 Found 66 hits of Enzyme Inhibition Constant Data
Found 66 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129541
(3-[5-(2',4'-Dichloro-biphenyl-4-yl)-3-tetrazol-1-y...)Show SMILES Clc1ccc(c(Cl)c1)-c1ccc(cc1)-c1cc(nn1-c1cccnc1)-n1cnnn1 Show InChI InChI=1S/C21H13Cl2N7/c22-16-7-8-18(19(23)10-16)14-3-5-15(6-4-14)20-11-21(29-13-25-27-28-29)26-30(20)17-2-1-9-24-12-17/h1-13H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129527
(5-(2',4'-Dichloro-biphenyl-4-yl)-1-pyridin-3-yl-1H...)Show SMILES Clc1ccc(c(Cl)c1)-c1ccc(cc1)-c1cc(nn1-c1cccnc1)C(=O)NCc1nnn[nH]1 Show InChI InChI=1S/C23H16Cl2N8O/c24-16-7-8-18(19(25)10-16)14-3-5-15(6-4-14)21-11-20(23(34)27-13-22-28-31-32-29-22)30-33(21)17-2-1-9-26-12-17/h1-12H,13H2,(H,27,34)(H,28,29,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human methionyl-tRNA synthetase (hMetRS). |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129528

(2-[5-(2',4'-Dichloro-biphenyl-4-yl)-3-tetrazol-1-y...)Show SMILES Clc1ccc(c(Cl)c1)-c1ccc(cc1)-c1cc(nn1-c1ccccn1)-n1cnnn1 Show InChI InChI=1S/C21H13Cl2N7/c22-16-8-9-17(18(23)11-16)14-4-6-15(7-5-14)19-12-21(29-13-25-27-28-29)26-30(19)20-3-1-2-10-24-20/h1-13H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129551
(CHEMBL74224 | {[5-(2',4'-Dichloro-biphenyl-4-yl)-1...)Show SMILES OC(=O)CNC(=O)c1cc(-c2ccc(cc2)-c2ccc(Cl)cc2Cl)n(n1)-c1cccnc1 Show InChI InChI=1S/C23H16Cl2N4O3/c24-16-7-8-18(19(25)10-16)14-3-5-15(6-4-14)21-11-20(23(32)27-13-22(30)31)28-29(21)17-2-1-9-26-12-17/h1-12H,13H2,(H,27,32)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129534

(5-(2',4'-Dichloro-biphenyl-4-yl)-1-pyridin-3-yl-1H...)Show SMILES OC(=O)c1cc(-c2ccc(cc2)-c2ccc(Cl)cc2Cl)n(n1)-c1cccnc1 Show InChI InChI=1S/C21H13Cl2N3O2/c22-15-7-8-17(18(23)10-15)13-3-5-14(6-4-13)20-11-19(21(27)28)25-26(20)16-2-1-9-24-12-16/h1-12H,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human methionyl-tRNA synthetase (hMetRS). |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129553
(5-(2',4'-Dichloro-biphenyl-4-yl)-1-pyridin-4-ylmet...)Show SMILES OC(=O)c1cc(-c2ccc(cc2)-c2ccc(Cl)cc2Cl)n(Cc2ccncc2)n1 Show InChI InChI=1S/C22H15Cl2N3O2/c23-17-5-6-18(19(24)11-17)15-1-3-16(4-2-15)21-12-20(22(28)29)26-27(21)13-14-7-9-25-10-8-14/h1-12H,13H2,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129539
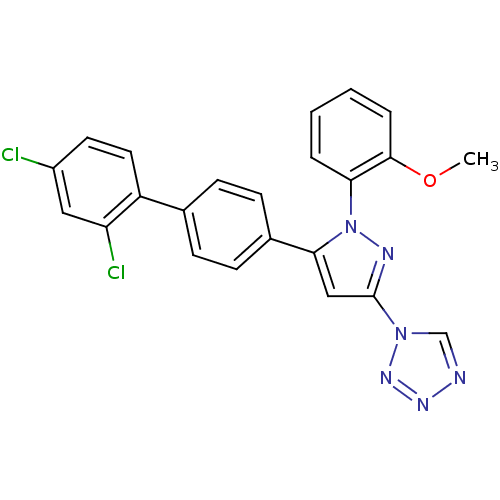
(1-[5-(2',4'-Dichloro-biphenyl-4-yl)-1-(2-methoxy-p...)Show SMILES COc1ccccc1-n1nc(cc1-c1ccc(cc1)-c1ccc(Cl)cc1Cl)-n1cnnn1 Show InChI InChI=1S/C23H16Cl2N6O/c1-32-22-5-3-2-4-20(22)31-21(13-23(27-31)30-14-26-28-29-30)16-8-6-15(7-9-16)18-11-10-17(24)12-19(18)25/h2-14H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Enterococci faecalis methionyl-tRNA synthetase (EfMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129546
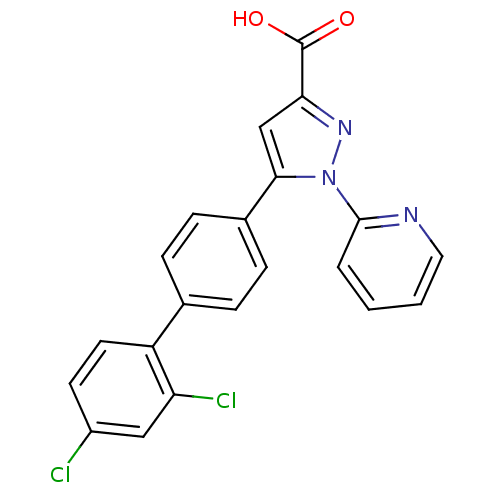
(5-(2',4'-Dichloro-biphenyl-4-yl)-1-pyridin-2-yl-1H...)Show SMILES OC(=O)c1cc(-c2ccc(cc2)-c2ccc(Cl)cc2Cl)n(n1)-c1ccccn1 Show InChI InChI=1S/C21H13Cl2N3O2/c22-15-8-9-16(17(23)11-15)13-4-6-14(7-5-13)19-12-18(21(27)28)25-26(19)20-3-1-2-10-24-20/h1-12H,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human methionyl-tRNA synthetase (hMetRS). |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129533
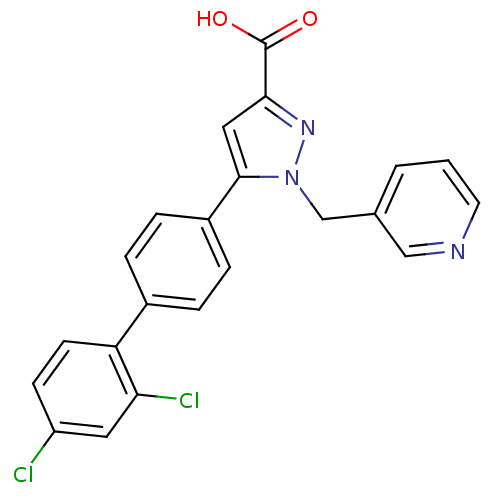
(5-(2',4'-Dichloro-biphenyl-4-yl)-1-pyridin-3-ylmet...)Show SMILES OC(=O)c1cc(-c2ccc(cc2)-c2ccc(Cl)cc2Cl)n(Cc2cccnc2)n1 Show InChI InChI=1S/C22H15Cl2N3O2/c23-17-7-8-18(19(24)10-17)15-3-5-16(6-4-15)21-11-20(22(28)29)26-27(21)13-14-2-1-9-25-12-14/h1-12H,13H2,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129550
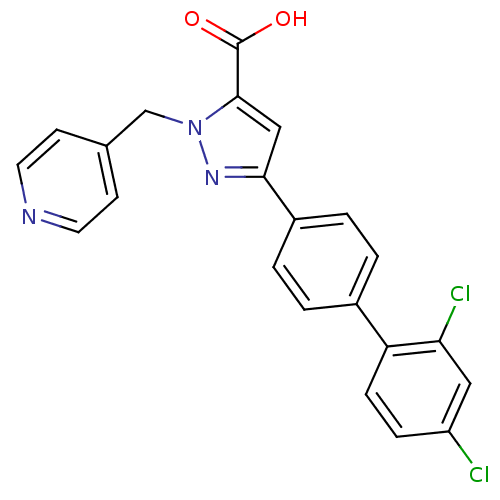
(5-(2',4'-Dichloro-biphenyl-4-yl)-2-pyridin-4-ylmet...)Show SMILES OC(=O)c1cc(nn1Cc1ccncc1)-c1ccc(cc1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C22H15Cl2N3O2/c23-17-5-6-18(19(24)11-17)15-1-3-16(4-2-15)20-12-21(22(28)29)27(26-20)13-14-7-9-25-10-8-14/h1-12H,13H2,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129532
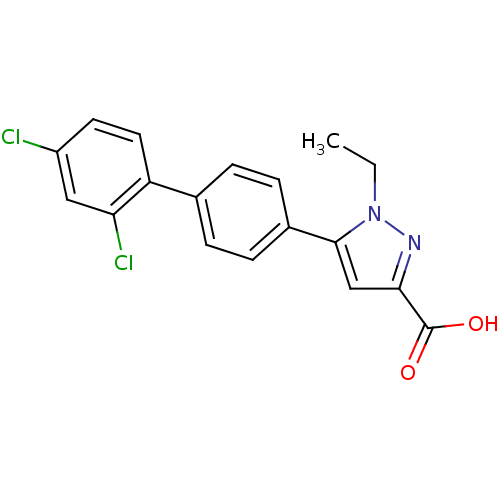
(5-(2',4'-Dichloro-biphenyl-4-yl)-1-ethyl-1H-pyrazo...)Show SMILES CCn1nc(cc1-c1ccc(cc1)-c1ccc(Cl)cc1Cl)C(O)=O Show InChI InChI=1S/C18H14Cl2N2O2/c1-2-22-17(10-16(21-22)18(23)24)12-5-3-11(4-6-12)14-8-7-13(19)9-15(14)20/h3-10H,2H2,1H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129543
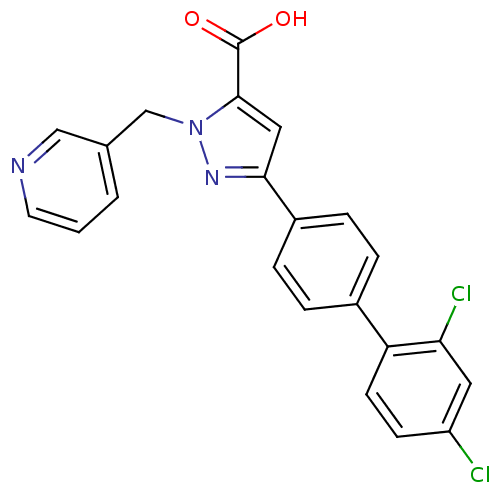
(5-(2',4'-Dichloro-biphenyl-4-yl)-2-pyridin-3-ylmet...)Show SMILES OC(=O)c1cc(nn1Cc1cccnc1)-c1ccc(cc1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C22H15Cl2N3O2/c23-17-7-8-18(19(24)10-17)15-3-5-16(6-4-15)20-11-21(22(28)29)27(26-20)13-14-2-1-9-25-12-14/h1-12H,13H2,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human methionyl-tRNA synthetase (hMetRS). |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129526
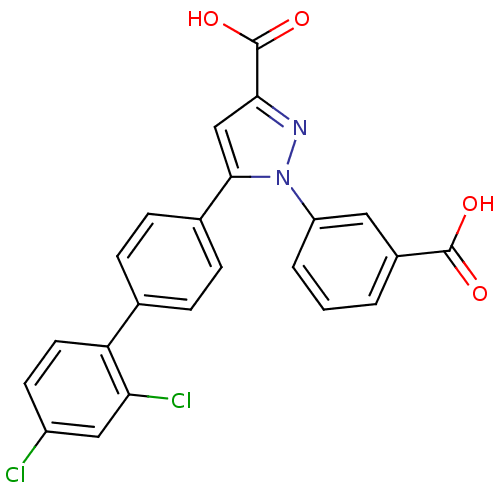
(1-(3-Carboxy-phenyl)-5-(2',4'-dichloro-biphenyl-4-...)Show SMILES OC(=O)c1cc(-c2ccc(cc2)-c2ccc(Cl)cc2Cl)n(n1)-c1cccc(c1)C(O)=O Show InChI InChI=1S/C23H14Cl2N2O4/c24-16-8-9-18(19(25)11-16)13-4-6-14(7-5-13)21-12-20(23(30)31)26-27(21)17-3-1-2-15(10-17)22(28)29/h1-12H,(H,28,29)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129548
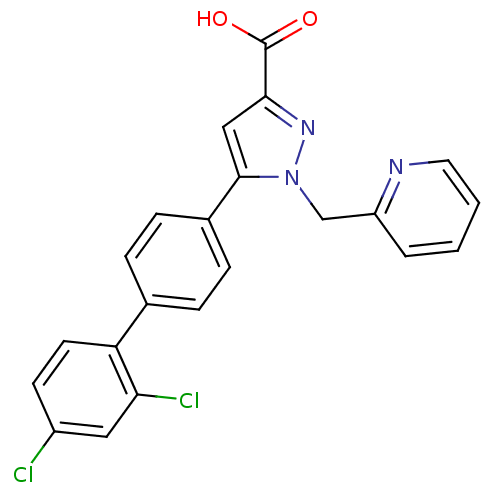
(5-(2',4'-Dichloro-biphenyl-4-yl)-1-pyridin-2-ylmet...)Show SMILES OC(=O)c1cc(-c2ccc(cc2)-c2ccc(Cl)cc2Cl)n(Cc2ccccn2)n1 Show InChI InChI=1S/C22H15Cl2N3O2/c23-16-8-9-18(19(24)11-16)14-4-6-15(7-5-14)21-12-20(22(28)29)26-27(21)13-17-3-1-2-10-25-17/h1-12H,13H2,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129544
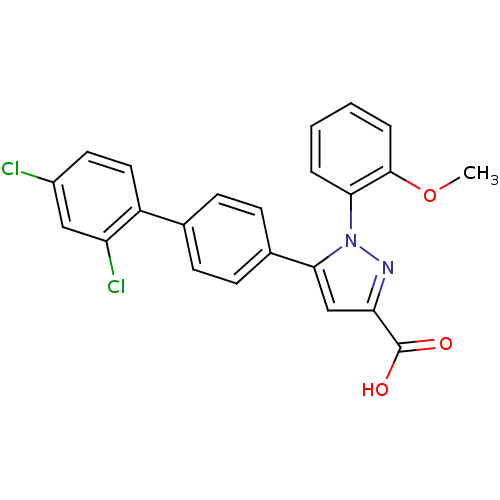
(5-(2',4'-Dichloro-biphenyl-4-yl)-1-(2-methoxy-phen...)Show SMILES COc1ccccc1-n1nc(cc1-c1ccc(cc1)-c1ccc(Cl)cc1Cl)C(O)=O Show InChI InChI=1S/C23H16Cl2N2O3/c1-30-22-5-3-2-4-20(22)27-21(13-19(26-27)23(28)29)15-8-6-14(7-9-15)17-11-10-16(24)12-18(17)25/h2-13H,1H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129528

(2-[5-(2',4'-Dichloro-biphenyl-4-yl)-3-tetrazol-1-y...)Show SMILES Clc1ccc(c(Cl)c1)-c1ccc(cc1)-c1cc(nn1-c1ccccn1)-n1cnnn1 Show InChI InChI=1S/C21H13Cl2N7/c22-16-8-9-17(18(23)11-16)14-4-6-15(7-5-14)19-12-21(29-13-25-27-28-29)26-30(19)20-3-1-2-10-24-20/h1-13H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Enterococci faecalis methionyl-tRNA synthetase (EfMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129546

(5-(2',4'-Dichloro-biphenyl-4-yl)-1-pyridin-2-yl-1H...)Show SMILES OC(=O)c1cc(-c2ccc(cc2)-c2ccc(Cl)cc2Cl)n(n1)-c1ccccn1 Show InChI InChI=1S/C21H13Cl2N3O2/c22-15-8-9-16(17(23)11-15)13-4-6-14(7-5-13)19-12-18(21(27)28)25-26(19)20-3-1-2-10-24-20/h1-12H,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Enterococci faecalis methionyl-tRNA synthetase (EfMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129542
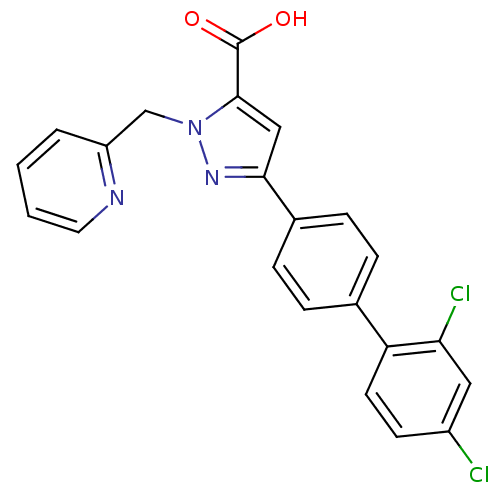
(5-(2',4'-Dichloro-biphenyl-4-yl)-2-pyridin-2-ylmet...)Show SMILES OC(=O)c1cc(nn1Cc1ccccn1)-c1ccc(cc1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C22H15Cl2N3O2/c23-16-8-9-18(19(24)11-16)14-4-6-15(7-5-14)20-12-21(22(28)29)27(26-20)13-17-3-1-2-10-25-17/h1-12H,13H2,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129529
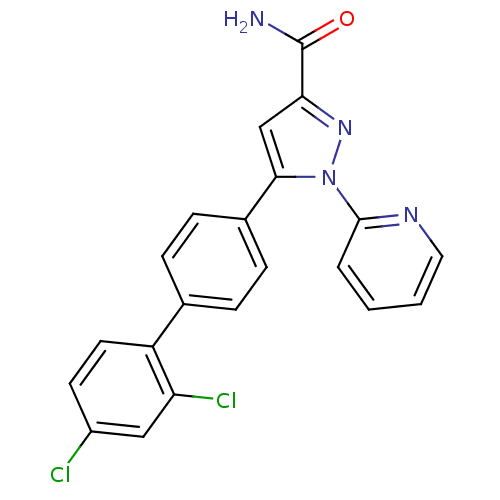
(5-(2',4'-Dichloro-biphenyl-4-yl)-1-pyridin-2-yl-1H...)Show SMILES NC(=O)c1cc(-c2ccc(cc2)-c2ccc(Cl)cc2Cl)n(n1)-c1ccccn1 Show InChI InChI=1S/C21H14Cl2N4O/c22-15-8-9-16(17(23)11-15)13-4-6-14(7-5-13)19-12-18(21(24)28)26-27(19)20-3-1-2-10-25-20/h1-12H,(H2,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129535
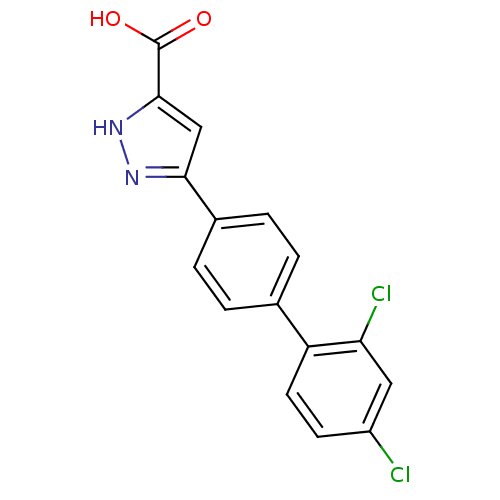
(5-(2',4'-Dichloro-biphenyl-4-yl)-1H-pyrazole-3-car...)Show SMILES OC(=O)c1cc(n[nH]1)-c1ccc(cc1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C16H10Cl2N2O2/c17-11-5-6-12(13(18)7-11)9-1-3-10(4-2-9)14-8-15(16(21)22)20-19-14/h1-8H,(H,19,20)(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129554
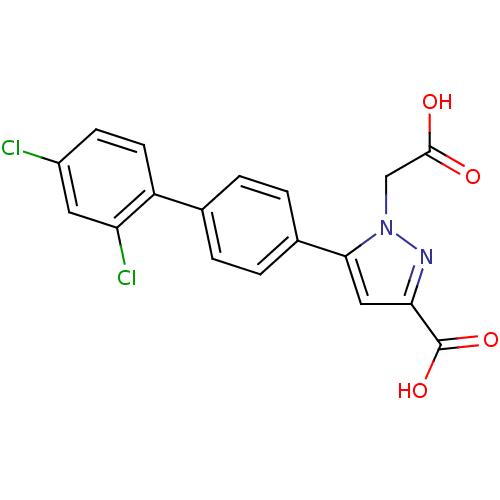
(1-Carboxymethyl-5-(2',4'-dichloro-biphenyl-4-yl)-1...)Show SMILES OC(=O)Cn1nc(cc1-c1ccc(cc1)-c1ccc(Cl)cc1Cl)C(O)=O Show InChI InChI=1S/C18H12Cl2N2O4/c19-12-5-6-13(14(20)7-12)10-1-3-11(4-2-10)16-8-15(18(25)26)21-22(16)9-17(23)24/h1-8H,9H2,(H,23,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129527

(5-(2',4'-Dichloro-biphenyl-4-yl)-1-pyridin-3-yl-1H...)Show SMILES Clc1ccc(c(Cl)c1)-c1ccc(cc1)-c1cc(nn1-c1cccnc1)C(=O)NCc1nnn[nH]1 Show InChI InChI=1S/C23H16Cl2N8O/c24-16-7-8-18(19(25)10-16)14-3-5-15(6-4-14)21-11-20(23(34)27-13-22-28-31-32-29-22)30-33(21)17-2-1-9-26-12-17/h1-12H,13H2,(H,27,34)(H,28,29,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Enterococci faecalis methionyl-tRNA synthetase (EfMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129531

(1-Benzyl-5-(2',4'-dichloro-biphenyl-4-yl)-1H-pyraz...)Show SMILES OC(=O)c1cc(-c2ccc(cc2)-c2ccc(Cl)cc2Cl)n(Cc2ccccc2)n1 Show InChI InChI=1S/C23H16Cl2N2O2/c24-18-10-11-19(20(25)12-18)16-6-8-17(9-7-16)22-13-21(23(28)29)26-27(22)14-15-4-2-1-3-5-15/h1-13H,14H2,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129540

(5-(2',4'-Dichloro-biphenyl-4-yl)-1-(2-hydroxy-ethy...)Show SMILES OCCn1nc(cc1-c1ccc(cc1)-c1ccc(Cl)cc1Cl)C(O)=O Show InChI InChI=1S/C18H14Cl2N2O3/c19-13-5-6-14(15(20)9-13)11-1-3-12(4-2-11)17-10-16(18(24)25)21-22(17)7-8-23/h1-6,9-10,23H,7-8H2,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human methionyl-tRNA synthetase (hMetRS). |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129536

(5-(2',4'-Dichloro-biphenyl-4-yl)-1-(2,4-dichloro-p...)Show SMILES OC(=O)c1cc(-c2ccc(cc2)-c2ccc(Cl)cc2Cl)n(n1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C22H12Cl4N2O2/c23-14-5-7-16(17(25)9-14)12-1-3-13(4-2-12)21-11-19(22(29)30)27-28(21)20-8-6-15(24)10-18(20)26/h1-11H,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human methionyl-tRNA synthetase (hMetRS). |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129545

(5-(2',4'-Dichloro-biphenyl-4-yl)-1-phenyl-1H-pyraz...)Show SMILES OC(=O)c1cc(-c2ccc(cc2)-c2ccc(Cl)cc2Cl)n(n1)-c1ccccc1 Show InChI InChI=1S/C22H14Cl2N2O2/c23-16-10-11-18(19(24)12-16)14-6-8-15(9-7-14)21-13-20(22(27)28)25-26(21)17-4-2-1-3-5-17/h1-13H,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129526

(1-(3-Carboxy-phenyl)-5-(2',4'-dichloro-biphenyl-4-...)Show SMILES OC(=O)c1cc(-c2ccc(cc2)-c2ccc(Cl)cc2Cl)n(n1)-c1cccc(c1)C(O)=O Show InChI InChI=1S/C23H14Cl2N2O4/c24-16-8-9-18(19(25)11-16)13-4-6-14(7-5-13)21-12-20(23(30)31)26-27(21)17-3-1-2-15(10-17)22(28)29/h1-12H,(H,28,29)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129541

(3-[5-(2',4'-Dichloro-biphenyl-4-yl)-3-tetrazol-1-y...)Show SMILES Clc1ccc(c(Cl)c1)-c1ccc(cc1)-c1cc(nn1-c1cccnc1)-n1cnnn1 Show InChI InChI=1S/C21H13Cl2N7/c22-16-7-8-18(19(23)10-16)14-3-5-15(6-4-14)20-11-21(29-13-25-27-28-29)26-30(20)17-2-1-9-24-12-17/h1-13H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Enterococci faecalis methionyl-tRNA synthetase (EfMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129536

(5-(2',4'-Dichloro-biphenyl-4-yl)-1-(2,4-dichloro-p...)Show SMILES OC(=O)c1cc(-c2ccc(cc2)-c2ccc(Cl)cc2Cl)n(n1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C22H12Cl4N2O2/c23-14-5-7-16(17(25)9-14)12-1-3-13(4-2-12)21-11-19(22(29)30)27-28(21)20-8-6-15(24)10-18(20)26/h1-11H,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human methionyl-tRNA synthetase (hMetRS). |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129537

(5-(2',4'-Dichloro-biphenyl-4-yl)-1-(4-methoxy-phen...)Show SMILES COc1ccc(cc1)-n1nc(cc1-c1ccc(cc1)-c1ccc(Cl)cc1Cl)C(O)=O Show InChI InChI=1S/C23H16Cl2N2O3/c1-30-18-9-7-17(8-10-18)27-22(13-21(26-27)23(28)29)15-4-2-14(3-5-15)19-11-6-16(24)12-20(19)25/h2-13H,1H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129541

(3-[5-(2',4'-Dichloro-biphenyl-4-yl)-3-tetrazol-1-y...)Show SMILES Clc1ccc(c(Cl)c1)-c1ccc(cc1)-c1cc(nn1-c1cccnc1)-n1cnnn1 Show InChI InChI=1S/C21H13Cl2N7/c22-16-7-8-18(19(23)10-16)14-3-5-15(6-4-14)20-11-21(29-13-25-27-28-29)26-30(20)17-2-1-9-24-12-17/h1-13H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Enterococci faecalis methionyl-tRNA synthetase (EfMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129540

(5-(2',4'-Dichloro-biphenyl-4-yl)-1-(2-hydroxy-ethy...)Show SMILES OCCn1nc(cc1-c1ccc(cc1)-c1ccc(Cl)cc1Cl)C(O)=O Show InChI InChI=1S/C18H14Cl2N2O3/c19-13-5-6-14(15(20)9-13)11-1-3-12(4-2-11)17-10-16(18(24)25)21-22(17)7-8-23/h1-6,9-10,23H,7-8H2,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129536

(5-(2',4'-Dichloro-biphenyl-4-yl)-1-(2,4-dichloro-p...)Show SMILES OC(=O)c1cc(-c2ccc(cc2)-c2ccc(Cl)cc2Cl)n(n1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C22H12Cl4N2O2/c23-14-5-7-16(17(25)9-14)12-1-3-13(4-2-12)21-11-19(22(29)30)27-28(21)20-8-6-15(24)10-18(20)26/h1-11H,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human methionyl-tRNA synthetase (hMetRS). |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129527

(5-(2',4'-Dichloro-biphenyl-4-yl)-1-pyridin-3-yl-1H...)Show SMILES Clc1ccc(c(Cl)c1)-c1ccc(cc1)-c1cc(nn1-c1cccnc1)C(=O)NCc1nnn[nH]1 Show InChI InChI=1S/C23H16Cl2N8O/c24-16-7-8-18(19(25)10-16)14-3-5-15(6-4-14)21-11-20(23(34)27-13-22-28-31-32-29-22)30-33(21)17-2-1-9-26-12-17/h1-12H,13H2,(H,27,34)(H,28,29,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Enterococci faecalis methionyl-tRNA synthetase (EfMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129538

(5-(3'-Chloro-4'-fluoro-biphenyl-4-yl)-1-(2,4-dichl...)Show SMILES OC(=O)c1cc(-c2ccc(cc2)-c2ccc(F)c(Cl)c2)n(n1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C22H12Cl3FN2O2/c23-15-6-8-20(17(25)10-15)28-21(11-19(27-28)22(29)30)13-3-1-12(2-4-13)14-5-7-18(26)16(24)9-14/h1-11H,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human methionyl-tRNA synthetase (hMetRS). |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129550

(5-(2',4'-Dichloro-biphenyl-4-yl)-2-pyridin-4-ylmet...)Show SMILES OC(=O)c1cc(nn1Cc1ccncc1)-c1ccc(cc1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C22H15Cl2N3O2/c23-17-5-6-18(19(24)11-17)15-1-3-16(4-2-15)20-12-21(22(28)29)27(26-20)13-14-7-9-25-10-8-14/h1-12H,13H2,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human methionyl-tRNA synthetase (hMetRS). |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129544

(5-(2',4'-Dichloro-biphenyl-4-yl)-1-(2-methoxy-phen...)Show SMILES COc1ccccc1-n1nc(cc1-c1ccc(cc1)-c1ccc(Cl)cc1Cl)C(O)=O Show InChI InChI=1S/C23H16Cl2N2O3/c1-30-22-5-3-2-4-20(22)27-21(13-19(26-27)23(28)29)15-8-6-14(7-9-15)17-11-10-16(24)12-18(17)25/h2-13H,1H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Enterococci faecalis methionyl-tRNA synthetase (EfMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129543

(5-(2',4'-Dichloro-biphenyl-4-yl)-2-pyridin-3-ylmet...)Show SMILES OC(=O)c1cc(nn1Cc1cccnc1)-c1ccc(cc1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C22H15Cl2N3O2/c23-17-7-8-18(19(24)10-17)15-3-5-16(6-4-15)20-11-21(22(28)29)27(26-20)13-14-2-1-9-25-12-14/h1-12H,13H2,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human methionyl-tRNA synthetase (hMetRS). |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129539

(1-[5-(2',4'-Dichloro-biphenyl-4-yl)-1-(2-methoxy-p...)Show SMILES COc1ccccc1-n1nc(cc1-c1ccc(cc1)-c1ccc(Cl)cc1Cl)-n1cnnn1 Show InChI InChI=1S/C23H16Cl2N6O/c1-32-22-5-3-2-4-20(22)31-21(13-23(27-31)30-14-26-28-29-30)16-8-6-15(7-9-16)18-11-10-17(24)12-19(18)25/h2-14H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human methionyl-tRNA synthetase (hMetRS). |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129551

(CHEMBL74224 | {[5-(2',4'-Dichloro-biphenyl-4-yl)-1...)Show SMILES OC(=O)CNC(=O)c1cc(-c2ccc(cc2)-c2ccc(Cl)cc2Cl)n(n1)-c1cccnc1 Show InChI InChI=1S/C23H16Cl2N4O3/c24-16-7-8-18(19(25)10-16)14-3-5-15(6-4-14)21-11-20(23(32)27-13-22(30)31)28-29(21)17-2-1-9-26-12-17/h1-12H,13H2,(H,27,32)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human methionyl-tRNA synthetase (hMetRS). |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129538

(5-(3'-Chloro-4'-fluoro-biphenyl-4-yl)-1-(2,4-dichl...)Show SMILES OC(=O)c1cc(-c2ccc(cc2)-c2ccc(F)c(Cl)c2)n(n1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C22H12Cl3FN2O2/c23-15-6-8-20(17(25)10-15)28-21(11-19(27-28)22(29)30)13-3-1-12(2-4-13)14-5-7-18(26)16(24)9-14/h1-11H,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human methionyl-tRNA synthetase (hMetRS). |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129542

(5-(2',4'-Dichloro-biphenyl-4-yl)-2-pyridin-2-ylmet...)Show SMILES OC(=O)c1cc(nn1Cc1ccccn1)-c1ccc(cc1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C22H15Cl2N3O2/c23-16-8-9-18(19(24)11-16)14-4-6-15(7-5-14)20-12-21(22(28)29)27(26-20)13-17-3-1-2-10-25-17/h1-12H,13H2,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human methionyl-tRNA synthetase (hMetRS). |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129528

(2-[5-(2',4'-Dichloro-biphenyl-4-yl)-3-tetrazol-1-y...)Show SMILES Clc1ccc(c(Cl)c1)-c1ccc(cc1)-c1cc(nn1-c1ccccn1)-n1cnnn1 Show InChI InChI=1S/C21H13Cl2N7/c22-16-8-9-17(18(23)11-16)14-4-6-15(7-5-14)19-12-21(29-13-25-27-28-29)26-30(19)20-3-1-2-10-24-20/h1-13H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Enterococci faecalis methionyl-tRNA synthetase (EfMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129553

(5-(2',4'-Dichloro-biphenyl-4-yl)-1-pyridin-4-ylmet...)Show SMILES OC(=O)c1cc(-c2ccc(cc2)-c2ccc(Cl)cc2Cl)n(Cc2ccncc2)n1 Show InChI InChI=1S/C22H15Cl2N3O2/c23-17-5-6-18(19(24)11-17)15-1-3-16(4-2-15)21-12-20(22(28)29)26-27(21)13-14-7-9-25-10-8-14/h1-12H,13H2,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129531

(1-Benzyl-5-(2',4'-dichloro-biphenyl-4-yl)-1H-pyraz...)Show SMILES OC(=O)c1cc(-c2ccc(cc2)-c2ccc(Cl)cc2Cl)n(Cc2ccccc2)n1 Show InChI InChI=1S/C23H16Cl2N2O2/c24-18-10-11-19(20(25)12-18)16-6-8-17(9-7-16)22-13-21(23(28)29)26-27(22)14-15-4-2-1-3-5-15/h1-13H,14H2,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129549

(1-(2,4-Dichloro-phenyl)-5-(4'-trifluoromethyl-biph...)Show SMILES OC(=O)c1cc(-c2ccc(cc2)-c2ccc(cc2)C(F)(F)F)n(n1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C23H13Cl2F3N2O2/c24-17-9-10-20(18(25)11-17)30-21(12-19(29-30)22(31)32)15-3-1-13(2-4-15)14-5-7-16(8-6-14)23(26,27)28/h1-12H,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129549

(1-(2,4-Dichloro-phenyl)-5-(4'-trifluoromethyl-biph...)Show SMILES OC(=O)c1cc(-c2ccc(cc2)-c2ccc(cc2)C(F)(F)F)n(n1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C23H13Cl2F3N2O2/c24-17-9-10-20(18(25)11-17)30-21(12-19(29-30)22(31)32)15-3-1-13(2-4-15)14-5-7-16(8-6-14)23(26,27)28/h1-12H,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129525
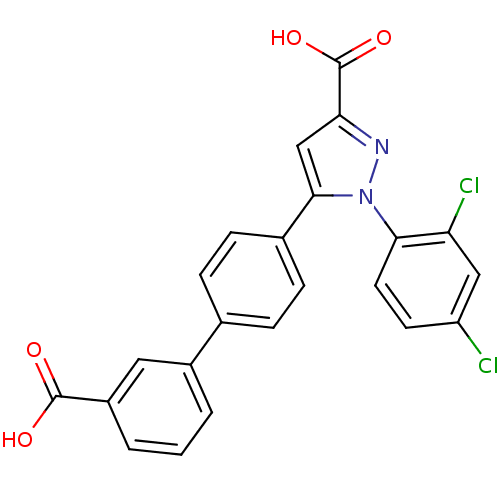
(5-(3'-Carboxy-biphenyl-4-yl)-1-(2,4-dichloro-pheny...)Show SMILES OC(=O)c1cc(-c2ccc(cc2)-c2cccc(c2)C(O)=O)n(n1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C23H14Cl2N2O4/c24-17-8-9-20(18(25)11-17)27-21(12-19(26-27)23(30)31)14-6-4-13(5-7-14)15-2-1-3-16(10-15)22(28)29/h1-12H,(H,28,29)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human methionyl-tRNA synthetase (hMetRS). |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129547
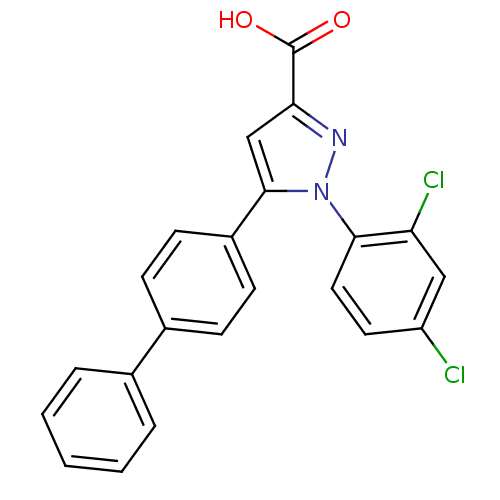
(5-Biphenyl-4-yl-1-(2,4-dichloro-phenyl)-1H-pyrazol...)Show SMILES OC(=O)c1cc(-c2ccc(cc2)-c2ccccc2)n(n1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C22H14Cl2N2O2/c23-17-10-11-20(18(24)12-17)26-21(13-19(25-26)22(27)28)16-8-6-15(7-9-16)14-4-2-1-3-5-14/h1-13H,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129525

(5-(3'-Carboxy-biphenyl-4-yl)-1-(2,4-dichloro-pheny...)Show SMILES OC(=O)c1cc(-c2ccc(cc2)-c2cccc(c2)C(O)=O)n(n1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C23H14Cl2N2O4/c24-17-8-9-20(18(25)11-17)27-21(12-19(26-27)23(30)31)14-6-4-13(5-7-14)15-2-1-3-16(10-15)22(28)29/h1-12H,(H,28,29)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human methionyl-tRNA synthetase (hMetRS). |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129552
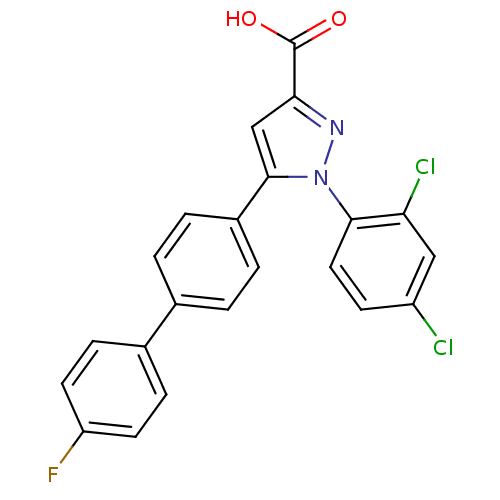
(1-(2,4-Dichloro-phenyl)-5-(4'-fluoro-biphenyl-4-yl...)Show SMILES OC(=O)c1cc(-c2ccc(cc2)-c2ccc(F)cc2)n(n1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C22H13Cl2FN2O2/c23-16-7-10-20(18(24)11-16)27-21(12-19(26-27)22(28)29)15-3-1-13(2-4-15)14-5-8-17(25)9-6-14/h1-12H,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human methionyl-tRNA synthetase (hMetRS). |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129548

(5-(2',4'-Dichloro-biphenyl-4-yl)-1-pyridin-2-ylmet...)Show SMILES OC(=O)c1cc(-c2ccc(cc2)-c2ccc(Cl)cc2Cl)n(Cc2ccccn2)n1 Show InChI InChI=1S/C22H15Cl2N3O2/c23-16-8-9-18(19(24)11-16)14-4-6-15(7-5-14)21-12-20(22(28)29)26-27(21)13-17-3-1-2-10-25-17/h1-12H,13H2,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129552

(1-(2,4-Dichloro-phenyl)-5-(4'-fluoro-biphenyl-4-yl...)Show SMILES OC(=O)c1cc(-c2ccc(cc2)-c2ccc(F)cc2)n(n1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C22H13Cl2FN2O2/c23-16-7-10-20(18(24)11-16)27-21(12-19(26-27)22(28)29)15-3-1-13(2-4-15)14-5-8-17(25)9-6-14/h1-12H,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human methionyl-tRNA synthetase (hMetRS). |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129547

(5-Biphenyl-4-yl-1-(2,4-dichloro-phenyl)-1H-pyrazol...)Show SMILES OC(=O)c1cc(-c2ccc(cc2)-c2ccccc2)n(n1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C22H14Cl2N2O2/c23-17-10-11-20(18(24)12-17)26-21(13-19(25-26)22(27)28)16-8-6-15(7-9-16)14-4-2-1-3-5-14/h1-13H,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129532

(5-(2',4'-Dichloro-biphenyl-4-yl)-1-ethyl-1H-pyrazo...)Show SMILES CCn1nc(cc1-c1ccc(cc1)-c1ccc(Cl)cc1Cl)C(O)=O Show InChI InChI=1S/C18H14Cl2N2O2/c1-2-22-17(10-16(21-22)18(23)24)12-5-3-11(4-6-12)14-8-7-13(19)9-15(14)20/h3-10H,2H2,1H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human methionyl-tRNA synthetase (hMetRS). |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129534

(5-(2',4'-Dichloro-biphenyl-4-yl)-1-pyridin-3-yl-1H...)Show SMILES OC(=O)c1cc(-c2ccc(cc2)-c2ccc(Cl)cc2Cl)n(n1)-c1cccnc1 Show InChI InChI=1S/C21H13Cl2N3O2/c22-15-7-8-17(18(23)10-15)13-3-5-14(6-4-13)20-11-19(21(27)28)25-26(20)16-2-1-9-24-12-16/h1-12H,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human methionyl-tRNA synthetase (hMetRS). |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129530

(5-(2',4'-Dichloro-biphenyl-4-yl)-1-(4-trifluoromet...)Show SMILES OC(=O)c1cc(-c2ccc(cc2)-c2ccc(Cl)cc2Cl)n(n1)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C23H13Cl2F3N2O3/c24-15-5-10-18(19(25)11-15)13-1-3-14(4-2-13)21-12-20(22(31)32)29-30(21)16-6-8-17(9-7-16)33-23(26,27)28/h1-12H,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129530

(5-(2',4'-Dichloro-biphenyl-4-yl)-1-(4-trifluoromet...)Show SMILES OC(=O)c1cc(-c2ccc(cc2)-c2ccc(Cl)cc2Cl)n(n1)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C23H13Cl2F3N2O3/c24-15-5-10-18(19(25)11-15)13-1-3-14(4-2-13)21-12-20(22(31)32)29-30(21)16-6-8-17(9-7-16)33-23(26,27)28/h1-12H,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129533

(5-(2',4'-Dichloro-biphenyl-4-yl)-1-pyridin-3-ylmet...)Show SMILES OC(=O)c1cc(-c2ccc(cc2)-c2ccc(Cl)cc2Cl)n(Cc2cccnc2)n1 Show InChI InChI=1S/C22H15Cl2N3O2/c23-17-7-8-18(19(24)10-17)15-3-5-16(6-4-15)21-11-20(22(28)29)26-27(21)13-14-2-1-9-25-12-14/h1-12H,13H2,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129540

(5-(2',4'-Dichloro-biphenyl-4-yl)-1-(2-hydroxy-ethy...)Show SMILES OCCn1nc(cc1-c1ccc(cc1)-c1ccc(Cl)cc1Cl)C(O)=O Show InChI InChI=1S/C18H14Cl2N2O3/c19-13-5-6-14(15(20)9-13)11-1-3-12(4-2-11)17-10-16(18(24)25)21-22(17)7-8-23/h1-6,9-10,23H,7-8H2,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human methionyl-tRNA synthetase (hMetRS). |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129554

(1-Carboxymethyl-5-(2',4'-dichloro-biphenyl-4-yl)-1...)Show SMILES OC(=O)Cn1nc(cc1-c1ccc(cc1)-c1ccc(Cl)cc1Cl)C(O)=O Show InChI InChI=1S/C18H12Cl2N2O4/c19-12-5-6-13(14(20)7-12)10-1-3-11(4-2-10)16-8-15(18(25)26)21-22(16)9-17(23)24/h1-8H,9H2,(H,23,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human methionyl-tRNA synthetase (hMetRS). |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129537

(5-(2',4'-Dichloro-biphenyl-4-yl)-1-(4-methoxy-phen...)Show SMILES COc1ccc(cc1)-n1nc(cc1-c1ccc(cc1)-c1ccc(Cl)cc1Cl)C(O)=O Show InChI InChI=1S/C23H16Cl2N2O3/c1-30-18-9-7-17(8-10-18)27-22(13-21(26-27)23(28)29)15-4-2-14(3-5-15)19-11-6-16(24)12-20(19)25/h2-13H,1H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human methionyl-tRNA synthetase (hMetRS). |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129546

(5-(2',4'-Dichloro-biphenyl-4-yl)-1-pyridin-2-yl-1H...)Show SMILES OC(=O)c1cc(-c2ccc(cc2)-c2ccc(Cl)cc2Cl)n(n1)-c1ccccn1 Show InChI InChI=1S/C21H13Cl2N3O2/c22-15-8-9-16(17(23)11-15)13-4-6-14(7-5-13)19-12-18(21(27)28)25-26(19)20-3-1-2-10-24-20/h1-12H,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Enterococci faecalis methionyl-tRNA synthetase (EfMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129526

(1-(3-Carboxy-phenyl)-5-(2',4'-dichloro-biphenyl-4-...)Show SMILES OC(=O)c1cc(-c2ccc(cc2)-c2ccc(Cl)cc2Cl)n(n1)-c1cccc(c1)C(O)=O Show InChI InChI=1S/C23H14Cl2N2O4/c24-16-8-9-18(19(25)11-16)13-4-6-14(7-5-13)21-12-20(23(30)31)26-27(21)17-3-1-2-15(10-17)22(28)29/h1-12H,(H,28,29)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human methionyl-tRNA synthetase (hMetRS). |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129529

(5-(2',4'-Dichloro-biphenyl-4-yl)-1-pyridin-2-yl-1H...)Show SMILES NC(=O)c1cc(-c2ccc(cc2)-c2ccc(Cl)cc2Cl)n(n1)-c1ccccn1 Show InChI InChI=1S/C21H14Cl2N4O/c22-15-8-9-16(17(23)11-15)13-4-6-14(7-5-13)19-12-18(21(24)28)26-27(19)20-3-1-2-10-25-20/h1-12H,(H2,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human methionyl-tRNA synthetase (hMetRS). |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129530

(5-(2',4'-Dichloro-biphenyl-4-yl)-1-(4-trifluoromet...)Show SMILES OC(=O)c1cc(-c2ccc(cc2)-c2ccc(Cl)cc2Cl)n(n1)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C23H13Cl2F3N2O3/c24-15-5-10-18(19(25)11-15)13-1-3-14(4-2-13)21-12-20(22(31)32)29-30(21)16-6-8-17(9-7-16)33-23(26,27)28/h1-12H,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data