-c1cnc(Nc2ccc(-c3cnco3)c(Cl)c2)o1 Show InChI InChI=1S/C21H17ClN4O4/c1-26(20(28)11-27)17-5-3-2-4-15(17)19-10-24-21(30-19)25-13-6-7-14(16(22)8-13)18-9-23-12-29-18/h2-10,12,27H,11H2,1H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50129093
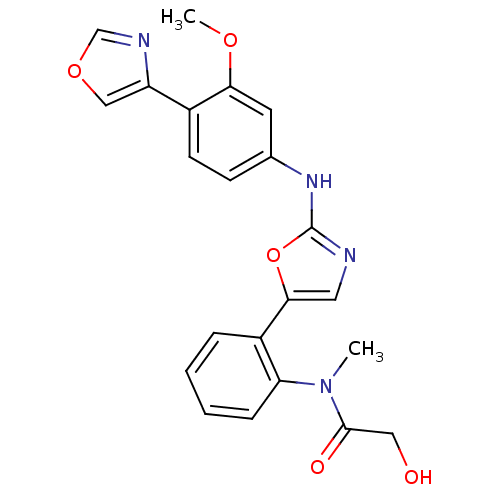
(2-Hydroxy-N-{2-[2-(3-methoxy-4-oxazol-4-yl-phenyla...)Show SMILES COc1cc(Nc2ncc(o2)-c2ccccc2N(C)C(=O)CO)ccc1-c1cocn1 Show InChI InChI=1S/C22H20N4O5/c1-26(21(28)11-27)18-6-4-3-5-16(18)20-10-23-22(31-20)25-14-7-8-15(19(9-14)29-2)17-12-30-13-24-17/h3-10,12-13,27H,11H2,1-2H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50129095
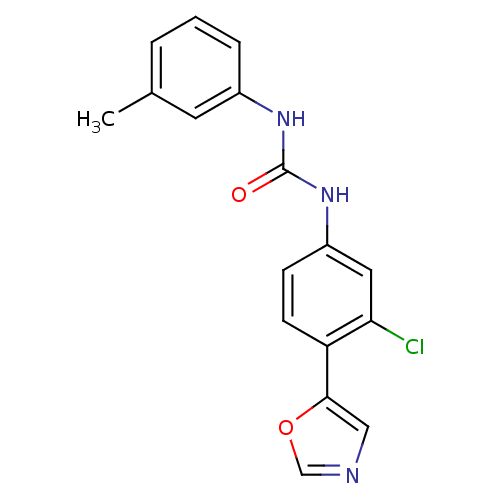
(1-(3-Chloro-4-oxazol-5-yl-phenyl)-3-m-tolyl-urea |...)Show InChI InChI=1S/C17H14ClN3O2/c1-11-3-2-4-12(7-11)20-17(22)21-13-5-6-14(15(18)8-13)16-9-19-10-23-16/h2-10H,1H3,(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50129091

(CHEMBL62496 | N-Methyl-N-{2-[2-(3-methyl-4-oxazol-...)Show SMILES CN(C(=O)CN1CCOCC1)c1ccccc1-c1cnc(Nc2ccc(-c3cnco3)c(C)c2)o1 Show InChI InChI=1S/C26H27N5O4/c1-18-13-19(7-8-20(18)23-14-27-17-34-23)29-26-28-15-24(35-26)21-5-3-4-6-22(21)30(2)25(32)16-31-9-11-33-12-10-31/h3-8,13-15,17H,9-12,16H2,1-2H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50129092

(CHEMBL293162 | N-{2-[2-(3-Chloro-4-oxazol-5-yl-phe...)Show SMILES CN(C(=O)CN1CCOCC1)c1ccccc1-c1cnc(Nc2ccc(-c3cnco3)c(Cl)c2)o1 Show InChI InChI=1S/C25H24ClN5O4/c1-30(24(32)15-31-8-10-33-11-9-31)21-5-3-2-4-19(21)23-14-28-25(35-23)29-17-6-7-18(20(26)12-17)22-13-27-16-34-22/h2-7,12-14,16H,8-11,15H2,1H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50112558

(CHEMBL24866 | N-(3-Bromo-4-oxazol-5-yl-phenyl)-N'-...)Show InChI InChI=1S/C15H16BrN3O3/c1-15(2,3)19-14(21)13(20)18-9-4-5-10(11(16)6-9)12-7-17-8-22-12/h4-8H,1-3H3,(H,18,20)(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50112568
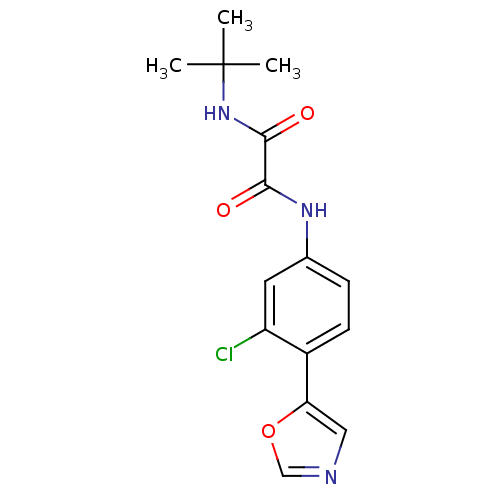
(CHEMBL24092 | N-tert-Butyl-N'-(3-chloro-4-oxazol-5...)Show InChI InChI=1S/C15H16ClN3O3/c1-15(2,3)19-14(21)13(20)18-9-4-5-10(11(16)6-9)12-7-17-8-22-12/h4-8H,1-3H3,(H,18,20)(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50129103
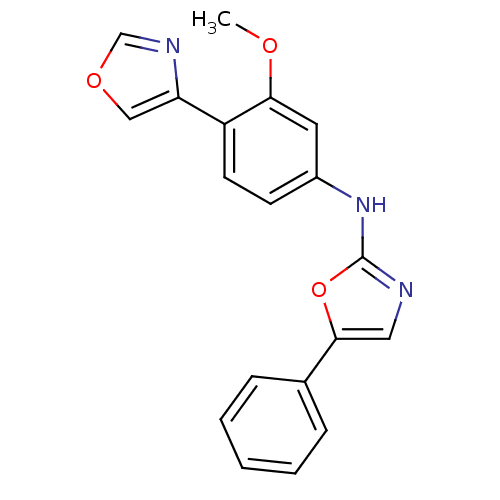
((3-Methoxy-4-oxazol-4-yl-phenyl)-(5-phenyl-oxazol-...)Show InChI InChI=1S/C19H15N3O3/c1-23-17-9-14(7-8-15(17)16-11-24-12-21-16)22-19-20-10-18(25-19)13-5-3-2-4-6-13/h2-12H,1H3,(H,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >57 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50116124
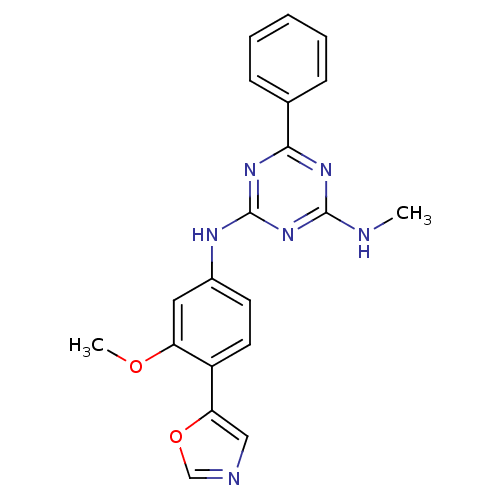
(CHEMBL433062 | N-(3-Methoxy-4-oxazol-5-yl-phenyl)-...)Show SMILES CNc1nc(Nc2ccc(-c3cnco3)c(OC)c2)nc(n1)-c1ccccc1 Show InChI InChI=1S/C20H18N6O2/c1-21-19-24-18(13-6-4-3-5-7-13)25-20(26-19)23-14-8-9-15(16(10-14)27-2)17-11-22-12-28-17/h3-12H,1-2H3,(H2,21,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50129097
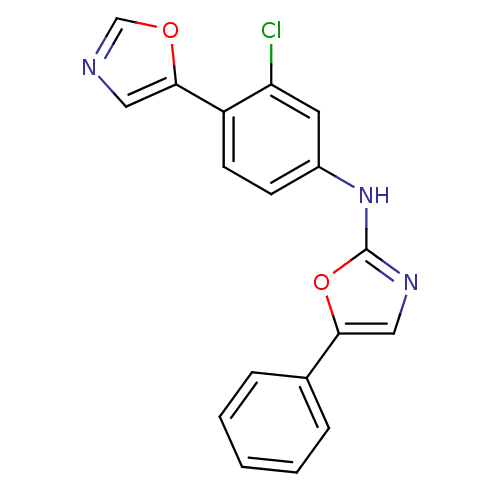
((3-Chloro-4-oxazol-5-yl-phenyl)-(5-phenyl-oxazol-2...)Show InChI InChI=1S/C18H12ClN3O2/c19-15-8-13(6-7-14(15)17-9-20-11-23-17)22-18-21-10-16(24-18)12-4-2-1-3-5-12/h1-11H,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50129118

(CHEMBL302609 | N-{2-[2-(3-Methoxy-4-oxazol-4-yl-ph...)Show SMILES COc1cc(Nc2ncc(o2)-c2ccccc2N(C)C(=O)CN2CCOCC2)ccc1-c1cocn1 Show InChI InChI=1S/C26H27N5O5/c1-30(25(32)15-31-9-11-34-12-10-31)22-6-4-3-5-20(22)24-14-27-26(36-24)29-18-7-8-19(23(13-18)33-2)21-16-35-17-28-21/h3-8,13-14,16-17H,9-12,15H2,1-2H3,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50129100

(2-Hydroxy-N-methyl-N-{2-[2-(3-methyl-4-oxazol-5-yl...)Show SMILES CN(C(=O)CO)c1ccccc1-c1cnc(Nc2ccc(-c3cnco3)c(C)c2)o1 Show InChI InChI=1S/C22H20N4O4/c1-14-9-15(7-8-16(14)19-10-23-13-29-19)25-22-24-11-20(30-22)17-5-3-4-6-18(17)26(2)21(28)12-27/h3-11,13,27H,12H2,1-2H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50129089

(CHEMBL62467 | N-{2-[2-(3-Ethyl-4-oxazol-5-yl-pheny...)Show SMILES CCc1cc(Nc2ncc(o2)-c2ccccc2N(C)C(=O)CO)ccc1-c1cnco1 Show InChI InChI=1S/C23H22N4O4/c1-3-15-10-16(8-9-17(15)20-11-24-14-30-20)26-23-25-12-21(31-23)18-6-4-5-7-19(18)27(2)22(29)13-28/h4-12,14,28H,3,13H2,1-2H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50129107
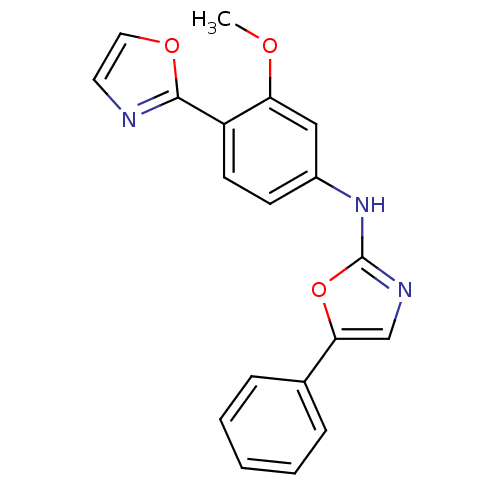
((3-Methoxy-4-oxazol-2-yl-phenyl)-(5-phenyl-oxazol-...)Show InChI InChI=1S/C19H15N3O3/c1-23-16-11-14(7-8-15(16)18-20-9-10-24-18)22-19-21-12-17(25-19)13-5-3-2-4-6-13/h2-12H,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50129120
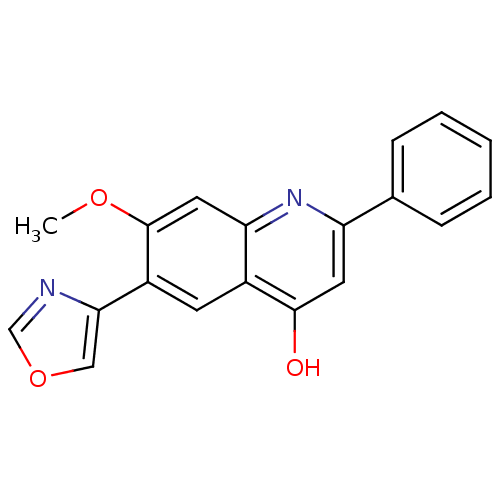
(7-Methoxy-6-oxazol-4-yl-2-phenyl-1H-quinolin-4-one...)Show InChI InChI=1S/C19H14N2O3/c1-23-19-9-16-13(7-14(19)17-10-24-11-20-17)18(22)8-15(21-16)12-5-3-2-4-6-12/h2-11H,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50119130

(CHEMBL60730 | N''-cyano-N-[3-methoxy-4-(1,3-oxazol...)Show SMILES COc1cc(NC(Nc2ccccc2)=NC#N)ccc1-c1cnco1 |w:14.15| Show InChI InChI=1S/C18H15N5O2/c1-24-16-9-14(7-8-15(16)17-10-20-12-25-17)23-18(21-11-19)22-13-5-3-2-4-6-13/h2-10,12H,1H3,(H2,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50129110
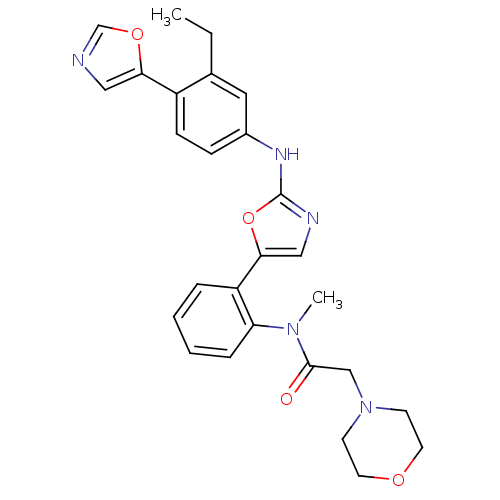
(CHEMBL62747 | N-{2-[2-(3-Ethyl-4-oxazol-5-yl-pheny...)Show SMILES CCc1cc(Nc2ncc(o2)-c2ccccc2N(C)C(=O)CN2CCOCC2)ccc1-c1cnco1 Show InChI InChI=1S/C27H29N5O4/c1-3-19-14-20(8-9-21(19)24-15-28-18-35-24)30-27-29-16-25(36-27)22-6-4-5-7-23(22)31(2)26(33)17-32-10-12-34-13-11-32/h4-9,14-16,18H,3,10-13,17H2,1-2H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50129113

(CHEMBL65970 | N-(3-Chloro-4-oxazol-5-yl-phenyl)-N'...)Show SMILES CNc1nc(Nc2ccc(-c3cnco3)c(Cl)c2)nc(n1)-c1ccccc1 Show InChI InChI=1S/C19H15ClN6O/c1-21-18-24-17(12-5-3-2-4-6-12)25-19(26-18)23-13-7-8-14(15(20)9-13)16-10-22-11-27-16/h2-11H,1H3,(H2,21,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >340 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50129099

((3-Methoxy-4-[1,2,4]triazol-1-yl-phenyl)-(5-phenyl...)Show InChI InChI=1S/C18H15N5O2/c1-24-16-9-14(7-8-15(16)23-12-19-11-21-23)22-18-20-10-17(25-18)13-5-3-2-4-6-13/h2-12H,1H3,(H,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50129112

(1-[4-(4-Methyl-oxazol-5-yl)-phenyl]-3-m-tolyl-urea...)Show InChI InChI=1S/C18H17N3O2/c1-12-4-3-5-16(10-12)21-18(22)20-15-8-6-14(7-9-15)17-13(2)19-11-23-17/h3-11H,1-2H3,(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50129101

(1-(4-Oxazol-5-yl-phenyl)-3-m-tolyl-urea | CHEMBL62...)Show InChI InChI=1S/C17H15N3O2/c1-12-3-2-4-15(9-12)20-17(21)19-14-7-5-13(6-8-14)16-10-18-11-22-16/h2-11H,1H3,(H2,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50129114

(2-Oxazol-5-yl-5-(5-phenyl-oxazol-2-ylamino)-phenol...)Show InChI InChI=1S/C18H13N3O3/c22-15-8-13(6-7-14(15)17-9-19-11-23-17)21-18-20-10-16(24-18)12-4-2-1-3-5-12/h1-11,22H,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50129111
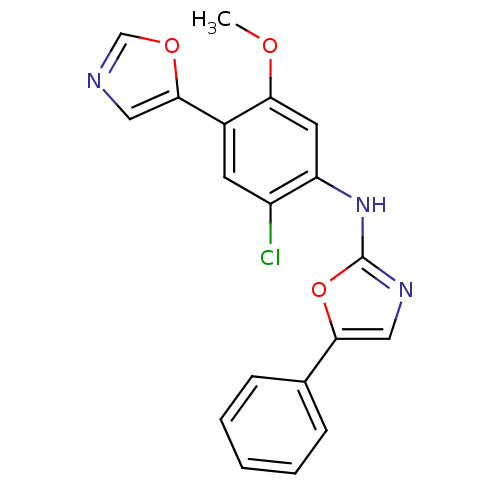
((2-Chloro-5-methoxy-4-oxazol-5-yl-phenyl)-(5-pheny...)Show SMILES COc1cc(Nc2ncc(o2)-c2ccccc2)c(Cl)cc1-c1cnco1 Show InChI InChI=1S/C19H14ClN3O3/c1-24-16-8-15(14(20)7-13(16)18-9-21-11-25-18)23-19-22-10-17(26-19)12-5-3-2-4-6-12/h2-11H,1H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50129121
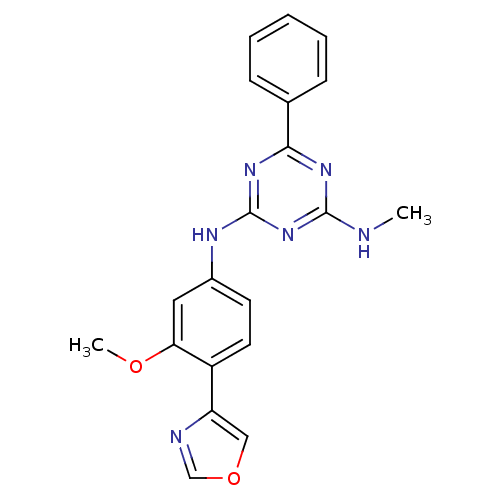
(CHEMBL302626 | N-(3-Methoxy-4-oxazol-4-yl-phenyl)-...)Show SMILES CNc1nc(Nc2ccc(-c3cocn3)c(OC)c2)nc(n1)-c1ccccc1 Show InChI InChI=1S/C20H18N6O2/c1-21-19-24-18(13-6-4-3-5-7-13)25-20(26-19)23-14-8-9-15(17(10-14)27-2)16-11-28-12-22-16/h3-12H,1-2H3,(H2,21,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50129123

(CHEMBL63466 | [3-Methoxy-4-(2-methyl-oxazol-5-yl)-...)Show InChI InChI=1S/C20H17N3O3/c1-13-21-12-19(25-13)16-9-8-15(10-17(16)24-2)23-20-22-11-18(26-20)14-6-4-3-5-7-14/h3-12H,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50129098

(CHEMBL63501 | [4-(2,4-Dimethyl-oxazol-5-yl)-phenyl...)Show SMILES Cc1nc(C)c(o1)-c1ccc(Nc2ncc(o2)-c2ccccc2)cc1 Show InChI InChI=1S/C20H17N3O2/c1-13-19(24-14(2)22-13)16-8-10-17(11-9-16)23-20-21-12-18(25-20)15-6-4-3-5-7-15/h3-12H,1-2H3,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50129094

(3-Furan-2-yl-N-[4-(4-methyl-oxazol-5-yl)-phenyl]-a...)Show InChI InChI=1S/C17H14N2O3/c1-12-17(22-11-18-12)13-4-6-14(7-5-13)19-16(20)9-8-15-3-2-10-21-15/h2-11H,1H3,(H,19,20)/b9-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50129104

((2-Chloro-3-methoxy-4-oxazol-5-yl-phenyl)-(5-pheny...)Show SMILES COc1c(Cl)c(Nc2ncc(o2)-c2ccccc2)ccc1-c1cnco1 Show InChI InChI=1S/C19H14ClN3O3/c1-24-18-13(16-9-21-11-25-16)7-8-14(17(18)20)23-19-22-10-15(26-19)12-5-3-2-4-6-12/h2-11H,1H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50112571
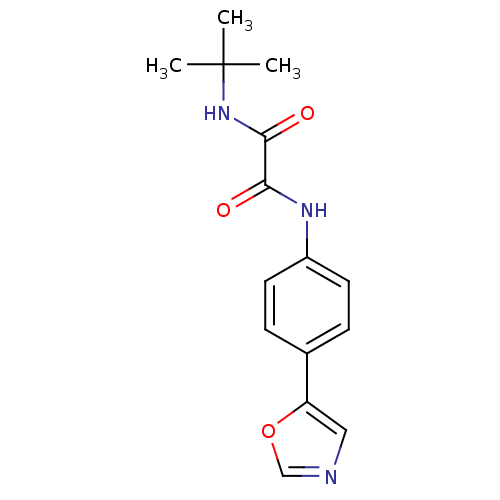
(CHEMBL27985 | N-tert-Butyl-N'-(4-oxazol-5-yl-pheny...)Show InChI InChI=1S/C15H17N3O3/c1-15(2,3)18-14(20)13(19)17-11-6-4-10(5-7-11)12-8-16-9-21-12/h4-9H,1-3H3,(H,17,19)(H,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50129102

(CHEMBL63968 | [3-Methoxy-4-(4-methyl-oxazol-5-yl)-...)Show InChI InChI=1S/C20H17N3O3/c1-13-19(25-12-22-13)16-9-8-15(10-17(16)24-2)23-20-21-11-18(26-20)14-6-4-3-5-7-14/h3-12H,1-2H3,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50129122

((3-Methyl-4-oxazol-4-yl-phenyl)-(5-phenyl-oxazol-2...)Show InChI InChI=1S/C19H15N3O2/c1-13-9-15(7-8-16(13)17-11-23-12-21-17)22-19-20-10-18(24-19)14-5-3-2-4-6-14/h2-12H,1H3,(H,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50129105

((4-Oxazol-5-yl-phenyl)-(5-phenyl-oxazol-2-yl)-amin...)Show InChI InChI=1S/C18H13N3O2/c1-2-4-13(5-3-1)17-11-20-18(23-17)21-15-8-6-14(7-9-15)16-10-19-12-22-16/h1-12H,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50129096

((4-Oxazol-4-yl-phenyl)-(5-phenyl-oxazol-2-yl)-amin...)Show InChI InChI=1S/C18H13N3O2/c1-2-4-14(5-3-1)17-10-19-18(23-17)21-15-8-6-13(7-9-15)16-11-22-12-20-16/h1-12H,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50112570
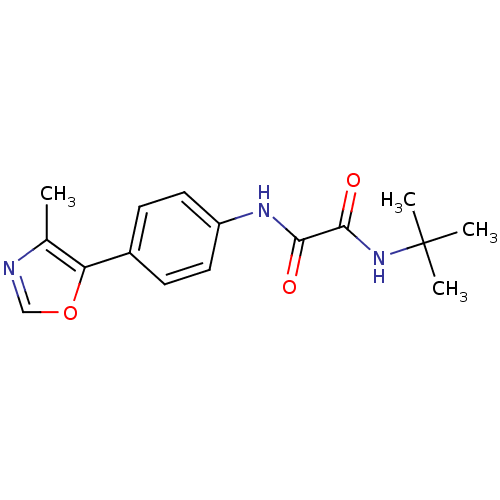
(CHEMBL279957 | N-tert-Butyl-N'-[4-(4-methyl-oxazol...)Show InChI InChI=1S/C16H19N3O3/c1-10-13(22-9-17-10)11-5-7-12(8-6-11)18-14(20)15(21)19-16(2,3)4/h5-9H,1-4H3,(H,18,20)(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50129108

((3-Chloro-4-oxazol-4-yl-phenyl)-(5-phenyl-oxazol-2...)Show InChI InChI=1S/C18H12ClN3O2/c19-15-8-13(6-7-14(15)16-10-23-11-21-16)22-18-20-9-17(24-18)12-4-2-1-3-5-12/h1-11H,(H,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50129088

(CHEMBL62355 | N-Methyl-N'-(4-oxazol-5-yl-phenyl)-6...)Show InChI InChI=1S/C19H16N6O/c1-20-18-23-17(14-5-3-2-4-6-14)24-19(25-18)22-15-9-7-13(8-10-15)16-11-21-12-26-16/h2-12H,1H3,(H2,20,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50129119

(CHEMBL64898 | [4-(4-Methyl-oxazol-5-yl)-phenyl]-(5...)Show InChI InChI=1S/C19H15N3O2/c1-13-18(23-12-21-13)15-7-9-16(10-8-15)22-19-20-11-17(24-19)14-5-3-2-4-6-14/h2-12H,1H3,(H,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50129087
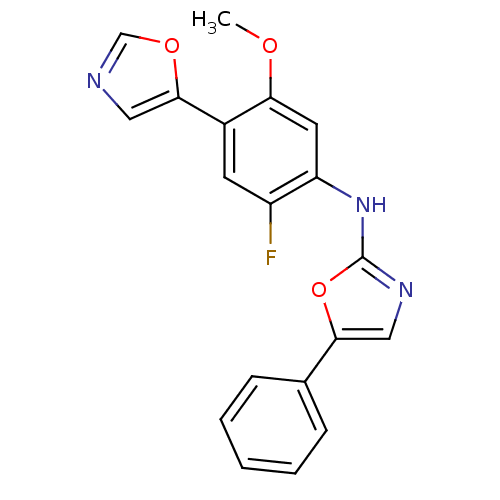
((2-Fluoro-5-methoxy-4-oxazol-5-yl-phenyl)-(5-pheny...)Show InChI InChI=1S/C19H14FN3O3/c1-24-16-8-15(14(20)7-13(16)18-9-21-11-25-18)23-19-22-10-17(26-19)12-5-3-2-4-6-12/h2-11H,1H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50129115
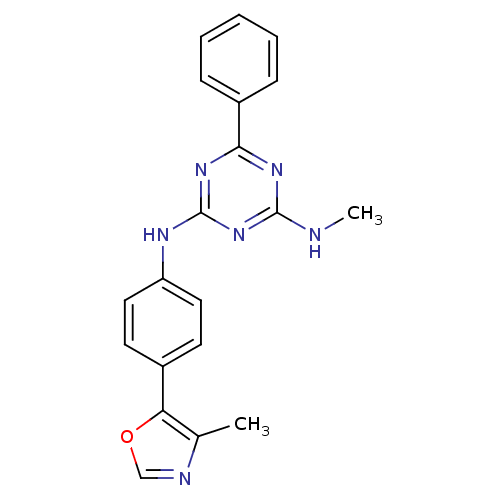
(CHEMBL62691 | N-Methyl-N'-[4-(4-methyl-oxazol-5-yl...)Show SMILES CNc1nc(Nc2ccc(cc2)-c2ocnc2C)nc(n1)-c1ccccc1 Show InChI InChI=1S/C20H18N6O/c1-13-17(27-12-22-13)14-8-10-16(11-9-14)23-20-25-18(24-19(21-2)26-20)15-6-4-3-5-7-15/h3-12H,1-2H3,(H2,21,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50129117
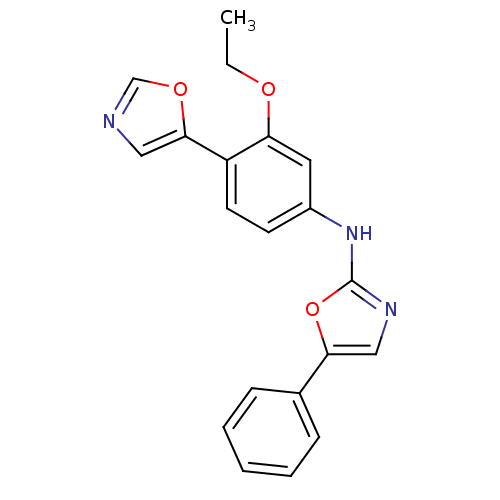
((3-Ethoxy-4-oxazol-5-yl-phenyl)-(5-phenyl-oxazol-2...)Show InChI InChI=1S/C20H17N3O3/c1-2-24-17-10-15(8-9-16(17)19-11-21-13-25-19)23-20-22-12-18(26-20)14-6-4-3-5-7-14/h3-13H,2H2,1H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of inosine-5'-monophosphate dehydrogenase 2. |
Bioorg Med Chem Lett 13: 2059-63 (2003)
BindingDB Entry DOI: 10.7270/Q22Z14X1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data